Abstract
Background
The use of vitamin K antagonists is an independent risk factor for the development of intracerebral hemorrhage (ICH). Four-factor prothrombin complex concentrate (4F-PCC) is recommended for urgent reversal of anticoagulation in this setting. The safety and efficacy of 4F-PCC in ICH with subtherapeutic levels of anticoagulation is yet to be determined.
Methods
This was a retrospective, observational study of 4F-PCC administration data from September 2013 to July 2015. Patients with spontaneous or traumatic ICH with initial INR 1.4–1.9 were compared to those with INR 2–3.9. A Fisher’s exact test was used to compare the difference between the two groups in the effectiveness of 4F-PCC in reversing the INR to ≤1.3 and in the occurrence of thrombotic events within 7 days of administration.
Results
A total of 131 patients with a presenting INR between 1.4 and 3.9 received 4F-PCC during the study period. Twenty-three of 29 patients (79 %) in the INR <2 group achieved an INR reduction to ≤1.3 after 4F-PCC administration compared to 47 of 92 patients (51 %) in the INR 2–4 group, p = 0.03. There was no difference in thrombotic complications within 7 days after administration (6.7 % in INR 1.4–1.9 group, 10 % in INR 2–3.9 group, p = 0.73).
Conclusion
The use of 4F-PCC in patients with INR between 1.4 and 1.9 results in an effective reduction in INR with similar thrombotic risks compared to patients presenting with an INR of 2–3.9.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In both primary and secondary intracerebral hemorrhage (ICH), vitamin K antagonist (VKA) therapy is an independent risk factor associated with increased mortality and hematoma expansion [1–7]. Hematoma expansion is associated with neurologic deterioration as measured by Glasgow Coma Scale (GCS) and the National Institute of Health Stroke Scale [8]. While there is a clear and consistent link between mortality and hematoma expansion in ICH and VKA therapy, there is no proven pharmacologic management that both decreases hematoma expansion and improves mortality.
Current guidelines and consensus opinion support the use of four-factor prothrombin complex concentrate (4F-PCC) over fresh-frozen plasma (FFP) for urgent correction of international normalized ratio (INR) in ICH [9–12]. However, it is less well-defined as to what degree of INR elevation requires the need for reversal or to what extent the INR should be reversed. There is emerging data suggesting rapid correction of INR to levels below 1.3 may have some beneficial effects on hematoma expansion [13–15].
Both fatal and nonfatal arterial and venous thromboembolic complications have been reported with 4F-PCC in clinical trials and postmarketing surveillance [16–18]. Thromboembolic complications have been reported in up to 7.8 % of patients receiving 4F-PCC with an INR >2 [17, 18]. It is unclear if reversal of patients who present with an INR <2 leads to an increased thrombotic risk. To determine if 4F-PCC is safe and effective in patients with ICH presenting with INR >2, we compared outcomes in patients presenting with INR 1.4–1.9 to those with INR 2–3.9.
Methods
This was a retrospective, observational study completed at the University of Pittsburgh Medical Center (UPMC) Presbyterian Hospital, a large, academic, urban, tertiary medical center with over 700 beds. It was approved by the UPMC Quality Improvement Committee.
The cohort consisted of all 4F-PCC medication administrations (KCentra®) occurring from UPMC formulary approval in September 2013 to July 2015. Patients were included if 4F-PCC was used to reverse elevated INRs secondary to warfarin usage in traumatic or spontaneous ICH with a presenting INR between 1.4 and 3.9. Patients were then divided into two groups: initial INR 1.4–1.9 and INR 2–3.9. Institutional guidelines recommended the same dose of 4F-PCC (25 U/kg to a maximum of 2500 U) for all patients presenting with an INR range of 1.4–3.9. The comparator group of INR 2–3.9 was selected to remove any bias through patients receiving different doses of 4F-PCC.
All data were obtained through review of the electronic health record. The initial INR was defined as the INR immediately preceding the administration of 4F-PCC with follow-up INR being the first INR after administration. Determination of hematoma expansion was made by the original reading radiologist. Venous thrombotic events were determined via review of either Doppler ultrasound or computed tomography (CT) angiogram. Ischemic stroke was defined as new infarction on any follow-up CT or magnetic resonance imaging (MRI) of the brain through reports of reading radiologist. Each patient with elevated troponins after 4F-PCC administration was further examined through review of electrocardiogram and physician progress notes for a diagnosis of myocardial infarction. Definite thrombotic events were defined as those with supporting documentation (e.g., CT, MRI, Doppler), and probable thrombotic events were defined as those with a high clinical suspicion through retrospective review of health record. Each thrombotic event was reviewed retrospectively by a neurocritical care attending physician.
The primary outcomes were to evaluate if there was a difference between the two groups in the effectiveness of 4F-PCC defined as INR reversal to ≤1.3 at the time of follow-up INR and development of thrombotic events. Patients without a follow-up INR postadministration were excluded from the primary efficacy analysis. We examined all thrombotic events occurring within 7 days of administration, hospital discharge, or death, whichever came first. All patients were included in the thrombotic event analysis. A multivariate regression analysis was performed to evaluate the predictors of INR reversal to ≤1.3.
Statistical analysis was performed using IBM SPSS version 22 (Chicago, IL). Normally distributed data are reported as mean ± standard deviation. Nonparametric data are reported as median (interquartile range). Fisher’s exact test, Student’s t–test, and Mann–Whitney U tests were used where appropriate. A logistic regression analysis evaluating INR reversal to ≤1.3 with the following variables was completed: initial INR, FFP administration, vitamin K administration, mechanism of bleed, time from 4F-PCC administration to follow-up INR, and age. Significance was set at p = 0.05.
Results
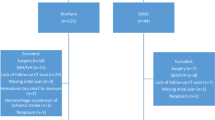
A total of 131 patients with spontaneous or traumatic ICH with a presenting INR between 1.4 and 3.9 received 4F-PCC during the study period (Fig. 1). The two groups were similar at baseline with the exception of a higher number of patients in the INR 1.4–1.9 group having a history of venous or atrial thromboembolism (Table 1). Twenty-nine patients in the INR 1.4–1.9 group and 92 patients in the INR 2–3.9 group had a repeat INR performed (Fig. 2). There was no difference in regard to dose of 4F-PCC administered and time between 4F-PCC and repeat INR (Tables 1, 2). The mean repeat INR in the INR 1.4–1.9 group was 1.3 ± 0.1 compared to 1.4 ± 0.2 in the INR 2–3.9 group. Twenty-three patients (79.3 %) in the INR 1.4–1.9 group achieved an INR reduction to ≤1.3 after 4F-PCC administration compared to 47 patients (51 %) in the INR 2–3.9 group, p = 0.03 (Table 2). Notably, 87 % in the INR 1.4–1.9 group and 93 % in the INR 2–3.9 group achieved an INR ≤1.5. When controlling for age, 4F-PCC dose, vitamin K administration, FFP administration, mechanism of ICH, and time to follow-up INR, the initial INR remained a predictor of achieving a repeat INR of ≤1.3 (odds ratio: 0.3; 95 % confidence interval: 0.14–0.66).
To determine if there is an increased risk of thrombotic complications in patients receiving 4F-PCC with an INR of 1.4–1.9, we examined all thrombotic events occurring within 7 days of administration, hospital discharge, or death, whichever came first. There was no difference in the number of patients experiencing a probable or definite thrombotic complication within this period (6.7 % in INR 1.4–1.9 group, 10 % in INR 2–3.9 group, p = 0.73; Table 3). One patient in the INR 2–3.9 group was diagnosed with both a deep venous thromboembolism (VTE) and myocardial infarction. Of the probable thrombotic events, one patient experienced a pulseless electrical activity cardiac arrest 36 h after 4F-PCC administration; this was considered to be secondary to pulmonary embolism. The second probable event was an acute mental status change with aphasia 24 h after 4F-PCC administration; this was considered to be an ischemic stroke.
Twenty-six patients (87 %) in the INR 1.4–1.9 and 80 patients (79 %) in the INR 2–3.9 group received a follow-up CT scan. The median time to follow-up CT scan was 270 min in the INR 1.4–1.9 group and 220 min in the INR 2–3.9 group. Four patients (15.4 %) in the INR 1.4–1.9 group and 18 patients (22.5 %) in the INR 2–3.9 group were noted to have hemorrhage expansion on the first CT scan after 4F-PCC administration (Table 4).
Discussion
This evaluation demonstrates that 4F-PCC is safe and effective for reversing INR elevations in patients with spontaneous and traumatic ICH presenting with subtherapeutic INRs. Since there appears to be a link between VKA therapy and an increased risk of mortality and hematoma expansion, recent guidelines from the Neurocritical Care Society and Society of Critical Care Medicine recommend administration of 4F-PCC for patients with ICH and an INR >1.3 [2, 3, 7, 11, 12]. The guidelines do not comment on the severity of brain injury that warrants anticoagulation reversal, and these data represent real-world utilization of 4F-PCC with both mild (GCS >12) and severe (GCS <9) patients. Here we provide additional safety and efficacy data to support the guidelines, though additional prospective trials will be valuable. A primary concern with rapid INR reversal is the development of thrombotic complications after the administration of 4F-PCC.
While some patients with INR between 1.5 and 1.9 have been included in prior trials, this subgroup has not been specifically examined for dosing or to determine if there is a heightened risk of thrombotic complications compared to patients with INR ≥2 [13, 17, 18]. By comparing the incidence of thrombotic complications in patients presenting with INR 1.4–1.9 to those with INR 2–3.9, we demonstrate that there is no evidence of an increased risk in using 4F-PCC in patients with subtherapeutic INR. Due to the heterogeneous populations previously examined and variability in surveillance for thrombotic events, the rate of 4F-PCC-associated thrombotic events in real-world practice is yet to be elucidated. In a large retrospective cohort study, the incidence of symptomatic VTE was 6.7, 3.8, and 2.9 % in patients presenting with subarachnoid hemorrhage, ICH, and traumatic brain injury (TBI), respectively [20]. The rates of thrombotic complications (arterial and venous) in our full cohort, 9.2 %, are slightly higher than those previously reported but represent real-world utilization in a population at known elevated risk of thrombotic complication [17–21].
While our data suggest there is not an increased risk of thrombotic complications in reversing subtherapeutic INRs compared to INRs of 2–3.9, the literature evaluating the relationship between the degree of anticoagulation at the time of injury and the resulting risks have yielded mixed results [2, 3, 7]. In a non-randomized prospective cohort of spontaneous ICH patients taking warfarin, Rosand et al. found patients to be at an increased risk for 3-month mortality compared to those not receiving warfarin therapy (OR: 3.0; 95 % CI: 1.9–4.7) [2]. However, a post hoc analysis demonstrated that those results did not remain significant when evaluating a cohort of patients with subtherapeutic INRs (OR: 1.5; 95 % CI: 0.6–3.7), questioning the indication for INR reversal in this subgroup [2]. Pieracci et al. confirmed these results in a TBI population [3]. However, Allard and colleagues noted an increased risk for mortality in severe TBI patients with an INR ≥1.3 (OR: 6.27; 95 % CI: 1.84–21.35) in a post hoc subgroup univariate analysis [7]. However, these trials did not evaluate the efficacy nor the safety of reversing VKA therapy.
Emerging evidence has revealed the importance of achieving complete INR reversal after ICH. Kuramatsu et al. demonstrated that it was not the degree of initial coagulopathy (2.8 vs. 2.7, p = 0.13) but rather the rapid correction of INR levels to ≤1.3 that may have some beneficial effects on hematoma expansion [13]. The authors did not report adverse events associated with INR reversal nor evaluate patients presenting with an INR <2 separately. The results of our study attempt to fill a void in the current literature by directly evaluating the effectiveness and safety of 4F-PCC in a cohort of subtherapeutic INRs.
Despite its strengths, this evaluation is not without limitations. The percentage of patients presenting with an INR 1.4–1.9 that did not receive 4F-PCC is unknown, as the decision to treat was based on physician preference. The likelihood exists that 4F-PCC was only administered in patients presenting with INR 1.4–1.9 if physician risk is stratified to low risk of thrombosis. However, a greater proportion of patients in the INR 1.4–1.9 group had a history of a thrombotic event prior to administration (70 % vs. 47.5 %). There is not a standardized venous or arterial thrombosis screening protocol after 4F-PCC administration, so the true rate of thrombotic events may be underrepresented in the study. However, the thrombotic rates noted in our data represent clinically significant thrombotic events. This study provides background data and motivation to complete a prospective trial to address the above limitations. Another limitation is the use of INR reversal as an efficacy outcome for stable hemostasis. VKA therapy is routinely monitored by a coagulation analysis (INR) based on the prothrombin time. This coagulation process is a plasma-based model and does not reflect the entire hemostatic process, which is known to be a cell-based model [22].
Conclusion
This evaluation reflects the current therapeutic dilemma because no standardized treatment regimen exists for ICH in patients with elevated but subtherapeutic INRs. The results demonstrate that the use of 4F-PCC in this patient population results in an effective reduction in INR with similar thrombotic risks as compared to a patient presenting with an INR of 2–3.9. A randomized prospective trial would be needed to confirm results as well as evaluate additional measures of efficacy.
References
Franke CL, de Jonge J, van Swieten JC, Opde Coul AA, van Gijn J. Intracerebral hematomas during anticoagulant treatment. Stroke. 1990;21:726–30.
Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164:880–4.
Pieracci FM, Eachempati SR, Shou J, Hydo LJ, Barie PS. Degree of anticoagulation, but not warfarin use itself, predicts adverse outcomes after traumatic brain injury in elderly trauma patients. J Trauma. 2007;63:525–30.
Karni A, Holtzman R, Bass T, et al. Traumatic head injury in the anticoagulated elderly patient: a lethal combination. Am Surg. 2001;67:1098–100.
Cucchiara B, Messe S, Sansing L, Kasner S, Lyden P, CHANT Investigators. Hematoma growth in oral anticoagulant related intracerebral hemorrhage. Stroke. 2008;39:2993–6.
Flibotte JJ, Hagan N, O’Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63:1059–64.
Allard B, Scarpelini S, Rhind SG, et al. Abnormal coagulation tests are associated with progression of traumatic intracranial hemorrhage. J Trauma. 2009;67:959–67.
Brott T, Broderick J, Kothari R, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5.
Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(Suppl 1):e152S–84S.
Hemphill JC 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–60.
Frontera JA, Lewin JJ, Rabinstein AA, Aisiku IP, Alexandrov AW, Cook AM, del Zoppo GJ, et al. Guideline for reversal of antithrombotics in intracranial hemorrhage. Neurocrit Care. 2015;24(1):6–46.
Fontera JA, Gordon E, Zach V, et al. Reversal of coagulopathy using prothrombin complex concentrates is associated with improved outcome compared to fresh frozen plasma in warfarin-associated intracranial hemorrhage. Neurocrit Care. 2014;21:397–406.
Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA. 2015;313:824–36.
Huttner HB, Schellinger PD, Hartmann M, et al. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke. 2006;37:1465–70.
Ivascu FA, Howells GA, Junn FS, et al. Rapid warfarin reversal in anticoagulated patients with traumatic intracranial hemorrhage reduces hemorrhage progression and severity. J Trauma. 2005;59:1131–9.
Kcentra© [package insert]. Kankakee: CSL Behring LLC; 2013.
Sarode R, Milling TJ Jr, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128:1234–43.
Goldstein JN, Refaai MA, Milling TJ Jr, Lewis B, Goldbert-Alberts R, Hug BA, Sarode R. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. Lancet. 2015;23(385):2077–87.
Goldstein JN, Fazen LE, Wendell L, et al. Risk of thromboembolism following acute intracerebral hemorrhage. Neurocrit Care. 2009;10:28–34.
Kim KS, Brophy GM. Symptomatic venous thromboembolism: incidence and risk factors in patients with spontaneous or traumatic intracranial hemorrhage. Neurocrit Care. 2009;11(1):28–33.
Bratton SL, Chesnut RM, Ghajar J, et al. J Neurotrauma. 2007;24(Suppl 1):S32–6.
Sølbeck S, Ostrowski SR, Johansson PI. A review of the clinical utility of INR to monitor and guide administration of prothrombin complex concentrates to orally anticoagulated patients. Thromb J. 2012;10:5–12.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethical approval
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Rivosecchi, R.M., Durkin, J., Okonkwo, D.O. et al. Safety and Efficacy of Warfarin Reversal with Four-Factor Prothrombin Complex Concentrate for Subtherapeutic INR in Intracerebral Hemorrhage. Neurocrit Care 25, 359–364 (2016). https://doi.org/10.1007/s12028-016-0271-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-016-0271-9