Abstract
Background
Infections are a common medical complication in hemorrhagic stroke patients, with vancomycin commonly used as empiric therapy. The purpose of this study was to evaluate the pharmacokinetic parameters of vancomycin in hemorrhagic stroke patients.
Methods
This was a retrospective study of adult patients with aneurysmal subarachnoid hemorrhage (aSAH) or intracerebral hemorrhage (ICH) admitted between May 2010 and February 2015 who received vancomycin. Predicted pharmacokinetic parameters based on population data were compared with calculated pharmacokinetic parameters based on serum trough concentrations.
Results
Eighty aSAH patients and 66 ICH patients met inclusion criteria. In the aSAH group, the mean dosing regimen was 17.6 ± 4 mg/kg every 12 (8–12) h. The mean measured trough concentration was lower than the predicted trough concentration (9.9 ± 4.1 vs. 19 ± 8.7 μg/mL; p < 0.001). The mean calculated elimination rate constant was higher than the predicted value (0.135 ± 0.04 vs. 0.092 ± 0.03 h−1; p < 0.001), and the mean calculated half-life was lower than predicted (5.7 ± 1.8 vs. 8.3 ± 2.9 h; p < 0.001). In the ICH group, the mean dosing regimen was 15.9 ± 4.3 mg/kg every 12 (8–12) h. Similarly, the mean measured trough concentration was lower than the predicted trough concentration (10.7 ± 4.6 vs. 17.5 ± 8.5 μg/mL; p < 0.001). The mean calculated elimination rate constant was higher than the predicted value (0.106 ± 0.03 vs. 0.079 ± 0.02 h−1; p < 0.001), and the mean calculated half-life was lower than predicted (7.2 ± 2.3 vs. 9.6 ± 3.2 h; p < 0.001).
Conclusions
Patients with hemorrhagic stroke exhibited pharmacokinetic alterations favoring increased elimination of vancomycin when compared to predicted pharmacokinetic parameters based on population data. This may result in underexposure to vancomycin, leading to treatment failure and other medical complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemorrhagic stroke is considered a neurologic emergency, with high disability and mortality rates [1–3]. In addition to the direct effects of the initial bleeding event and secondary neurologic complications, patients with aneurysmal subarachnoid hemorrhage (aSAH) or intracerebral hemorrhage (ICH) are predisposed to medical complications that can have a direct impact on outcome and increased ICU and hospital length of stay, along with increased costs of care [4–6]. Furthermore, patients with an acute brain injury have been shown to experience alterations in the levels of acute phase proteins, an acute surge of sympathetic activity, and changes in vascular tone, fluid status and administration, cardiac output, and major organ blood flow, resulting in a hyperdynamic state [7, 8]. This hyperdynamic state may lead to alterations in glomerular filtration rate with resultant enhanced elimination of renally eliminated medications.
Infection is one of the most common medical complications that occur after aSAH or ICH [6, 9]. The development of infections is of significant concern since recent reports found infections as one of the strongest drivers of length of stay and the most common reason for readmission to the hospital within 30 days following discharge after aSAH or ICH [10–12]. Vancomycin is an antibiotic often used empirically in these patient populations, which is primarily renally eliminated and often undergoes routine therapeutic drug monitoring to optimize clinical efficacy and limit toxicity.
The described metabolic and physiologic alterations in patients with an acute brain injury may suggest that vancomycin elimination is enhanced, necessitating an increased vancomycin dosing regimen compared to predicted estimates in order to achieve therapeutic serum concentrations [13, 14]. However, to our knowledge, no studies exist investigating alterations in vancomycin pharmacokinetic parameters in patients with hemorrhagic stroke. Therefore, the purpose of this study was to evaluate the pharmacokinetic parameters calculated from serum concentrations in patients with hemorrhagic stroke to those predicted based on population-based equations.
Materials and Methods
Patients
This was a retrospective study that included adult patients with ICH or aSAH admitted to UNC Hospitals between May 2010 and February 2015. The study was approved by the Institutional Review Board with a waiver of informed consent. Patients must have received vancomycin and had one steady-state vancomycin serum concentration recorded. Patients were excluded if they were pregnant, had renal dysfunction (chronic kidney disease stages 3–5 and/or SCr >1.4 mg/dL), received renal replacement therapy during admission, had a history of nephrectomy, or had a body mass index <18 kg/m2.
Management of ICH and aSAH patients at UNC Hospitals is consistent with published guidelines [3, 15]. As symptomatic cerebral vasospasm has been shown to be associated with reduced instrumental activities of daily living, cognitive impairment, and poor quality of life, that was the definition used for this study in identifying patients who experienced cerebral vasospasm [16].
Vancomycin Dosing Regimens
Clinical pharmacists are consulted for vancomycin initiation for patients admitted to the Neurosciences Intensive Care Unit at UNC Hospitals. The consulted clinical pharmacists are responsible for determining the vancomycin dosing regimen. The goal serum vancomycin trough concentration at steady state is 10–20 ug/mL, depending on the indication. Clinical pharmacists are also responsible for determining the optimal time to obtain steady-state vancomycin serum concentrations, which is generally considered to be at least four times the half-life of the drug after administration.
Clinical Variables
Medical records were retrospectively reviewed for the following baseline patient characteristics: age, gender, weight, admitting diagnosis, Glasgow Coma Scale (GCS) score, Sequential Organ Failure Assessment (SOFA) Score, and serum creatinine. Specifically for the ICH patients, admission ICH score and ICH volume were collected. For the aSAH patients, the Hunt and Hess scale grade and modified Fisher grade were collected. Additionally for the aSAH patients, symptomatic cerebral vasospasm occurrence was recorded. Data collected for characterizing the vancomycin regimen include days from injury to the steady-state vancomycin serum trough concentration, vancomycin dose, vancomycin frequency, temperature modulation use, fluid balance, and infection source.
Pharmacokinetic Measures
To determine alterations in pharmacokinetic parameters of vancomycin therapy following an aSAH or ICH, predicted pharmacokinetic parameters based on population data were compared with pharmacokinetic parameters calculated based on available steady-state vancomycin serum trough concentrations. Predicted pharmacokinetic parameters were calculated using the following equations: [17]
where Vd is the volume of distribution, Ke is the first-order elimination rate constant, and CrCl is the estimated creatinine clearance based on the Cockroft and Gault equation [18]. Ideal body weight was used in the Cockroft and Gault equation, except if the actual body weight was less than the ideal body weight, then the actual body weight was used, or if the actual body weight was greater than 125 % of the ideal body weight, then the adjusted body weight was used [19, 20]. The predicted pharmacokinetic parameters calculated by the equations were then used to determine the estimated vancomycin serum trough concentration, which was compared with the measured vancomycin serum trough concentration.
The following equation was used to calculate patient pharmacokinetic parameters based on steady-state vancomycin serum trough concentrations: [21]
where Vd is the volume of distribution, Ke is the first-order elimination rate constant, Cmin is the steady-state vancomycin serum trough concentration, and τ is the dosing frequency.
Statistical Analysis
Descriptive statistics were used to characterize baseline patient characteristics and vancomycin regimen characteristics. Continuous variables are represented as mean (standard deviation), ordinal variables are represented as median (interquartile range), and categorical variables are represented as n (%). Predicted pharmacokinetic parameters based on population data were compared with pharmacokinetic parameters based on available steady-state vancomycin serum trough concentrations using the Student’s t test. Statistical significance was defined as a p value <0.05. All analyses were carried out with Stata version 13.1 (StataCorp LP, College Station, TX).
Results
There were 1004 patients with hemorrhagic stroke identified during the study period. Of these, 447 patients were identified with an admission diagnosis of aSAH and 557 patients were identified with an admission diagnosis of ICH. A total of 80 aSAH patients and 66 ICH patients were included in the final analyses (see Fig. 1).
Baseline Patient Characteristics
Table 1 displays the baseline characteristics for all patients with hemorrhagic stroke included in the study. The majority of patients were female (52.7 %) with a mean age of 58.8 ± 11.9 years. The median admission GCS was 7 (6–10) and the median admission SOFA score was 4 (3–5). Patients had a mean admission creatinine clearance of 95 ± 33 mL/min, calculated using the Cockcroft and Gault equation.
Of the patients with an admission diagnosis of aSAH, the majority of patients were female (72.5 %) with a mean age of 57.2 ± 10.9 years and had their aneurysm secured by a coiling procedure (67.5 %). The median Hunt and Hess scale grade was 4 (3–4) and the median modified Fisher grade was 3 (3–4). Fifty of the patients included (62.5 %) developed symptomatic cerebral vasospasm. Calculated from the Cockroft and Gault equation, patients had a mean admission creatinine clearance of 91.9 ± 28.1 mL/min.
Specifically for the patients with an admission diagnosis of ICH, the majority of patients were male (71.2 %) with a mean age of 60.7 ± 12.9 years. The median admission ICH score was 2 (2–3) and mean admission ICH volume was 44.6 ± 42.2 mL. Patients had a mean admission creatinine clearance of 98.7 ± 38.1 mL/min based on the Cockcroft-Gault equation.
Vancomycin Characteristics
Patient vancomycin characteristics are displayed in Table 2. The mean estimated vancomycin volume of distribution was 58.3 ± 18.5 L. The mean number of days from injury to the steady-state vancomycin serum concentration was 9.4 ± 5.4 days. Pneumonia was the most frequently reported infection source [n = 77 (52.7 %)]. The mean vancomycin dosing regimen was 16.8 ± 4.2 mg/kg every 12 (8–12) h in order to achieve a predicted trough concentration of 18.3 ± 8.6 ug/mL.
Patients with an admission diagnosis of aSAH had a mean estimated volume of distribution of 53.5 ± 14.6 L with a mean number of 9.5 ± 5.7 days from injury to the steady-state vancomycin serum concentration. Pneumonia was reported as the infection source in 51.2 % of patients. The mean vancomycin dosing regimen was 17.6 ± 4 mg/kg every 12 (8–12) h to achieve a predicted trough concentration of 19 ± 8.7 υg/mL.
The mean estimated vancomycin volume of distribution was 64.2 ± 21 L with a mean number of 9.3 ± 5.1 days from injury to the steady-state vancomycin serum concentration for patients with an admission diagnosis of ICH. The highest reported infection source was pneumonia (54.6 % of patients) with a mean vancomycin dosing regimen of 15.9 ± 4.3 υg/mL every 12 (8–12) h to achieve a predicted trough concentration of 17.5 ± 8.5 ug/mL.
Vancomycin Pharmacokinetic Parameters
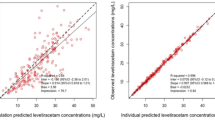
Table 3 displays the predicted pharmacokinetic parameters based on population data and the pharmacokinetic parameters calculated from steady-state vancomycin serum trough concentrations. The mean calculated elimination rate constant was significantly higher than the predicted value (0.122 ± 0.04 vs. 0.086 ± 0.03 h−1; p < 0.001) and the mean calculated half-life was significantly lower than the predicted half-life (6.4 ± 2.2 vs. 8.9 ± 3.1 h; p < 0.001). The mean measured steady-state vancomycin serum trough concentration was also significantly lower than the vancomycin serum trough concentration estimated from the predicted pharmacokinetic parameters based on population data (10.3 ± 4.3 vs. 18.3 ± 8.6 υg/mL; p < 0.001).
For patients with an admission diagnosis of aSAH, the mean calculated elimination rate constant was significantly higher than the predicted value (0.135 ± 0.04 vs. 0.092 ± 0.03 h−1; p < 0.001) and the mean calculated half-life was significantly lower than the predicted half-life (5.7 ± 1.8 vs. 8.3 ± 2.9 h; p < 0.001). The mean measured steady-state vancomycin serum trough concentration of 9.9 ± 4.1 υg/mL was significantly lower than the vancomycin serum trough concentration of 19 ± 8.7 υg/mL estimated from the predicted pharmacokinetic parameters based on population data (p < 0.001). When evaluating the patients with aSAH who experienced symptomatic vasospasm, these findings were slightly more pronounced (Table 4; n = 50).
Patients with an admission diagnosis of ICH had a mean calculated elimination rate constant significantly higher than the predicted value (0.106 ± 0.03 vs. 0.079 ± 0.02 h−1; p < 0.001) and a mean calculated half-life significantly lower than the predicted half-life (7.2 ± 2.3 vs. 9.6 ± 3.2 h; p < 0.001). The mean measured steady-state vancomycin serum trough concentration was also significantly lower than the vancomycin serum trough concentration estimated from the predicted pharmacokinetic parameters based on population data (10.7 ± 4.6 vs. 17.5 ± 8.5 υg/mL; p < 0.001).
Discussion
This study is the first to our knowledge to evaluate alterations in vancomycin pharmacokinetics in patients with hemorrhagic stroke. Patients with aSAH or ICH, collectively and separately, exhibited pharmacokinetic alterations and serum trough concentrations favoring an increased elimination of vancomycin when compared to predicted pharmacokinetic parameters and serum trough concentrations based on population data. When considering the significance and impact of these findings, it is important to recognize the potential impact of acute brain injury on the pharmacokinetic parameters of vancomycin.
While there are currently no known studies investigating alterations in pharmacokinetic parameters in patients with aSAH or ICH, there have been recent data suggesting that an increased dosage of vancomycin is needed in critically ill patients with acute brain injury. A case report of a 22-year-old female with a traumatic brain injury was published by Cook and colleagues evaluating serum monitoring of vancomycin [13]. They found that when using standard dosing of vancomycin, typical target therapeutic concentrations were not obtained. Additionally, our research team also recently published a study evaluating the impact of temperature modulation on vancomycin pharmacokinetic parameters in patients with acute brain injury [14]. In patients whose core body temperature was reduced to a median of 34 °C through induced therapeutic hypothermia or pentobarbital infusion, there was no significant difference between calculated vancomycin pharmacokinetic parameters and pharmacokinetic parameters estimated from population-based equations. However, patients who underwent controlled normothermia exhibited pharmacokinetic alterations favoring an increased elimination of vancomycin and subtherapeutic vancomycin serum trough concentrations when using standard dosing regimens.
Reasoning as to why standard doses of vancomycin do not achieve therapeutic serum trough concentrations in critically ill patients with acute brain injury may relate to the concept of augmented renal clearance. In a study evaluating 20 patients with traumatic brain injury, Udy and colleagues found that 85 % of the patients experienced augmented renal clearance, defined as a CrCl >150 mL/min/1.73 m2 in women and >160 mL/min/1.73 m2 in men [22]. May and colleagues also evaluated the actual creatinine clearance through a 24-h urine collection in 20 patients with subarachnoid hemorrhage [23]. The mean actual creatinine clearance among the patients enrolled was 325.9 ± 135.2 mL/min/1.73 m2, which was significantly higher than the Cockcroft and Gault estimated Creatinine clearance of 144.9 ± 42.8 mL/min/1.73 m2 (p < 0.001). Four of the patients in their group experienced cerebral vasospasm, resulting in a higher mean actual creatinine clearance (558.4 ± 356.1 mL/min/1.73 m2). In patients who experience augmented renal clearance, it has been proposed that renally eliminated drugs may be excreted more quickly than is expected, resulting in subtherapeutic concentrations [24–27].
While there is currently a lack of literature demonstrating a direct association between elevations in creatinine clearance in patients with aSAH or ICH and increased elimination of vancomycin, our study suggests that the augmented renal clearance experienced by patients with aSAH cause pharmacokinetic alterations that result in a quicker elimination than predicted. Furthermore, although a study evaluating the actual creatinine clearance of patients with ICH is not currently available, our study suggests that patients with ICH also experience augmented renal clearance. This subsequently results in pharmacokinetic alterations causing an increased elimination of vancomycin. These data reflect an important finding, as subtherapeutic vancomycin serum trough concentrations may lead to treatment failure and other serious patient complications. This finding is especially critical for patients with ICH, as these patients may generally be considered to be less likely to experience augmented renal clearance, given their older age and higher likelihood of co-morbidities that result in renal dysfunction, such as hypertension and diabetes [28].
This study has several limitations that are worthy for discussion. First, during the study period, only steady-state vancomycin serum trough concentrations were obtained by our institution through routine vancomycin therapeutic drug monitoring. Therefore, the pharmacokinetic parameters were calculated using a one-compartment model and one-level vancomycin concentration equation that uses the population volume of distribution to calculate an elimination rate constant. Although these methods may be less reliable than obtaining vancomycin serum peak and trough concentrations, our findings reflect a real-world clinical setting where vancomycin therapeutic drug monitoring routinely uses one-compartment model, population-based equations. Furthermore, the Cockcroft and Gault equation was used to estimate creatinine clearance. This equation was principally designed for use in an ambulatory or ward-based setting, and has been shown to be a poor predictor of renal function in patients who are critically ill [7]. A more accurate predictor of renal function, such as a 24-h urine creatinine measurement, is needed for critically ill patients. However, this is not considered standard practice at our institution. Additionally, minimal information was available on follow-up vancomycin dosing regimens and subsequent serum concentrations, and the impact on infectious outcomes as vancomycin was frequently discontinued in an effort to narrow antibiotic therapy. Nevertheless, while this study illustrates that vancomycin pharmacokinetic alterations occur in patients with aSAH and ICH, it also raises questions regarding the proper dosing of other renally eliminated medications that do not undergo routine therapeutic drug monitoring. This is an important area for future evaluation. Also, due to the study’s retrospective nature, it was also not possible to collect data on vasopressor doses in the study patients. This is critical information to capture in future studies, as vasopressors have been shown to influence renal function [29]. Specifically for the aSAH patients, the incidence of cerebral vasospasm seen in our study was higher then reported in the literature, which may impair the generalizability of our results to patients with aSAH without cerebral vasospasm [16, 30]. However, when evaluating only the aSAH patients with cerebral vasospasm in our study, there was only a slightly more enhanced alteration of the pharmacokinetic parameters. Moreover, patients who develop cerebral vasospasm generally have a longer ICU length of stay, leading to the potential of a higher likelihood for vancomycin to be prescribed for infectious complications than patients without cerebral vasospasm.
Conclusion
This is the first study evaluating alterations in vancomycin pharmacokinetic parameters in patients with hemorrhagic stroke. Patients with aSAH or ICH exhibited pharmacokinetic alterations favoring a quicker elimination of vancomycin than predicted, resulting in subtherapeutic vancomycin serum trough concentrations. This results in underexposure to vancomycin, potentially leading to treatment failure and other serious patient complications. These findings highlight the importance of close therapeutic drug monitoring and suggest that future studies are needed to determine optimal dosing strategies in these patient populations.
References
Claassen J, Vu A, Kreiter KT, et al. Effect of acute physiologic derangements on outcome after subarachnoid hemorrhage. Crit Care Med. 2004;32:832–8.
Wartenberg KE, Mayer SA. Medical complications after subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21:325–38.
Hemphill JC III, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–60.
Wartenberg KE, Mayer SA. Medical complications after subarachnoid hemorrhage: new strategies for prevention and management. Curr Opin Crit Care. 2006;12(2):78–84.
Wartenberg KE, Schmidt JM, Claassen J, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34:617–23.
Naidech AM, Bendok BR, Tamul P, et al. Medical complications drive length of stay after brain hemorrhage: a cohort study. Neurocrit Care. 2009;10:11–9.
Udy AA, Baptista JP, Lim NL, et al. Augmented renal clearance in the ICU: results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations. Crit Care Med. 2014;42:520–7.
Udy AA, Roberts JA, Lipman J. Implications of augmented renal clearance in critically ill patients. Nat Rev Nephrol. 2011;7:539–43.
Balami JS, Buchan AM. Complications of intracerebral haemorrhage. Lancet Neurol. 2012;11:101–18.
Liotta EM, Singh M, Kosteva AR, et al. Predictors of 30-day readmission after intracerebral hemorrhage: a single-center approach for identifying potentially modifiable associations with readmission. Crit Care Med. 2013;41:2762–9.
Singh M, Guth JC, Liotta E, et al. Predictors of 30-day readmission after subarachnoid hemorrhage. Neurocrit Care. 2013;19:306–10.
Ohwaki K, Yano E, Nagashima H, Nakagomi T, Tamura A. Impact of infection on length of intensive care unit stay after intracerebral hemorrhage. Neurocrit Care. 2008;8:271–5.
Cook AM, Arora S, Davis J, Pittman T. Augmented renal clearance of vancomycin and levetiracetam in a traumatic brain injury patient. Neurocrit Care. 2013;19:210–4.
Morbitzer KA, Jordan JD, Rhoney DH. Vancomycin pharmacokinetic parameters in patients with acute brain injury undergoing controlled normothermia, therapeutic hypothermia, or pentobarbital infusion. Neurocrit Care. 2015;22:258–64.
Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43:1711–37.
Frontera JA, Fernandez A, Schmidt JM, et al. Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke. 2009;40:1963–8.
Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984;25:433–7.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
Wurtz R, Itokazu G, Rodvold K. Antimicrobial dosing in obese patients. Clin Infect Dis. 1997;25:112–8.
Sawyer WT, Canaday BR, Poe TE, et al. Variables affecting creatinine clearance prediction. Am J Hosp Pharm. 1983;40:2175–80.
Ambrose PJ, Winter ME. Basic clinical pharmacokinetics. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2004. p. 451–76.
Udy A, Boots R, Senthuran S, et al. Augmented creatinine clearance in traumatic brain injury. Anesth Analg. 2010;111:1505–10.
May CC, Arora S, Parli SE, Fraser JF, Bastin MT, Cook AM. Augmented renal clearance in patients with subarachnoid hemorrhage. Neurocrit Care. 2015;23(3):374–9.
Lin Wu FL, Liu SS, Yang TY, et al. A larger dose of vancomycin is required in adult neurosurgical intensive care unit patients due to augmented clearance. Ther Drug Monit. 2015;37(5):609–18.
Campassi ML, Gonzalez MC, Masevicius FD, et al. Augmented renal clearance in critically ill patients: incidence, associated factors and effects on vancomycin treatment. Rev Bras Ter Intensiva. 2014;26:13–20.
Baptista JP, Sousa E, Martins PJ, Pimentel JM. Augmented renal clearance in septic patients and implications for vancomycin optimisation. Int J Antimicrob Agents. 2012;39:420–3.
Udy AA, Roberts JA, Boots RJ, Paterson DL, Lipman J. Augmented renal clearance: implications for antibacterial dosing in the critically ill. Clin Pharmacokinet. 2010;49:1–16.
Andaluz N, Zuccarello M. Recent trends in the treatment of spontaneous intracerebral hemorrhage: analysis of a nationwide inpatient database. J Neurosurg. 2009;110:403–10.
McQueen EG, Morrison RB. The effects of synthetic angiotensin and noradrenaline on blood pressure and renal function. Br Heart J. 1961;23(1):1–6.
Dorsch NW, King MT. A review of cerebral vasospasm in aneurysmal subarachnoid haemorrhage Part I: incidence and effects. J Clin Neurosci. 1994;1:19–26.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The IRB at the University of North Carolina at Chapel Hill granted a waiver of informed consent for the study procedures.
Rights and permissions
About this article
Cite this article
Morbitzer, K.A., Jordan, J.D., Sullivan, K.A. et al. Vancomycin Pharmacokinetic Parameters in Patients with Hemorrhagic Stroke. Neurocrit Care 25, 250–257 (2016). https://doi.org/10.1007/s12028-016-0264-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-016-0264-8