Abstract
The aim of this study was to assess the current state of automated pupillometry technology and its application in the neurointensive care unit (neuroICU). We performed a literature search using the PubMed, MEDLINE, and EMBASE databases from database inception through a search end date of October 18, 2018, to identify studies reporting on the use of automated pupillometry in the care of critically ill patients with neurological impairment. Two independent reviewers reviewed all titles and abstracts in two filtering phases. Data were extracted independently. One hundred and forty-one articles/abstracts have been published on the use of automated pupillometry in critical care since inception of the PubMed, MEDLINE, and EMBASE databases. We selected and reviewed 22 full-text articles and 8 abstracts, of which 26 were prospective, 2 were retrospective, and 2 were larger case series. Automated pupillometry increased precision, reliability, and reproducibility compared with the manual pupillary examination; detected subtle and early pupillary changes; detected pupillary changes that indicate a rise, or impending rise, in intracranial pressure detected level of analgesia and depth of sedation; served as a prognostic indicator; estimated the clinical severity of aneurysmal subarachnoid hemorrhage; and served as a noninvasive monitor of response to osmotic therapy. At present, no consensus guidelines exist endorsing routine use of automated pupillometry in the neuroICU. However, an increasing quantity of research supports the usefulness of automated pupillometry in this setting. Further large-scale prospective studies are needed before updated consensus guidelines recommending widespread adoption of automated pupillometry are produced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Close monitoring of neurological status is employed in multiple clinical settings, as many conditions predispose to neurological deterioration related to cerebral edema and increasing intracranial pressure (ICP). These include, but are not limited to, postoperative neurosurgery, hypothermia after cardiac arrest (CA), acute ischemic or hemorrhagic stroke, brain tumors, and head injury [1]. Traumatic brain injury (TBI) alone affects more than 2.8 million Americans annually [2].
Neuromonitoring is essential to the detection of abnormalities in cerebral perfusion, oxygenation, chemistry, and function to support measures for regeneration from primary brain damage and to prevent secondary brain damage [3]. Additionally, intense clinical monitoring is an integral part of the management of patients at risk of neurological deterioration because neurological worsening may occur rapidly and signs and symptoms may be subtle [4, 5]. Neurological deterioration is associated with poor outcomes; therefore, at-risk patients are routinely monitored every 30–60 min, or more frequently. These serial neurological examinations provide important information that guides clinical decision making [4]. Thus, nursing and medical staff who regularly attend to these patients must be adept at performing thorough neurological assessments [5].
Neurointensive care units (neuroICUs) apply various methods for monitoring neurological function, as patients are often unable to participate in clinical examinations due to their critical condition and/or sedation [3]. The adoption of new technologies to assist clinical staff in their serial assessments of neurological status has been common practice in neuroICUs [3]. In 2014, a Neurocritical Care Society (NCS) expert group, in collaboration with the European Society of Intensive Care Medicine, the Society of Critical Care Medicine, and the Latin American Brain Injury Consortium, assessed widely used methods for neuromonitoring and published consensus summary recommendations to guide healthcare professionals who routinely apply these technologies. Invasive methods for neuromonitoring, such as by probes and catheters, were prioritized in severe brain injury and thought to be fairly accurate, reliable, and valid [6,7,8]. However, the disadvantages of invasive methods are noted to be numerous. Invasive methods are expensive, cannot easily be changed or readapted, may require neurosurgical assistance, often require imaging to control the probe location, and, importantly, carry a risk of bleeding and infection [3]. A recent descriptive review of commonly applied noninvasive neuromonitoring methods, including transcranial ultrasound, evoked potentials, electroencephalography, near-infrared spectroscopy, and the emerging noninvasive technologies of bispectral index, bioimpedance, cranial accelerometry, and pupillometry concluded that, in addition to their noninvasive nature, these methods provide important advantages of repeatability, adjustability, low cost, and easy execution and interpretation [3].
Pupillometry is an emerging technology of particular interest because the pupillary examination is established as a fundamental element of any neurological assessment [9]. Abnormalities of pupillary response are associated with neurological deterioration and correlated with poor neurological outcomes [10]. In the clinical setting, pupil size, shape, symmetry, and the pupillary light reflex are most commonly tested manually by clinicians and nurses [11, 12]. However, there are intra- and inter-observer disparities in interpreting the results, especially in the extremities of pupil size [12,13,14]. This has led to the development of automated objective pupillometers. The pupillometer is a handheld, portable, user-friendly, automated, accessible, inexpensive device with the capability to perform reproducible, precise, and quantitative measurements [14]. Although there are variations in the make of automated pupillometers available on the market, the working principle is the same and most devices assess similar parameters. Common parameters evaluated include the pupillary light reflex, pupil diameter and shape, onset latency, constriction and dilatation velocities, and percentage/ratio reduction in amplitude [3].
The 2014 NCS consensus conference did not recommend the use of automated pupillometry for routine neuromonitoring and instead stressed that automated pupillometry needs more development and validation through randomized controlled trials and careful observational studies [6]. However, various studies published both prior to and since 2014 demonstrate automated pupillometry’s value in the clinical setting. Since the conference, hospitals have quickly adopted a practice that includes automated pupillometry technology, with neurocritical care at the forefront of this trend [12, 15, 16]. As of the last fiscal quarter, 295 hospitals in the USA have adopted the use of NeurOptics® pupillometers, one of the leading commercial brands. The company now has a presence in over 23 countries worldwide [17]. This has led to a corresponding increase in the quantity of research published on automated pupillometry and its usefulness as an assessment tool. However, despite the increase in available research and continued advancements in pupillometry technology, there have been no new consensus guidelines published as to the use of automated pupillometers in the neurointensive care setting.
With this systematic review, we aim to assess the specific outcomes associated with the use of automated quantitative pupillometry in neuromonitoring of critically ill patients with neurological impairment who receive care in a critical care setting. Additionally, we examine whether the specific outcomes associated with the use of automated quantitative pupillometers in this patient population have any effect on patient outcomes and assess potential limitations to wider adoption of automated pupillometry technology. We also consider whether there is now sufficient evidence to validate routine use of automated quantitative pupillometry in the care of these patients.
Methods
We performed a systematic review according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. A literature search using the PubMed, MEDLINE, and EMBASE databases was performed from database inception through a search end date of October 18, 2018. Original studies with human subjects reporting on specific outcomes associated with the use of automated quantitative pupillometry to monitor critically ill patients with neurological impairment in a critical care setting were eligible for inclusion. Articles and abstracts published in English with study populations of 15 patients or more were included. Smaller case reports and case series were excluded. There was no limitation on type of publication.
We used the keyword “pupillometry” and at least one of the following terms or phrases in our search: neurocritical care, intracranial pathology, neurological monitoring, intracranial pressure, herniation, head trauma, intensive care, critical care, intracranial lesions, critically ill patients, outcomes, and prognosis. This primary database search provided 135 records after removal of duplicates. Six additional records were identified through text references and communication with researchers in the field, for a total of 141 titles/abstracts. Two independent reviewers (S.P. and C.M.) reviewed all titles and abstracts in the first of two filtering phases, providing 8 abstracts and 24 full-text manuscripts for further review and inclusion.
In the second filtering phase, the same reviewers evaluated full-text manuscripts for eligibility, providing 22 articles for inclusion, in addition to the 8 abstracts, for a total of 30 records. The first of two excluded full-text articles did not meet criteria for an original study and the second failed to control for intra- and inter-patient variability, rendering results inconclusive. All 30 records comprising original articles and abstracts were included and thoroughly reviewed by the same reviewers with no discrepancies. Data extraction was performed independently.
We determined the study quality for each study using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach, with randomized trials given a high grade, observational studies a low grade, and any other evidence, a very low grade. Applying the patient–intervention–comparison–outcome (PICO) format to our data, the patient problem was the pupillary examination, the intervention was the automated quantitative pupillometer, and the comparison was the manual examination. Primary outcomes assessed included the ability of automated pupillometry to increase precision, reliability, and reproducibility compared with the manual pupillary examination; detect subtle and early pupillary changes; detect pupillary changes that indicate a rise, or impending rise, in intracranial pressure (ICP) detect level of analgesia and depth of sedation; serve as a prognostic indicator; estimate the clinical severity of aneurysmal subarachnoid hemorrhage (aSAH); and serve as a noninvasive monitor of response to osmotic therapy. Secondary outcomes assessed included the ability of automated quantitative pupillometry to affect clinical outcomes and whether sufficient evidence exists to validate routine use of automated quantitative pupillometry in the care of critically ill patients in the neuroICU. We also examined how cost, variety of pathology, environmental factors, and presence of medical comorbidities may influence the use of automated pupillometry and serve to limit wider adoption of the technology. Our systematic review includes relevant data from all 30 records and provides an updated assessment of the utility of automated pupillometry in the care of critically ill patients with neurological impairment based on the current literature.
Results
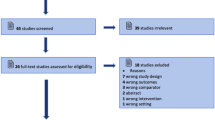
One hundred and forty-one articles and abstracts have been published on this subject since inception of the PubMed, MEDLINE, and EMBASE databases. After review, we selected 22 manuscripts and 8 abstracts that met eligibility criteria for inclusion according to the PRISMA Flow Diagram (Fig. 1). All 30 records were original studies with human subjects, of which 26 were prospective, 2 were retrospective, and 2 were larger case series. Table 1 summarizes the studies and main findings.
PRISMA flow diagram. From: From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097. For more information, visit www.prisma-statement.org
Increased Precision, Reliability, and Reproducibility
The increased precision, reliability, and reproducibility of automated pupillometry compared to manual examinations were evaluated by six studies included in this review [11, 14, 18,19,20,21]. In all cases, automated pupillometry was superior to manual pupillometry. Meeker et al. [11] found that the median absolute error in measuring pupil size using manual examination is over twice as large when compared to an automated pupillometer (0.54 mm, 95% CI 0.51–0.62 vs. 0.23 mm, 95% CI 0.20–0.31). Couret et al. [20] showed a 19% rate of discordance in pupil size measurements between manual examination by nurses and automated pupillometry for pupils between 2 and 4 mm in size. This discrepancy increased to 39% for pupils less than 2 mm. Additionally, trained neurocritical care nurses did not detect 50% of cases of anisocoria and wrongly detected 16 cases of anisocoria.
Olson et al. [14] measured inter-examiner reliability using Cohen’s kappa coefficient (k) and showed that reliability between two independent practitioners performing manual pupillary assessments was only moderate for pupil size (k = 0.54, 95% CI 0.50–0.57) and only fair between the two independent practitioners and pupillometers (k = 0.29, 95% CI 0.27–0.32 and k = 0.31, 95% CI 0.28–0.34) for the first and second practitioners, respectively. Yan et al. [19] also found lower inter-examiner disagreement using the automated pupillometer compared to manual pupillometry when measuring pupil size in patients with absent pupil reactivity (4.5% vs. 27.3%, p < 0.05) and sluggish pupil reactivity (4.5% vs. 31.8%, p < 0.05).
Assessing inter-device reliability, Zhao et al. [21] determined that Cohen’s kappa assessments (k) for pupil size and reactivity showed almost perfect agreement between two identical NeurOptics® NPi™-100 pupillometers for maximum pupil size (k = 0.97 left eye, k = 0.91 right eye), minimum pupil size (k = 0.96 left eye, k = 0.98 right eye), and pupil reactivity (k = 0.99 left eye, k = 0.90 right eye).
Detection of Subtle Pupillary Changes
Several studies examined the ability of automated pupillometry to detect subtle pupillary changes and do so earlier than manual examination [14, 19, 22, 23]. Prior research has shown that routine manual evaluation with traditional penlight is unable to detect the presence of a pupillary light reflex (PLR) when the amplitude is < 0.3 mm [22]. Olson et al. [14] found that automated pupillometry could detect a light reflex in 66.7% of pupils that were scored as non-reactive by practitioners. Larson et al. [22] determined that 44% of comatose ICU patients assessed to have no PLR on manual examination were found to have a detectable PLR using automated pupillometry.
Additionally, Shoyombo et al. [23] found that automated pupillometers were better able to detect subtle and unexpected changes in the PLR by capturing many variables simultaneously and normalizing data using pupillary index technology, specifically the neurological pupil index (NPi) function of automated pupillometers manufactured by NeurOptics®. They found that when measuring NPi and constriction velocity (CV), two distinct variables for characterizing the PLR, an NPi of less than 3.0 (abnormal), did not automatically correspond to lower CV values (sluggish pupils), as would be expected. 30.9% of patients had either one or both eyes showing normal NPi with slow CV, or abnormal NPi with brisk CV, mismatches that are often missed on manual pupillary examination.
Detection of Pupillary Changes that Indicate a Rise, or Impending Rise, in Intracranial Pressure
The ability of automated pupillometers to detect pupillary changes that indicate a rise, or impending rise, in ICP was evaluated by four studies included in this review [18, 24,25,26]. Taylor et al. [18] showed that pupillary size asymmetry detected by automated pupillometry of at least 0.5 mm was present in 81% of observations when ICP rose above 30 mmHg. Similarly, Giede-Jeppe et al. [25] showed an association between pupillary index technology and increased ICP as an NPi < 4.15 at the time of ICP measurement was associated with a 7.7-fold higher rate of ICP crises when compared to an NPi > 4.15. Additionally, Natzeder et al. [26] confirmed prior findings of a statistically significant inverse correlation between NPi and ICP (Spearman r = − 0.551, p < 0.001) using data from patients that had undergone continuous ICP monitoring.
Detection of Level of Analgesia and Depth of Sedation
There are six studies included in this review that assess the ability of automated pupillometry to detect the level of analgesia or depth of sedation in critically ill patients [27,28,29,30,31,32]. Lukaszewicz et al. [27] examined patients who were unable to communicate verbally and found that a percentage variation in pupil size (> 19%) during a noxious stimulus (i.e., dressing change) was predictive of the presence of pain as determined by a Behavioral Pain Scale score > 3 with 100% sensitivity and 77% specificity. Rouche et al. [28] compared maximum contraction velocity of the pupil (Vmax) and variation in pupil diameter (PD) between three groups based on evaluation of bispectral index (BIS): heavy sedation (< 40), acceptable sedation (40–60), and light sedation (> 60). They found a statistically significant difference in Vmax (1.14 mm/s vs. 1.35 mm/s, p < 0.0001) and variation in PD (0.3 mm vs. 0.4 mm, p < 0.0001) between heavy sedation and acceptable sedation groups. There was also a statistically significant difference in Vmax (1.14 mm/s vs. 1.40 mm/s, p < 0.0001) and variation in PD (0.3 mm vs. 0.43 mm, p < 0.0001) between heavy sedation and light sedation groups.
Paulus et al. [29] evaluated the performance of the pupillary dilatation reflex (PDR), an autonomic response that results from a noxious stimulus, in predicting insufficient analgesia before endotracheal suctioning of deeply sedated mechanically ventilated ICU patients by applying tetanic stimulations of 10, 20, and 40 mA to elicit the PDR. The areas under the receiver operating characteristic (ROC) curve for predicting insufficient analgesia were 0.70 (95% CI 0.54–0.85), 0.78 (95% CI 0.61–0.91), and 0.85 (95% CI 0.721–0.954) with 10, 20, and 40 mA tetanic stimulations, respectively. However, Gaillard et al. [30] performed a similar study in sedated and mechanically ventilated surgical ICU patients with tetanic stimulations of 5, 10, 20, 40 and 60 mA and found no difference in the areas under the ROC curves between different intensity levels, and PDR at different stimulation levels never exceeded 0.6.
Utility as a Prognostic Indicator
Of the 30 studies included in this review, 14 explore the ability of the automated pupillometer to be used as a prognostic indicator [24, 26, 33,34,35,36,37,38,39,40,41,42,43,44]. Tamura et al. [33] showed that automated quantitative PLR values were consistently higher in survivors of CA within the first 72 h of return of spontaneous circulation (ROSC) compared to non-survivors at 90 days. PLR value was associated with 90-day survival (p < 0.001). PLR values were also consistently higher in patients with a good neurological outcome determined by a cerebral performance category (CPC) of 1 or 2 compared to those with poor neurological outcomes (CPC 3–5) at 90 days. PLR value was associated with 90-day favorable neurological outcomes (p < 0.001). ROC curve analysis for the comparison of area under the curve (AUC) values at each measurement time point (0, 6, 12, 24, 48, and 72 h after ROSC) showed that 0-h PLR value was the best predictor of 90-day survival with an AUC value of 0.82 using a PLR cutoff value of 3%. Additionally, 0-h PLR value was the best predictor of good neurological outcome at 90 days with an AUC value of 0.84 and PLR cutoff value of 6%.
Similarly, Solari et al. [34] were the first to use a blinded approach to analyze the value of quantitative automated pupillometry to predict neurologic recovery in CA. The blinded approach was taken to limit “self-fulfilling prophecy.” A total of 103 comatose adult patients who were unconscious 48 h after CA underwent repeated measurements of quantitative PLR using an automated pupillometer. It was discovered that a quantitative PLR < 13% at a relatively early stage after CA (48 h) was 100% predictive of mortality, irrespective of the amount of sedatives, analgesics, and vasopressors.
The prognostic value of automated pupillometry has also been demonstrated in clinical conditions other than CA. Park et al. [44] evaluated the utility of automated pupillometry to predict clinical outcomes in patients with acute brain lesions. They showed that there is a definite difference in initial NPi values between patients with poor neurological outcomes as determined by a Glascow Outcome Scale (GOS) < 3 and those with favorable (GOS ≥ 3) outcomes (mean ± SD) (0.88 ± 1.68 vs. 3.89 ± 0.97, p = 0.001) at 1-month follow-up from injury. With an NPi cutoff value of 3.4, the initial NPi value of the automated pupillometer had 86% sensitivity and 84.6% specificity in predicting the clinical outcome at 1 month after the acute brain injury. Furthermore, Natzeder et al. [26] demonstrated the utility of automated pupillometry as a prognostic indicator in aSAH. They found that mean NPi tended to be lower in patients with unfavorable (GOS 1–3) compared to favorable (GOS 4–5) outcomes at the time of discharge (mean ± SE) (3.64 ± 0.48 vs. 4.50 ± 0.08, p = 0.198) and pathologic NPi values were recorded more frequently in patients with unfavorable compared to favorable outcomes (19.2% ± 10.6% vs. 0.7% ± 0.6%, p = 0.017). Subgroup analysis of patients with clinically severe aSAH as determined by a World Federation of Neurological Surgeons (WFNS) grade of 4–5 showed a significantly lower mean NPi for patients who died compared to those who survived (2.5 ± 1.2 vs. 4.2 ± 0.2, p = 0.041).
Estimation of Clinical Severity in Aneurysmal Subarachnoid Hemorrhage
The study by Natzeder et al. [26] examined the ability of quantitative automated pupillometry to estimate the clinical severity of aSAH. Although not statistically significant, they found that mean NPi tended to be lower in patients with clinically severe (WFNS 4–5) compared with non-severe (WFNS 1–3) aSAH (mean ± SE) (3.75 ± 0.40 vs. 4.56 ± 0.06, p = 0.171). A statistically significant difference was found between the frequency of pathological NPi values in clinically severe (WFNS 4–5) and non-severe (WFNS 1–3) aSAH (16.3% ± 8.8% vs. 0.0% ± 0.0%, p = 0.002).
Utility as a Noninvasive Monitor of Response to Osmotic Therapy
Ong et al. [45] evaluated the utility of quantitative automated pupillometry as a noninvasive biomarker to monitor reduction in ICP after administration of an osmotic agent in patients admitted to the neuroICU with increased ICP and brain herniation. They showed that there was a statistically significant improvement in NPi within 2 h of administration of either 20% mannitol or 23.4% hypertonic saline in patients with abnormal NPi (< 3) prior to medication delivery (median [IQR]) [2.4 (1.75–2.65) vs. 3.0 (1.9–3.45), p < 0.0004]. This improvement was also seen in patients with normal NPi (> 3) prior to receiving osmotic therapy [4.4 (3.8–4.7) vs. 4.5 (4.0–4.7), p < 0.0322]. Results remained significant for the total patient cohort (p = 0.0168) and for patients with abnormal NPi (< 3) prior to therapy initiation (p = 0.0235) when controlling for other interventions to reduce ICP including cerebrospinal fluid diversion, blood pressure management strategies, initiation or increase in anesthetic and analgesic agents, and hyperventilation.
Discussion
Assessment of primary outcomes confirms that the automated pupillometer has utility in the care of critically ill patients with neurological impairment. The importance of serial pupillary examinations to a thorough neurologic assessment is well established [9]. However, the traditional approach of using manual serial pupillary examinations performed by trained healthcare professionals has come under scrutiny due to its inherent subjectivity demonstrated by low intra- and inter-observer reliability and errors in detecting a light reflex in very small and dilated pupils [12,13,14]. Automated pupillometry devices provide an objective measure of pupillary size and reactivity [15]. We conducted a literature review to explore the evidence assessing the usefulness of automated pupillometry in the care of critically ill patients with neurological impairment. After an exhaustive review, 30 records of original studies with human subjects were identified that assessed outcomes of the use of automated pupillometry in a critical care setting. Although a variety of pupillometers from common commercial brands were used in the studies that we reviewed, including NeurOptics® and IDMed®, the basic functionality was the same. Each pupillometer was capable of assessing multiple variables commonly associated with the PLR including pupillary size, constriction velocity, dilation velocity, and latency period from light exposure to start of constriction [15]. However, it is important to note that although a study examining inter-device reliability of the NeurOptics® NPi™-100 Pupillometer has been published [21], to date there has been no head-to-head trial comparing the relative reliability of different brands and models of automated pupillometers.
Summary of Primary Outcomes Data
Data extracted from studies evaluating the increased precision, reliability, and reproducibility of automated pupillometry compared with manual examinations show consistently lower inter-observer discrepancy and increased agreement in pupillary measurements with the use of automated pupillometry, suggesting that standard practice in pupillary monitoring yields inaccurate data. The evidence supports automated quantitative pupillometry as a more reliable method with which to collect pupillary measurements at the bedside for populations of critically ill patients requiring neuromonitoring [11, 14, 18,19,20,21].
Multiple studies provide evidence that automated pupillometry can detect subtle pupillary changes and do so earlier than manual examination [14, 19, 22, 23]. The NPi is a function of specific automated pupillometers manufactured by NeurOptics® that accurately grades a pupil’s response to light using a proprietary algorithm based on normalized variables of the PLR including size, latency, constriction velocity, and dilatation velocity. The NPi has been shown to be particularly useful in pupillary monitoring. The study by Shoyombo et al. [23] examined NPi and CV values simultaneously. Interestingly, they found that 30.9% of observations had either one or both eyes showing a normal NPi with slow CV, or abnormal NPi with brisk CV, suggesting that a briskly reactive pupil is insufficient to conclude that the PLR is normal. The authors concluded that practitioners performing manual pupillary examinations might equate brisk CV with a normal examination, and miss subtle changes in the PLR that could be detected by automated pupillometry. Additionally, the CV–NPi relationship has the potential to be an intriguing, noninvasive biomarker of neurological status, thus lending support to the idea that automated pupillometers may allow for a more accurate representation of neurological status and function.
Evidence also supports the ability of automated pupillometers to detect pupillary changes that indicate a rise, or impending rise, in ICP. Increased asymmetry of both pupillary size and dilation velocity appears to be associated with increased ICP [18, 24]. An NPi < 4.15 at the time of ICP measurement in neuroICU patients is also associated with a higher risk of developing ICP crisis [25].
Studies examining the ability of automated pupillometry to detect the level of analgesia and depth of sedation show that pupillometry technology may be useful in determining whether analgesia levels need to be adjusted in critically ill patients that are unable to verbally communicate prior to a noxious procedure [27, 29, 31]. Additionally, data show that automated pupillometry may be more useful in the evaluation of sedation in the ICU when compared to BIS [28]. Wildemeersch et al. [32] showed that automated pupillometry may have utility in evaluating patients’ specific analgesic needs, especially in those who are not able to report pain levels themselves.
Numerous studies demonstrate the value of automated pupillometry as a prognostic indicator post-cardiac arrest [24, 33,34,35,36,37,38,39,40,41,42,43]. These studies conclude that quantitative measurement of pupillary light reflexes has prognostic value as early as within 1 h after return of spontaneous circulation [33]. Additionally, they showed that quantitative PLR post-cardiac arrest may equal the prognostic value of electroencephalography and somatosensory evoked potentials for poor outcomes [34,35,36, 38, 39, 41] and predict favorable outcome if PLR is detected during resuscitation [37, 42]. Park et al. [44] also looked at prognosis in acute brain injury and found that initial NPi value had good sensitivity and specificity in predicting clinical outcome at 1 month after injury. Furthermore, Natzeder et al. [26] demonstrated that there is a statistically significant association between the frequency of pathological NPi values and poor clinical outcome in patients with aSAH. This same study provided evidence that NPi reflects the clinical severity of aSAH and may be useful to estimate the clinical severity of other neurological injuries.
Finally, Ong et al. [45] found that NPi values show statistically significant improvement after administration of osmotic therapy in patients admitted to the neuroICU with increased ICP and brain herniation. These results suggest that improvement in pupillary reactivity measured by serial quantitative pupillometry could serve as a noninvasive biomarker for reduction in cerebral edema, intracranial pressure, and/or displacement of midbrain and medullary structures after osmotic therapy.
Secondary Outcomes
Several authors have reported that pupillary signs detected by automated pupillometry have led to changes in therapy. However, no high-quality study has evaluated whether these changes improve clinical outcomes. Although not meeting inclusion criteria for this review, Chen et al. [10] described the case of one patient initially presenting after TBI with an abnormal NPi score of 0.7 for the right pupil, which in the context of a concerning neurological examination, raised questions about impending ICP issues. Repeated NPi measurements over the course of the next 8 h showed improvement in the pupillary function of her right eye. However, over the same monitoring period her pupillary asymmetry did not improve, and a decision had to be made to follow an observational treatment paradigm or pursue neurosurgical invasive techniques. Based off of the improving NPi measurements, the care team decided to decrease sedation, and she subsequently awoke, followed commands, and was extubated according to protocol. This case report suggests that there may be added value to patient care by examining pupils with automated pupillometry technology; however, proof of this added value is difficult to demonstrate.
Potential Limitations of Pupillometry Technology
Potential limitations to the use of objective pupillometers have been raised by several studies. Multiple authors have questioned whether different pathologies may be more or less susceptible to PLR changes [12, 16, 23]. Additionally, Kramer et al. [46] reported a case study wherein a neurologist that observed the pupil for 7–9 s was able to detect a 1-mm size change that was undetectable by pupillometry. They advised that the combined use of the manual examination and automated pupillometry may optimize the accuracy of the assessment of pupillary reactivity. However, the authors proposed that because the light stimulus delivered by the specific pupillometer used in the study lasts only 0.8 s, and subsequent recording of the pupillary constriction occurs for 3.2 s, the relatively brief stimulus and narrow window of recording may miss detection of very slow light reactivity. This can be addressed by changing the parameters of the pupillometer.
Additionally, several authors describe multiple clinical conditions that limit the ability of automated pupillometry to accurately measure pupillary function. These include periorbital edema [14, 47], sporadic movement in the patient with impaired cognition [14], cataract or a prosthetic eye [14], and other facial and ocular injuries that prevent visualization of the pupil [16, 47].
Although it has been claimed that the NPi is less vulnerable to confounding environmental factors such as variable ambient light conditions than standard methods of assessing pupil reactivity, Ong et al. [48] showed that changes in NPi levels under varying light conditions differ significantly in critically ill subjects. Given these findings, the authors recommended that practitioners should standardize lighting conditions to testing in dimly lit conditions in order to maximize measurement reliability and to achieve an optimal assessment of pupillary reactivity.
Financial cost has also been identified as a possible limiting factor to widespread adoption of automated pupillometry technology. Emelifeonwu et al. [12] described the costs involved in purchasing one specific brand of automated pupillometer as approximately $8000 (£5000) for the handheld machine, and $80 (£50) for each single-use detachable headrest, which facilitates placement of the pupillometer device in front of the eye. Regular use of this specific automated pupillometer would have significant cost implications. However, over time the retail cost of pupillometry technology has decreased, and a single unit from a leading commercial brand (NeurOptics®) can currently be purchased for less than $5000 [49].
A study assessing the length of time nurses at a high volume neuroICU spent completing a manual pupil examination showed that, on average, nurses spent 45.8 min per patient per day performing manual hourly pupil examinations and entering the data into the patient’s chart [50]. On average, nurses saved 19.8 min per patient per day using an automated pupillometer capable of uploading data directly to the patient’s chart [50]. The amount of time saved by use of automated pupillometers only increases with increasing frequency of serial pupillary examinations. This suggests that any improvement in workforce efficiency provided by the use of automated pupillometers may translate into increased productivity and, ultimately, cost-saving benefits. Further research is needed to explore the cost implications of automated pupillometry technology in the neuroICU.
Study Limitations and Recommendations
With this systematic review, we set out to examine whether sufficient evidence now exists to validate routine use of automated quantitative pupillometry in the care of critically ill patients in the neuroICU. In considering the evidence in support of our primary outcomes, every study had a study grade of low to very low according to GRADE criteria. Although an increasing number of studies continue to show that automated pupillometry could have value in assessing our primary outcomes, the low grade of the reviewed studies calls this into question. Automated pupillometry should be considered for use in all critically ill patients with neurological impairment, but at this time there is insufficient high-quality evidence from randomized controlled trials and careful observational studies to strongly recommend routine use of pupillometry technology.
Another limitation of our study is that we only reviewed publications in English, limiting generalizability. Our eligibility criteria may also have caused us to exclude studies examining outcomes in healthy and non-neurological critically ill patients that could be applicable to the neuroICU patient population. Additionally, a major limitation of our study is that baseline pupillometry values for populations in the neurocritical care setting have not been published, which limits application of the technology. However, the increasing use of registries, such as the Establishing Normative Data for Pupillometer Assessments in Neuroscience Intensive Care Registry, provides a large data set of pupillary size, reactivity, and speed of contraction in a cohort of patients admitted to a neuroscience ICU with a variety of conditions. These registries are helping to establish normative data for pupillometer readings for neurologically impaired patients [51].
Conclusion
This extensive literature review has aimed to explore the current state of automated pupillometry technology and its application in the neuroICU. At present, no consensus guidelines exist endorsing routine use of automated pupillometry in this setting. However, an increasing quantity of research supports the usefulness of automated pupillometry to: increase precision, reliability, and reproducibility compared with the manual pupillary examination; detect subtle and early pupillary changes; detect pupillary changes that indicate a rise, or impending rise, in ICP; detect level of analgesia and depth of sedation; serve as a prognostic indicator; estimate the clinical severity of aSAH; and serve as a noninvasive monitor of response to osmotic therapy. Yet, limitations of the current technology are evident and provide direction for future research and development. Additional large-scale prospective studies and randomized controlled trials evaluating the capabilities of automated pupillometry technology and the practicality and cost implications of its routine use in the neuroICU are needed before updated consensus guidelines recommending wider adoption are produced.
References
St. Joseph Health. Clinical Guidelines: Pupillometer in Critical Neuro Patients, Use of [Internet]. Mission Viejo, California: 2015 [cited 2018 June 14]. Available from: http://aann.org/uploads/Clinical_Guidelines_Pupillometer.pdf.
Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1–16.
Vinciguerra L, Bösel J. Noninvasive neuromonitoring: current utility in subarachnoid hemorrhage, traumatic brain injury, and stroke. Neurocrit Care. 2017;27(1):122–40.
Marklund N. The neurological wake-up test—a role in neurocritical care monitoring of traumatic brain injury patients? Front Neurol. 2017;17(8):540.
Adoni A, McNett M. The pupillary response in traumatic brain injury: a guide for trauma nurses. J Trauma Nurs. 2007;14(4):191–6.
Le Roux P, Menon DK, Citerio G, et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit Care. 2014;21(Suppl 2):S1–26.
Le Roux P, Menon DK, Citerio G, et al. The International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a list of recommendations and additional conclusions: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit Care. 2014;21(Suppl 2):S282–96.
Le Roux P, Menon DK, Citerio G, et al. The International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: evidentiary tables: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit Care. 2014;21(Suppl 2):S297–361.
Hickey J. The clinical practice of neurological and neurosurgical nursing. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2003.
Chen JW, Gombart ZJ, Rogers S, Gardiner SK, Cecil S, Bullock RM. Pupillary reactivity as an early indicator of increased intracranial pressure: the introduction of the neurological pupil index. Surg Neurol Int. 2011;2:82.
Meeker M, Du R, Baccheti P, et al. Pupil examination: validity and clinical utility of an automated pupillometer. J Neurosci Nurs. 2005;37(1):34–40.
Emelifeonwu JA, Reid K, Rhodes JK, Myles L. Saved by the pupillometer!—a role for pupillometry in the acute assessment of patients with traumatic brain injuries? Brain Inj. 2018;32(5):675–7.
Kerr RG, Bacon AM, Baker LL, et al. Underestimation of pupil size by critical care and neurosurgical nurses. Am J Crit Care. 2016;25(3):213–9.
Olson DM, Stutzman S, Saju C, Wilson M, Zhao W, Aiyagari V. Interrater reliability of pupillary assessments. Neurocrit Care. 2016;24(2):251–7.
Zafar SF, Suarez JI. Automated pupillometer for monitoring the critically ill patient: a critical appraisal. J Crit Care. 2014;29(4):599–603.
McNett M, Moran C, Janki C, Gianakis A. Correlations between hourly pupillometer readings and intracranial pressure values. J Neurosci Nurs. 2017;49(4):229–34.
NeurOptics—The Leader in the Science of Pupillometry. NeurOptics, 18 August 2018. https://neuroptics.com.
Taylor WR, Chen JW, Meltzer H, et al. Quantitative pupillometry, a new technology: normative data and preliminary observations in patients with acute head injury. J Neurosurg. 2003;98(1):205–21.
Yan S, Tu Z, Lu W, et al. Clinical utility of an automated pupillometer for assessing and monitoring recipients of liver transplantation. Liver Transpl. 2009;15(12):1718–27.
Couret D, Boumaza D, Grisotto C, et al. Reliability of standard pupillometry practice in neurocritical care: an observational, double-blinded study. Crit Care. 2016;13(20):99.
Zhao W, Stutzman S, Olson D, et al. Inter-device reliability of the NPi-100 pupillometer. J Clin Neurosci. 2016;33:79–82.
Larson MD, Muhiudeen I. Pupillometric analysis of the “absent light reflex”. Arch Neurol. 1995;52(4):369–72.
Shoyombo I, Aiyagari V, Stutzman SE, et al. Understanding the relationship between the neurologic pupil index and constriction velocity values. Sci Rep. 2018;8:6992.
Ong C, Kim A, Zafar S, et al. Range of quantitative pupillometry measurements and its association with traditional monitoring metrics in the neuro ICU. Neurocrit Care. 2017;27(2 Suppl 1):(S252-).
Giede-Jeppe A, Koehn J, Gerner S, et al. Serial pupillometer readings predicting intracranial pressure crisis in neurocritical-care patients. Neurocrit Care. 2017;27(2 Suppl 1):(S254-).
Natzeder S, Mack DJ, Maissen G, et al. Portable infrared pupillometer in patients with subarachnoid hemorrhage: prognostic value and circadian rhythm of the neurological pupil index (NPi). J Neurosurg Anesthesiol. 2018. https://doi.org/10.1097/ANA.0000000000000553.
Lukaszewicz A, Dereu D, Gayat E, Payen D. The relevance of pupillometry for evaluation of analgesia before noxious procedures in the intensive care unit. Anesth Analg. 2015;120(6):1297–300.
Rouche O, Wolak-Thierry A, Destoop Q, et al. Evaluation of the depth of sedation in an intensive care unit based on the photo motor reflex variations measured by video pupillometry. Ann Intensive Care. 2013;3(1):5.
Paulus J, Roquilly A, Beloeil H, et al. Pupillary reflex measurement predicts insufficient analgesia before endotracheal suctioning in critically ill patients. Crit Care. 2013;17(4):R161.
Gaillard T, Gergaud S, Grayot C, et al. Can pupillometry diagnose a lack of analgesia prior to nursing in critically ill patients? Intensive Care Med Exp. 2015;3(Suppl 1):A326.
Li D, Miaskowski C, Burkhardt D, Puntillo K. Evaluations of physiologic reactivity and reflexive behaviors during noxious procedures in sedated critically ill patients. J Crit Care. 2009;24(3):472.e9–13.
Wildemeersch D, Gios J, Jorens P, Hans G. Objective nociceptive assessment in ventilated ICU patients: a feasibility study using pupillometry and the nociceptive flexion reflex. J Vis Exp. 2018. https://doi.org/10.3791/57972.
Tamura T, Namiki J, Sugawara Y, et al. Quantitative assessment of pupillary light reflex for early prediction of outcomes after out-of-hospital cardiac arrest: a multicentre prospective observational study. Resuscitation. 2018;131:108–13.
Solari D, Rossetti A, Carteron L, et al. Early prediction of coma recovery after cardiac arrest with blinded pupillometry. Ann Neurol. 2017;81(6):804–10.
Suys T, Bouzat P, Marques-Vidal P, et al. Automated quantitative pupillometry for the prognostication of coma after cardiac arrest. Neurocrit Care. 2014;21(2):300–8.
Beuchat I, Solari D, Novy J, Oddo M, Rossetti A. Standardized EEG interpretation in patients after cardiac arrest: correlation with other prognostic predictors. Resuscitation. 2018;126:143–6.
Behrends M, Niemann C, Larson M. Infrared pupillometry to detect the light reflex during cardiopulmonary resuscitation: a case series. Resuscitation. 2012;83(10):1223–8.
Heimburger D, Durand M, Gaide-Chevronnay L, et al. Quantitative pupillometry and transcranial Doppler measurements in patients treated with hypothermia after cardiac arrest. Resuscitation. 2016;103:88–93.
Sawyer M, Riker R, May T, et al. 287: Very early pupillometry, EEG suppression, and bis data predict outcome 6 hours after cardiac arrest. Crit Care Med. 2018;46(1):126.
Riker R, Sawyer M, Fischman V, et al. 726: Quantitative pupillometry data after adult cardiac arrest an observational study. Crit Care Med. 2018;46(1):349.
Sawyer M, Lucas L, May T, et al. Neurological pupil index predicts neurological outcome early after cardiac arrest: an observational study. Neurocrit Care. 2017;27(2 Suppl 1):(S47-).
Reynolds J, Chassee T, Fankhauser M, Uber A. Abstract 231: preliminary experience with prehospital pupillometry: a prospective, observational study in out-of-hospital cardiac arrest. Circulation. 2018;130:A231.
Suys T, Sala N, Rossetti A, Oddo M. Infrared pupillometry for outcome prediction after cardiac arrest and therapeutic hypothermia. Crit Care. 2013;17(Suppl 2):P310.
Park J, Moon C, Park D, Song S. Clinical utility of an automated pupillometer in patients with acute brain lesion. J Korean Neurosurg Soc. 2015;58(4):363–7.
Ong C, Hutch M, Barra M, et al. Effects of osmotic therapy on pupil reactivity: quantification using pupillometry in critically III neurologic patients. Neurocrit Care. 2018. https://doi.org/10.1007/s12028-018-0620-y.
Kramer C, Rabinstein A, Wijdicks E, Hocker S. Neurologist versus machine: is the pupillometer better than the naked eye in detecting pupillary reactivity. Neurocrit Care. 2014;21(2):309–11.
Papangelou A, Zink E, Chang W, et al. Automated pupillometry and detection of clinical transtentorial brain herniation: a case series. Mil Med. 2018;183(1–2):e113–21.
Ong C, Hutch M, Smirnakis S. The effect of ambient light conditions on quantitative pupillometry. Neurocrit Care. 2018. https://doi.org/10.1007/s12028-018-0607-8.
Phillips S (2018) Phone interview with NeurOptics® representative.
Wilkie AC (2015) Pupillary assessment times [Internet]. Irvine, CA: NeuroSurgical ICU, University of California Irvine Medical Center; 2015 [cited 2018 June 14]. https://neuroptics.com/wp-content/uploads/2017/02/Wilkie-A.-Pupillar-Assessment-Poster-Pres.-2015.pdf.
Olson DM, Stutzman SE, Atem F, et al. Establishing normative data for pupillometer assessment in neuroscience intensive care: the “END-PANIC” registry. J Neurosci Nurs. 2017;49(4):251–4.
Funding
The study received no direct funding.
Author information
Authors and Affiliations
Contributions
SSP and CMM provided substantial contributions to the conception and design of the work and the acquisition, analysis, and interpretation of data. RGN and YMK provided substantial contributions to the analysis and interpretation of data for the work. SSP and CMM drafted and critically revised the work for important intellectual content. RGN and YMK critically revised the work for important intellectual content. SSP, CMM, RGN, and YMK provided final approval of the version to be published. SSP, CMM, RGN, and YMK agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
Raul G. Nogueira declares that he has no conflict of interest related to the topic or work under consideration. Other unrelated disclosures include Stryker Neurovascular (DAWN Trial Principal Investigator—no compensation, TREVO Registry Steering Committee—no compensation, Trevo-2 Trial Principal Investigator—modest; Consultant—modest); Medtronic (SWIFT Trial Steering Committee—modest; SWIFT-Prime Trial Steering Committee—no compensation; STAR Trial Angiographic Core Lab—significant); Penumbra (3D Separator Trial Executive Committee—no compensation); Cerenovus/Neuravi (ENDOLOW Trial Principal Investigator, ARISE-2 trial Steering Committee—no compensation, Physician Advisory Board, modest); Phenox (Physician Advisory Board, modest); Anaconda (Physician Advisory Board, modest); Genentech (Physician Advisory Board—modest); Biogen (Physician Advisory Board—modest); Prolong Pharmaceuticals (Physician Advisory Board—modest); and Allm Inc. (Physician Advisory Board—no compensation). Editor-In-Chief Interventional Neurology Journal (no compensation) and remaining authors declare that they have no conflict of interest.
Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Phillips, S.S., Mueller, C.M., Nogueira, R.G. et al. A Systematic Review Assessing the Current State of Automated Pupillometry in the NeuroICU. Neurocrit Care 31, 142–161 (2019). https://doi.org/10.1007/s12028-018-0645-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-018-0645-2