Abstract
Background
The neurological prognosis of patients after cardiopulmonary resuscitation (CPR) is difficult to assess. GFAP is an astrocytic intermediate filament protein released into bloodstream in case of cell death. We performed a prospective study aiming to compare the predictive potential of GFAP after resuscitation to the more widely used biomarker neuron-specific enolase (NSE).
Methods
One hundred patients were included at 48 h (tolerance interval ±12 h) after cardiac arrest. A serum sample was collected immediately after study inclusion. We determined serum levels of GFAP and NSE by means of immunoassays. Primary outcome was the modified Glasgow outcome scale at 4 weeks. Values below four were considered as a poor functional outcome.
Results
Median GFAP levels in poor outcome (n = 61) and good outcome (n = 39) patients were 0.03 μg/L (interquartile range 0.01–0.07 μg/L) and 0.02 μg/L (0.01–0.03 μg/L; p = 0.014), respectively. GFAP revealed a sensitivity of 60.7% and a specificity of 66.7% to predict a poor functional outcome. All patients having a GFAP level >0.08 µg/L had a poor functional outcome. For NSE, sensitivity was 44.3% and specificity was 100.0% for predicting a poor outcome. Multivariate regression analysis revealed GFAP, NSE, and the Karnofsky index to be independent predictors of outcome.
Conclusions
The release patterns of GFAP and NSE after CPR show differences. GFAP levels above 0.08 µg/L were associated with a poor outcome in all cases, and patients with strongly elevated values (>3 µg/L) consistently had severe brain damage on brain imaging. Both biomarkers independently contribute to outcome prediction after CPR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The prediction of functional outcome in patients after cardiopulmonary resuscitation (CPR) is challenging. Besides the neurological examination, brain imaging, EEG, and somatosensory evoked potentials (SEP) are used to assess brain status [16, 30]. Blood biomarkers could provide additional independent information on brain damage on a molecular basis and could therefore be helpful to stratify patients according to their prognosis [14, 26].
At present, neuron-specific enolase (NSE) is the most commonly used blood test for this indication [1, 10, 24, 25]. It has, however, little brain specificity. NSE is mainly located in neurons and neuroendocrine cells, but is also expressed by small-cell bronchial carcinoma and in some benign lung diseases. A general problem of NSE in outcome prediction after CPR is that sensitivity values are only moderate if specificity is set to 100%. Furthermore, the recommended NSE cutoff levels after resuscitation vary from guideline to guideline, and target temperature management (TTM) seems to also have an influence on NSE cutoff points [27]. This underlines the vulnerability of this marker, making a reliable outcome prediction based on NSE levels difficult.
In comparison with NSE, less data are available for glial fibrillary acidic protein (GFAP). This protein is a main component of intermediate filaments in the cytoplasm of glial cells (particularly astrocytes) [6, 7]. GFAP is considered to be highly brain specific. As a structural protein, it is not released from cells under physiologic conditions, and healthy individuals do not show detectable GFAP levels in their blood stream [2]. Events that cause astroglial cell necrosis, however, lead to the release of GFAP into plasma. Thus, GFAP was detected early in the time course of intracerebral hemorrhage [8, 9] and traumatic brain injury [4, 22]. It is released with some delay in ischemic stroke [2, 5]. In the context of resuscitation, two small studies did not find differences in GFAP levels between patients resuscitated from cardiac arrest with a good and a poor prognosis [12, 21]. In contrast, a retrospective study [15] and a recently published prospective evaluation in 125 post-cardiac arrest patients that was based on a newly developed prototype GFAP immunoassay [17] showed higher GFAP levels in patients with a poor prognosis.
The following study was performed to characterize the release pattern of GFAP in a prospective cohort of resuscitated patients and to evaluate the predictive potential of GFAP in comparison with NSE.
Materials and Methods
Study Design and Patients
This prospective study was performed between March 2011 and January 2013 in three secondary- and tertiary-care hospitals in Germany (Hochtaunus-Kliniken Bad Homburg, Kerckhoff-Klinik Bad Nauheim, and University Hospital Frankfurt am Main). It was approved from the ethics committee of the University Hospital Frankfurt am Main (No. 45/11). Consent for being included in the trial was obtained from legal representatives or, in case the patients had regained consciousness, from the patients themselves. Participation in the study did not alter any aspects of the patients’ clinical treatment, including the induction of TTM.
Sample size calculation was performed using an online tool for studies of diagnostic test accuracy (www.sample-size.net). At a confidence level of 0.95, we assumed 50 patients having a poor functional outcome and 50 patients having a good functional outcome [21]. Based on previously published data on biomarkers in patients resuscitated from cardiac arrest, we assumed a probability of a positive GFAP test result in the poor outcome group of 0.6 and a probability of a positive GFAP test result in the good outcome group of 0.06 [15]. This resulted in a likelihood ratio of 10.0 (3.3-30.7).
Inclusion criterion was a documented successful out-of-hospital or in-hospital CPR within the last 60 h. Exclusion criteria were: (1) age under 18 years, (2) previous stroke within the last 12 months, (3) traumatic brain injury within the last 12 months, (4) any brain tumor in medical history. The latter three exclusion criteria were chosen based on previous exploratory studies on GFAP. In ischemic stroke [13] and intracerebral hemorrhage [9], GFAP is released within the first few days after symptom onset. In traumatic brain injury, GFAP can immediately be detected in serum [29]. In patients with singular mass lesions in the brain, GFAP is a marker of glioblastoma [28]. Other neurological conditions were not found associated with GFAP release [19].
We documented whether the CPR was performed within a hospital (i.e., during a hospital stay) or outside a hospital. In addition, the following parameters were prospectively collected: age, sex, Karnofsky index [3] prior to resuscitation, cause of cardiac arrest, time span to CPR, duration of CPR, first documented rhythm, defibrillation during CPR, and TTM after CPR. Brain imaging was not scheduled as part of the study protocol. However, available brain scans (i.e., those administered in clinical routine) were evaluated for the presence of hypoxic brain damage, including pathologies such as ischemic stroke and intracerebral hemorrhage.
Blood Sampling
At 48 h (tolerance interval ±12 h) after CPR, blood was collected in a serum-separating tube and was rapidly transported to the laboratory facility of the respective hospitals. Blood tubes were centrifuged at 1500–2000g for 10 min as soon as possible but no longer than 1 h after blood collection. Serum was separated, immediately frozen, and stored below −80 °C. Samples were later on shipped on dry ice.
Measuring GFAP and NSE Serum Levels
Quantification of serum GFAP concentrations was performed at Roche Diagnostics, Penzberg, Germany. All scientists involved in the analysis were fully blinded to the clinical data. A prototype electro-chemiluminometric immunoassay for the in vitro quantification of GFAP in human serum and plasma was used on an Elecsys®17 platform [9, 17, 28].
NSE was measured on a fully automated analyzer (Cobas e®). Here, the “sandwich principle” was used for quantification of NSE serum levels.
Outcome Assessment
Mortality rate was determined at 4 weeks after CPR. The modified Glasgow outcome scale (MGOS) was used as the primary outcome measure [23]. The assessment was done by phone interview. The rater was blinded to the results of the laboratory testing. The MGOS distinguishes outcome into six categories and has been applied for outcome determination after cardiac arrest in the appraisal of biomarkers (MGOS 5: normal life, MGOS 4: disabled but independent, MGOS 3: conscious but disabled and dependent, MGOS 2: unconscious, MGOS 1: deceased with a documented hypoxic brain damage, MGOS 0: deceased with unknown cerebral status). Thus, compared to the “regular” Glasgow outcome scale, patients with an unknown cerebral status at their death are stratified in a separate group. For the primary endpoint, MGOS was dichotomized into a good (MGOS 4 + 5) and a poor (MGOS 0–3) functional outcome category.
Statistical Analysis
The Kolmogorow–Smirnov-test was used to evaluate whether data are normally distributed. Parametric data were then compared using mean values ± SD and the t test. Nonparametric data were displayed using median and interquartile (IQ) ranges, and comparisons between groups were performed by means of the Mann–Whitney U test. We used receiver operating characteristics (ROC) curve calculations to determine cutoff values for an optimal differentiation between patients with a good and poor functional outcome for each biomarker. Sensitivity, specificity and predictive values were then derived from cross-tabulations. Multivariate analysis (simultaneous inclusion model) was used to identify whether GFAP and NSE are independent predictors for a poor functional outcome. For doing so, biomarkers were grouped into quintiles, due to nonparametric distribution. Age, sex, Karnofsky index, time to CPR (dichotomized into ≤5 vs. >5 min [12]), duration of CPR (dichotomized into ≤5 vs. >5 min [15]), first determined cardiac rhythm (ventricular fibrillation vs. all others), TTM, and the performance of CPR within a hospital (vs. outside) were used as co-variables. The threshold of significance was set at p < 0.05. SPSS version 22 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
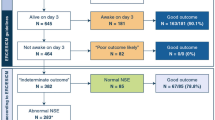
One hundred patients were prospectively included in this trial. Baseline variables of the study cohort are displayed in Table 1.
At 4 weeks after CPR, 15% of the patients had died (MGOS 0–1). Regarding the primary endpoint, 61% of patients had a poor functional outcome (MGOS 0–3), and 39% reached the good functional outcome category (MGOS 4–5). Median NSE serum levels at 48 h after CPR were significantly higher in the poor functional outcome group (26.3 μg/L [IQ range 10.8-60.8]) as compared to the good outcome group (16.1 μg/L [IQ range 10.4–21.2]; p = 0.025; see Fig. 1). Median GFAP serum levels were also significantly increased in the poor outcome group as compared to patients with a good functional outcome (0.03 μg/L [IQ range 0.01–0.07] vs. 0.02 μg/L [IQ range 0.01–0.03]; p = 0.014; see Fig. 2).
NSE serum levels in patients with poor and with good functional outcome (displayed by means of boxplots). The boundaries of the box indicate the 25th and 75th percentile, respectively. The whiskers indicate the 10th and 90th percentile, respectively. Outliers (between 1.5 and 3 times the interquartile range) are marked with circles. Extreme values (>3 times the interquartile range) are marked with asterisks. The y-axis is on a logarithmic scale
For GFAP, we noticed that most of the patients had low serum values, but nine patients showed levels more than 100-fold higher than the median GFAP levels (i.e., >3 µg/L, max. 144 µg/L). Of those, five patients had died at follow-up, and all survivors showed a poor functional outcome with a persistent vegetative state (MGOS 2). A detailed reevaluation of the available imaging data (all CT scans) in the acute phase (7 out of 9 patients) revealed large ischemic infarctions (e.g., bilaterally in the posterior cerebral artery territory) and brain swelling in all cases. NSE values in patients with massively elevated GFAP were also strongly increased (range 14.0–360.2 µg/L; mean 161.0 ± 132.7 µg/L).
Fifteen patients had high NSE values (above 60 µg/L), and the highest value was 360.2 µg/L. Hypoxic or ischemic brain damage was detected with CT in 13 of these 15 cases (in the remaining two patients, no brain imaging was available). In the 15 patients with strongly elevated NSE values, the mean GFAP values were also substantially increased (15.1 ± 38.5 µg/L). Overall, GFAP showed a more prominent positive skew (right-tailed) of the serum values as compared to NSE (see Figs. 1, 2). GFAP and NSE values revealed a significant correlation with each other (Pearson’s correlation coefficient r = 0.384, p < 0.001).
ROC analysis revealed a cutoff point of 34.0 μg/L for NSE for an optimized differentiation between the two endpoints (AUC of 0.633; 95% CI 0.524–0.742; p = 0.025; see Fig. 3). Sensitivity was determined to be 44.3%, and specificity was 100.0% for predicting a poor functional outcome (positive predictive value 100.0%, negative predictive value 53.4%). For GFAP, a cutoff point of 0.02 μg/L was determined (AUC 0.646; 95% CI 0.540–0.753; p = 0.014; see Fig. 4). Cross-tabulation revealed a sensitivity of 60.7% and a specificity of 66.7% for predicting a poor outcome (positive predictive value 74.0%, negative predictive value 52.0%). A GFAP cutoff point of 0.08 µg/L provided both specificity and a positive predictive value of 100% (at a sensitivity of 21.3% and a negative predictive value of 44.8%) for a poor functional outcome. If both biomarkers were analyzed in combination and were elevated above their respective cutoff values, sensitivity was 34.4%, and specificity was 100.0% (positive predictive value 100%, negative predictive value 49.3%).
In a subgroup analysis, we focused on only those patients who were treated with TTM following CPR (n = 36 in the poor outcome group and n = 29 in the good outcome group). As compared to the entire patient cohort, ROC analysis in this subgroup revealed a slightly higher diagnostic accuracy both for NSE and GFAP to differentiate between patients with a poor and a good outcome (NSE: AUC 0.786, p < 0.001; GFAP AUC 0.721, p = 0.002).
In a multivariate regression analysis, the Karnofsky index (pre-CPR) as well as GFAP- and NSE serum levels were found to be independent predictors of poor neurological outcome (see Table 2).
Discussion
Our prospective and multicenter study revealed that GFAP and NSE are both independent predictors of poor functional outcome in resuscitated patients. Both markers have their strength in high positive predictive values, meaning that the chance of having a good functional outcome in the case of elevated serum values (i.e., a false positive finding) is very low. On the other side, a clear drawback of both parameters is the reduced sensitivity, meaning that a significant proportion (depending on the cutoff levels used) of patients with a poor functional outcome are not detected by means of biomarker elevation.
Although being not brain specific and hampered by limitations as discussed above, NSE has been extensively studied as a prognostic biomarker in CPR [1, 10, 24, 25, 27]. The cutoff values for NSE for predicting a poor functional outcome in our study were comparable to those obtained in previously published investigation, which underlines that we investigated a representative sample of patients resuscitated from cardiac arrest.
Until now, little data are available for GFAP as a predictor of functional outcome in patients resuscitated from cardiac arrest [12, 15, 17, 21]. However, from a pathophysiological point of view, GFAP appears to be an interesting biomarker candidate for further evaluation in this context. It is not released into the blood under physiologic conditions [2, 9, 20]. It is also not released in patients with transient focal cerebral ischemia, where primarily functional but not structural deficits on a cellular level occur [31]. However, cell damage with the loss of structural integrity leads to GFAP release. Significant correlations of serum GFAP levels with lesion size and functional outcome were reported in patients with traumatic brain injury [4, 22], intracerebral hemorrhage [8, 9], and ischemic stroke [5, 13]. In contrast, patients with trauma without cerebral involvement and patients with other neurological diseases did not show elevated GFAP levels [18, 19]. Taken together, it appears worthwhile to study GFAP in CPR survivors, where a global ischemia of variable duration causes heterogeneous brain damage on a cellular level.
Our study unmasks differences of GFAP and NSE release after CPR. Elevated GFAP values were found in a smaller number of patients than elevated NSE values. But all patients with GFAP values >0.08 µg/L had a poor functional outcome, and those patients with strongly elevated values (>2 µg/L) consistently showed severe ischemic or hypoxic brain damage on brain imaging. In view of the pathophysiological considerations mentioned above, these findings are well understandable. GFAP as a robust structural protein is released only in cases of substantial cellular damage. Our multivariate statistics identified both markers to be independent predictors of outcome. However, the diagnostic accuracy did not improve in this study if both markers were used in combination. The most likely reason for this discrepancy is that our study was not statistically powered to demonstrate an additional value of GFAP in comparison with NSE alone in outcome prediction. The Karnofsky index as a performance status measure (“the general well being”) prior to CPR was the only clinical variable that independently contributed to outcome prediction in our dataset. Most likely due to the limited number of cases other predictors could not be identified, although a strong tendency was found for prolonged time intervals from cardiac arrest to CPR (p = 0.058).
According to the ROC analyses, the overall potential of GFAP and NSE to differentiate between the two outcomes is poor. Thus, our study does not have direct clinical implications on an individual patient level. However, prior to a “withdraw of life support” order based on prognostic parameters (including clinical variables and imaging), it may make sense to analyze both markers for the purpose of reassuring the results [11]. In other words, combining both markers will further reduce the change of having a false positive finding (i.e., elevated biomarkers despite a good functional outcome). On the other side, as mentioned above, both GFAP and NSE do not function as predictors of a poor functional outcome with a high sensitivity. Thus, in clinical routine, a substantial proportion of patients will remain having a poor neurological outcome despite low biomarker levels after CPR.
The results of our study are highly consistent with the findings of the recently published study by Larsson et al. [17]. They also showed that GFAP levels were significantly increased in the poor outcome group after a survived cardiac arrest. A 100% specificity at a cutoff value of 0.04 µg/L at 96 h after cardiac arrest was suggested. In contrast to our findings, however, Larsson et al. did not identify GFAP as an independent predictor of functional outcome, and differences in release kinetics based on pathophysiological considerations were not elaborated.
Strengths of our study are its prospective design and the measurement of GFAP with an advanced GFAP prototype assay. The levels and the cutoff point of NSE reported here (as the more established parameter) were found in the range to what has been previously published, thereby underlining the validity of our study. Clearly, the predictive value of serum levels of GFAP with respect to long-term outcome (e.g., 6 months after CPR) should be addressed in further studies.
Conclusion
Our study revealed differences in the release pattern of GFAP and NSE into serum after CPR. GFAP as a structural protein was found markedly increased only in patients with severe brain damage. Elevated GFAP values (>0.08 µg/L) were associated with a poor prognosis in all cases. The biomarker may therefore be used in combination with NSE, in order to reduce the number of false positive findings (i.e., patients having a good prognosis despite elevated biomarker levels). On the other side, low levels are not necessarily associated with a good functional outcome. Further studies are needed to confirm our results.
References
Ben-Hamouda N, Taccone FS, Rossetti AO, et al. Contemporary approach to neurologic prognostication of coma after cardiac arrest. Chest. 2014;146:1375–86.
Brunkhorst R, Pfeilschifter W, Foerch C. Astroglial proteins as diagnostic markers of acute intracerebral hemorrhage—pathophysiological background and clinical findings. Transl Stroke Res. 2010;1:246–51.
Conlon N, O’Brien B, Herbison GP, et al. Long-term functional outcome and performance status after intensive care unit re-admission: a prospective survey. Br J Anaesth. 2008;100:219–23.
Diaz-Arrastia R, Wang KK, Papa L, et al. Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J Neurotrauma. 2014;31:19–25.
Dvorak F, Haberer I, Sitzer M, et al. Characterisation of the diagnostic window of serum glial fibrillary acidic protein for the differentiation of intracerebral haemorrhage and ischaemic stroke. Cerebrovasc Dis. 2009;27:37–41.
Eng LF. Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. J Neuroimmunol. 1985;8:203–14.
Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem Res. 2000;25:1439–51.
Foerch C, Curdt I, Yan B, et al. Serum glial fibrillary acidic protein as a biomarker for intracerebral haemorrhage in patients with acute stroke. J Neurol Neurosurg Psychiatry. 2006;77:181–4.
Foerch C, Niessner M, Back T, et al. Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin Chem. 2012;58:237–45.
Fogel W, Krieger D, Veith M, et al. Serum neuron-specific enolase as early predictor of outcome after cardiac arrest. Crit Care Med. 1997;25:1133–8.
Geocadin RG, Buitrago MM, Torbey MT, et al. Neurologic prognosis and withdrawal of life support after resuscitation from cardiac arrest. Neurology. 2006;67:105–8.
Hayashida H, Kaneko T, Kasaoka S, et al. Comparison of the predictability of neurological outcome by serum procalcitonin and glial fibrillary acidic protein in postcardiac-arrest patients. Neurocrit Care. 2010;12:252–7.
Herrmann M, Vos P, Wunderlich MT, et al. Release of glial tissue-specific proteins after acute stroke: a comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke. 2000;31:2670–7.
Horn J, Cronberg T, Taccone FS. Prognostication after cardiac arrest. Curr Opin Crit Care. 2014;20:280–6.
Kaneko T, Kasaoka S, Miyauchi T, et al. Serum glial fibrillary acidic protein as a predictive biomarker of neurological outcome after cardiac arrest. Resuscitation. 2009;80:790–4.
Koenig MA. Brain resuscitation and prognosis after cardiac arrest. Crit Care Clin. 2014;30:765–83.
Larsson IM, Wallin E, Kristofferzon ML, et al. Post-cardiac arrest serum levels of glial fibrillary acidic protein for predicting neurological outcome. Resuscitation. 2014;85:1654–61.
Lumpkins KM, Bochicchio GV, Keledjian K, et al. Glial fibrillary acidic protein is highly correlated with brain injury. J Trauma. 2008;65:778–82 (discussion 774–82).
Mayer CA, Brunkhorst R, Niessner M, et al. Blood levels of glial fibrillary acidic protein (GFAP) in patients with neurological diseases. PLoS One. 2013;8:e62101.
Missler U, Wiesmann M, Wittmann G, et al. Measurement of glial fibrillary acidic protein in human blood: analytical method and preliminary clinical results. Clin Chem. 1999;45:138–41.
Mortberg E, Zetterberg H, Nordmark J, et al. S-100B is superior to NSE, BDNF and GFAP in predicting outcome of resuscitation from cardiac arrest with hypothermia treatment. Resuscitation. 2011;82:26–31.
Papa L, Silvestri S, Brophy GM, et al. GFAP out-performs S100beta in detecting traumatic intracranial lesions on computed tomography in trauma patients with mild traumatic brain injury and those with extracranial lesions. J Neurotrauma. 2014;31:1815–22.
Rana OR, Schroder JW, Kuhnen JS, et al. The modified Glasgow outcome score for the prediction of outcome in patients after cardiac arrest: a prospective clinical proof of concept study. Clin Res Cardiol. 2012;101:533–43.
Reisinger J, Hollinger K, Lang W, et al. Prediction of neurological outcome after cardiopulmonary resuscitation by serial determination of serum neuron-specific enolase. Eur Heart J. 2007;28:52–8.
Sandroni C, Cavallaro F, Callaway CW, et al. Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: a systematic review and meta-analysis. Part 2: patients treated with therapeutic hypothermia. Resuscitation. 2013;84:1324–38.
Scolletta S, Donadello K, Santonocito C, et al. Biomarkers as predictors of outcome after cardiac arrest. Expert Rev Clin Pharmacol. 2012;5:687–99.
Steffen IG, Hasper D, Ploner CJ, et al. Mild therapeutic hypothermia alters neuron specific enolase as an outcome predictor after resuscitation: 97 prospective hypothermia patients compared to 133 historical non-hypothermia patients. Crit Care. 2010;14:R69.
Tichy J, Spechtmeyer S, Mittelbronn M, et al. Prospective evaluation of serum glial fibrillary acidic protein (GFAP) as a diagnostic marker for glioblastoma. J Neurooncol. 2016;126(2):361–9.
Welch RD, Ayaz SI, Lewis LM, et al. Ability of serum glial fibrillary acidic protein, ubiquitin C-terminal hydrolase-L1, and S100B to differentiate normal and abnormal head computed tomography findings in patients with suspected mild or moderate traumatic brain injury. J Neurotrauma. 2016;33:203–14.
Wijdicks EF, Hijdra A, Young GB, et al. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67:203–10.
Wunderlich MT, Wallesch CW, Goertler M. Release of glial fibrillary acidic protein is related to the neurovascular status in acute ischemic stroke. Eur J Neurol. 2006;13:1118–23.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CF is designated as inventor in the following European patent application: Use of GFAP for identification of intracerebral hemorrhage (Patent Application 03021571.9). The other authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Helwig, K., Seeger, F., Hölschermann, H. et al. Elevated Serum Glial Fibrillary Acidic Protein (GFAP) is Associated with Poor Functional Outcome After Cardiopulmonary Resuscitation. Neurocrit Care 27, 68–74 (2017). https://doi.org/10.1007/s12028-016-0371-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-016-0371-6