Abstract
The proliferation of antigen-specific lymphocyte clones, the initial step in acquired immunity, is vital for effector functions. Proliferation tests both in immunology research and diagnosis are gaining attendance gradually, while the use of adult healthy individuals as controls of pediatric patients is a question. This study aimed to investigate and compare mitogen-stimulated proliferation responses of total lymphocytes and T- and B-lymphocyte subsets in adult and children healthy donors. Nineteen children and 20 adult healthy donors were enrolled in this study. Peripheral blood mononuclear cells (PBMCs) purified from peripheral blood samples of the donors, by Ficoll gradient centrifugation, were stained with CFSE and were cultured in a 37 ℃ CO2 incubator for 120 h with the absence or existence of polyclonal activators: PHA and CD-Mix. After cell culture, PBMCs were stained with monoclonal antibodies against CD4 and CD19, and proliferation percentages of CD4+ T and CD19+ B cells, together with total lymphocytes were determined by flow cytometry. This study revealed similarities between children and adult age groups, concerning mitogenic stimulation of the lymphocytes. The only difference was a significantly high proliferation of pediatric CD4+ T cells in response to PHA. CD4+ T cell responses against PHA were inversely correlated with altering age. When pediatric individuals were distributed into age groups of 0–2 years, 3–5 years, and 6–18 years, PHA responses of CD4+ cells were found to be diminished with advancing age. These findings propose the possibility of enrollment of adult healthy individuals as controls for pediatric patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The proliferation assays are gaining attendance in immunology studies [1]. In recent times, proliferation assays are applied to monitor the function of T and B lymphocytes in response to stimulants such as mitogens and are becoming a part of clinical practice. Furthermore, novel methodologies are being developed to provide easy, safe, and non-radioactive approaches and to render laboratory work reliable and easier [2].
T cell functions are extremely important for the avoidance of viral and fungal infections and for the provision of help to B cells for isotype switching of antibodies against protein antigens [3, 4]. The cells of adaptive immunity respond via antigen-specific receptors, and they clonally proliferate and rapidly differentiate into activated effector cells. Mitogen, a trigger to induce in vitro responsiveness, initiates mitotic activity through intracellular signaling that is transformed into cell division [5].
At present, besides laboratory screening tests, functional evaluation of T lymphocyte disturbance is essential for the investigation of combined T/B cell immunodeficiencies, most of which are commonly related to children. Proliferation assays have been exploited for the screening of patients with primary and secondary immunodeficiency disorders. The conventional method for the assessment of lymphocyte proliferation is accomplished by tritiated (H3) thymidine incorporation into recently divided cellular DNA [6]. However, this method is hard to exploit in routine laboratory practice due to some technical difficulties such as the usage of radioactivity and lack of information on proliferating lymphocyte subsets [7]. Lymphocyte proliferation analysis in vitro via dilution of a cytoplasmic dye called carboxyfluorescein succinimidyl ester (CFSE), which can enter the cell and integrate itself into the cell membrane and emit fluorescence, is efficient for practical work. CFSE attached to intracellular proteins is equally distributed between each divided daughter cell during mitosis, which causes a 50% drop of fluorescent signal in each cell division, that can be recognized via flow cytometry [8]. CFSE has been found effective for the investigation of lymphocyte division in many laboratories since its initial description by Lyons and Parish in 1994 [7, 9]. Research in immunology also uses CFSE proliferation assays to investigate cell functions and properties [10,11,12,13,14].
Flow cytometry has progressed during the last three decades, from being a laboratory technique to a routinely used tool for both basic and clinical immunologists, in a wide area from routine diagnosis to highly advanced research [15]. Flow cytometry has applications in molecular biology, cancer research, microbiology, and virology [16]. Additionally, flow cytometry is relevant to evaluate T cell functions by quantification of responses to mitogens. The utilization of flow cytometry for T cell proliferation analysis was reported to be applicable in the diagnosis and management of immune deficiency diseases [17, 18].
Proliferation analysis requires healthy controls both for the accuracy of the experiments and also for the comparison of the responses of the diseased individuals. Generally, the controls for the proliferation assays during clinical laboratory practice are adult healthy donors, which could be due to limitations in obtaining blood samples from healthy children. Together with this, proliferation tests were reported to be more relevant for neonates and elder children in the investigation of immune dysfunction [19]. However, antigen response in vitro may vary depending on the period and age of the patient, disease history and vaccination, and the absolute number of lymphocytes [20].
This study investigated and compared total, CD4+ T- and CD19+ B-lymphocyte proliferation values of adult and children healthy individuals in response to two major mitogens, by CFSE dilution method. The results of this study suggest that healthy controls in adults can be used in place of healthy controls in children and that pediatric individuals could be excluded as healthy controls, which could help to prevent children from blood sampling without any medical necessity.
Materials and methods
Study groups
Blood samples were obtained from 19 healthy children with ages between 0 and 18 and 20 healthy adults with ages between 26 and 46. Both participant groups were healthy with no chronic health problems. Individuals with allergic disorders, chronic inflammatory diseases, autoimmune diseases, primary and secondary immune deficiencies, and also with any infection during blood sampling were excluded from the study. The children’s samples were provided from Istanbul University-Cerrahpasa, Cerrahpasa Faculty of Medicine, Department of Pediatrics, Division of Pediatric Allergy and Immunology. Istanbul University, Aziz Sancar Institute of Experimental Medicine provided the blood samples of the adult volunteers. This study was approved by the local ethical committee of Istanbul University, Istanbul Faculty of Medicine. The signed consent forms were obtained from all participants.
Blood collection and purification of PBMCs
Peripheral blood was collected in lithium heparin tubes (3–5 ml vacutainer; BD Vacutainer, UK), and peripheral blood mononuclear cells (PBMCs) were purified by the Ficoll density gradient centrifugation method. Briefly, the blood sample was diluted with an equal volume of phosphate buffer saline solution (PBS) with a ratio of (1:1) and then layered over Ficoll–Hypaque (Capricorn, USA) with a ratio of (2:1) and centrifuged at 800G at 20 ℃ for 20 min. The mononuclear cell layer was collected with a Pasteur pipette and washed twice with PBS for 5 min at 20 ℃ at 300G. If necessary, erythrocytes were depleted with the addition of 2 ml of NH4Cl solution and incubation for 10 min and a washing step for 5 min at 20 ℃ at 300G. PBMCs were counted under light microscopy by trypan exclusion method.
CFSE staining and cell culture
Up to 2 × 107 cells were suspended in 1 ml of RPMI-1640 culture medium enriched with fetal bovine serum (FBS), penicillin, streptomycin, L-glutamine, non-essential amino acids, sodium pyruvate, and minimum essential medium (MEM) vitamins (All from Gibco, Paisley, UK). A 1 μl of 5 mM CFSE solution (Molecular Probes, Eugene, USA) was added to the PBMC suspension, incubated in the dark for 6 min at 4 ℃, and then washed twice for 5 min at 800G at 4 ℃. Following CFSE staining, cells were counted under light microscopy with the trypan exclusion method and were cultured in 48-well, flat bottom culture plates (NEST Biotechnology, China) for 120 h at 37 ℃ in a 5% CO2 environment, with the absence and existence of 5 µl/ml phytohaemagglutinin (PHA, Thermo Fisher, USA) and 1/500 monoclonal antibody cocktail encompassing anti-CD2, anti-CD3, and anti-CD28 (termed as CD-Mix; Stem Cell, USA). No specific agents for triggering B cell proliferation were used. Instead, the B cell proliferation following T cell activation was investigated. The doses of mitogens and the incubation time point of 120 h were either defined by preliminary experiments or previously described in several previous studies [11,12,13, 21,22,23].
Analysis of cell proliferation
After 120 h of incubation with the absence and presence of mitogens, 100 μl cell suspension was taken from the respective well (US, unstimulated; PHA, phytohemagglutinin-stimulated; CD-Mix, stimulated with CD-Mix) in three separate flow cytometry tubes. A 2.5 μl of anti-CD4 phycoerythrin-cyanin 7 (PE-Cy7) (BD Biosciences, USA) and 1 μl of anti-CD19 allophycocyanin (APC) (BD Biosciences, USA) fluorescent-labeled monoclonal antibodies were added to each tube and incubated at dark and room temperature for 20 min. After incubation, 5 ml of FACS buffer was added to each tube, and PBMCs were washed at 20 ℃ for 5 min at 300G. Following the washing step, supernatants were removed, and PBMCs were resuspended in 500 μl of FACS buffer and were analyzed in a FACSCalibur flow cytometry running CellQuest software (BD Biosciences, United States). Analysis of data was performed with CellQuest software. The obtained data were evaluated as (proliferation %). The percentages of total lymphocyte, CD4+ T- and CD19+ B-cell proliferation were evaluated separately for two different mitogens.
Statistical analysis
The group’s concordance to a normal distribution was evaluated by Kolmogorov–Smirnov test. Differences between normally distributed groups were evaluated by Student’s T-test, and differences between groups with no normal distribution were evaluated by Mann Whitney U test. Multiple groups’ statistical differences were evaluated by ANOVA followed by Tamhane’s test as post hoc. The correlations between age and proliferation levels were evaluated by Pearson test. The scatter plots reveal the means and values of each individuals. A p < 0.05 was accepted as the statistical significance level. S.P.S.S. version 19.0 was used for statistical analyses. GraphPad Prism 7.0 software was used for the construction of graphics.
Results
Nineteen healthy children (11 female and 8 male, age: 7.16 ± 6.07) and 20 healthy adult volunteers (8 female and 12 male, age: 35.95 ± 5.56) were enrolled in this study. Adult healthy volunteers were aged between 26 and 46 years, while children were aged between 0 and 18 years. All of the controls enrolled in this study were healthy during blood sampling.
Proliferation of total lymphocytes
In this study, the proliferation of total lymphocytes was initially investigated. Lymphocytes were gated according to forward scatter (FSc) and side scatter (SSc) properties. Spontaneous proliferation was marked on the unstimulated cells, and same marker was used for both stimulants (Fig. 1A). No significant differences were observed among adult and children healthy subjects. In other words, both children and adult healthy subjects displayed similar proliferative capabilities, in response to both PHA and CD-Mix (Fig. 1B).
Proliferation of total lymphocytes with the absence and existence of mitogens: PHA and CD-Mix. PBMCs were stimulated for 120 h in the absence and existence of PHA and CD-Mix mitogens, and proliferation was assayed by flow cytometry by gating of lymphocytes. A Demonstrative histogram of cell proliferation; graphs indicate proliferation percentages of PBMCs both unstimulated and in response to mitogens. B Cumulative data graph demonstrates proliferation percentages of children and adult healthy individuals in response to mitogens. Lines in the scatter graphs reveal the mean values of relevant groups. Circles indicate adult volunteers and triangles indicate child counterparts
Proliferation of CD4+ T cells
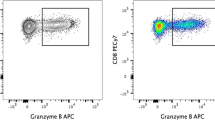
Next, we investigated the proliferation of CD4+ T cells. A density plot was used for the presentation of CD4+ T cell proliferation. Proliferating cell percentages were used as data. The quadrant was set according to the unstimulated condition and was used for PHA- and CD-Mix-stimulated conditions (Fig. 3A). CD4+ T cells of children were observed to respond significantly stronger to PHA than those of adult controls (40.35 ± 11.23% vs 29.66 ± 10.85%, respectively, p = 0.012). CD-Mix-induced proliferative responses of children and adult healthy subjects were observed to be similar (43.66 ± 10.15% vs 49.32 ± 14.26%, respectively) (Fig. 2B). The analysis of the relation between PHA-induced CD4+ T cell proliferation and age revealed an inverse and significant correlation (r = − 0.504, p < 0.001) (Fig. 2C) that exposed the declination of PHA-stimulated CD4+ T cell proliferation with advancing age.
Proliferation of CD4+ T cells in response to mitogens: PHA and CD-Mix. PBMCs were stimulated with the absence and existence of PHA, and CD-Mix mitogens for 120 h and CD4+ T cells in lymphocytes gate were demonstrated by surface staining with a fluorescent-labeled anti-CD4 monoclonal antibody. Lymphocyte proliferation was assayed by flow cytometry. A Demonstrative dot plot of CD4+ T cell proliferation; graphs indicate proliferation percentages of cells both unstimulated and in response to mitogens. B Cumulative data graph demonstrates CD4+ T cell proliferation percentages of children and adult healthy individuals in response to mitogens. Lines in the scatter graphs reveal the mean values of relevant groups. Circles indicate adult volunteers and triangles indicate child counterparts (*p = 0.018, Student’s T-test). C The analysis of the relation between PHA-induced CD4+ T cell proliferation and age. Pearson test was used for correlation analysis between CD4+ T cell proliferation and age in both adults and children
Proliferation of CD19+ B cells
A density plot was used for the exhibition of CD19+ B lymphocyte proliferation. The gate was set according to the unstimulated condition and was used for PHA- and CD-Mix-stimulated conditions (Fig. 3A). When proliferation levels of CD19+ B cells were investigated, heterogeneous B cell proliferation was observed both in adult and children control groups in response to both mitogens. No significant differences were observed among the adult and children healthy groups in response to both PHA and CD-Mix stimulations. In other words, B cell responses following cell culture with PHA- and CD-Mix were concordant in adult and children healthy individuals (Fig. 3B).
Proliferation of CD19+ B cells in response to mitogens: PHA and CD-Mix. PBMCs were stimulated with the absence and existence of PHA, and CD-Mix mitogens for 120 h and CD19+ T cells in lymphocytes gate were demonstrated by surface staining with a fluorescent-labeled anti-CD19 monoclonal antibody. Cell proliferation was assayed by flow cytometry. A Demonstrative dot plot of CD19+ B cell proliferation; graphs indicate proliferation percentages of cells both unstimulated and in response to mitogens. B The cumulative data graph demonstrates CD19+ B cell proliferation percentages of child and adult healthy individuals in response to mitogens. Lines in the scatter graphs reveal the mean values of relevant groups. Circles indicate adult volunteers and triangles indicate child counterparts
Proliferation in age groups of healthy children
The healthy children were grouped with respect to ages as < 2 years (n = 4), 2–5 years (n = 6), and 5–18 years (n = 9) (Fig. 4A). The investigation of the responses of total lymphocytes, CD4+ T, and CD19+ B cells to two polyclonal activators, PHA and CD-Mix, in different age groups of healthy children revealed variations only in PHA-stimulated CD4+ T cells. Accordingly, the children within the 0–2 years age group had the strongest proliferation values, which were significantly higher in comparison both with the 5–18 years group and with healthy adults. No significant differences were observed in the proliferative values of PBMCs as well as CD19+ B cells (Fig. 4B) among different age groups.
Proliferation values of children healthy control groups with respect to age. Nineteen children volunteers enrolled in this study were grouped according to age groups as < 2 years, 2–5 years, and > 5 years (A). Cumulative data graphs demonstrate proliferation percentages of total lymphocytes, CD4+ T cells, and CD19+ B cells of pediatric individuals in response to mitogens (B). Lines in the scatter graphs reveal the mean values of relevant groups. Circles indicate < 2 years age group, up-triangles indicate 2–5 years age group, down-triangles indicate > 5 years age group, and angled squares indicate adult age group (*p = 0.021, **p < 0.0001)
Discussion
The proliferation of the antigen-specific lymphocyte clones is the initial step in acquired immunity, which is important for developing the effector functions of the immune cells. Proliferation tests both in immunologic research topics such as studies investigating immune tolerance, antigen responsiveness and determination of the efficacy of inhibitory molecules, and diagnosis of immune deficiency diseases is gaining attendance day by day, while the use of adult healthy controls for comparison with pediatric patients is an important yet unanswered question. No previous study used CFSE staining to determine the proliferation levels of total lymphocytes and CD4+ T- and CD19+ B-lymphocytes in response to CD-Mix and PHA, and compare those levels in children and adult healthy control groups.
In this study, no specific agents for triggering B cell proliferation such as IgM, IL-4 + IL-21, nor TLR-9 agonists were used. Instead, this effect was dependent on T cell activation in response to PHA or CD-Mix, which in turn triggered B cells. The proliferation of B cell works normally by CD40-CD40L as co-stimulation following T cell activation by TCR-MHC II linking, and this signal is enough for secretion of lymphokines like IL-4 and sufficient expression of IL-21R which could initiate the B cell proliferation, together with the possibility of minimal cross-linking of B cell receptor with the mentioned mitogens. Although we demonstrated that healthy adults have similarities in proliferation function that can be used as the control for diseased pediatrics that suffer from primary immunodeficiencies, we did not use B cell-specific stimulants because that would not evaluate the dysfunction of combined deficiency for each T and B cell proliferation. At the molecular level, all diseases related to primary immune deficiencies have defects in receptors of the lymphocytes such as gamma chain receptors and CD40-CD40L defect in severe combined immune deficiency and hyper IgM syndrome [24,25,26].
In this study, the rates of mitogen-induced proliferation of total lymphocytes as well as CD4+ and CD19+ lymphocyte sub-groups were evaluated in adult healthy individuals and different age groups of pediatric individuals. Our results revealed similar proliferative properties of the lymphocyte subsets in all pediatric age groups. All but one parameter were concordant in adult and child groups, while PHA-responsiveness of CD4+ T cells was observed to be stronger in children, compared with adult healthy individuals. This variability could be attributed to some facts. A previous study reported diminished PHA- and anti-CD3-triggered proliferative responses of CD4+ T cells in elder adults with ages over 65 years old, compared with young adults aged between 20 and 30 years [27]. While another study reported a slightly decreased growth of CD4+ T cells in elder adults following PHA stimulation, which was observed to be completely similar among elder and younger adults under anti-CD3 stimulation. The counts of activated cells in both age groups that continue to live and proliferate in the culture were observed to be similar [28]. The downregulated proliferative responses could be attributed to the decreased efficiency of the signal transduction, which was assayed by the mobilization of intracellular free calcium activity in response to stimulation with PHA. Calcium was measured in CD4+ T cells of young and older adults and compared with that of young adults, by a flow cytometry assay with the dye indo-l. Accordingly, CD4+ T cells from older adults showed a calcium response that was up to ten percent lower following PHA stimulation. This appeared to be related to decreased calcium channel activity in older adults, rather than the mobilization of intracellular calcium stores. The reduced proliferation of T cells in older adults was not related to the decreased efficiency of transmembrane signal transduction [27]. Also, in a study investigating the expression of co-stimulatory molecules CD27 and CD28 on CD4+ T cells, no functional differences were observed between healthy young and elder persons [29]. In our study, the adult population (35.95 ± 5.56 years, range: 26–46 years) fits in the young adult group; therefore, the observed variability of CD4+ T cell responses could be a property of the pediatric age group.
Overall, our data support the hypothesis that healthy blood samples of relatively young adults could be used as control samples for pediatric patients, in lymphocyte proliferation studies, which use PHA and CD-Mix as polyclonal activators. This may avoid unnecessary blood sampling from healthy children to be used as a control for a diseased counterpart. Diminished responses of adult CD4+ T cells in response to PHA should be kept in mind and should further be investigated. The most important limitation of our study was the limited number of individuals, especially at younger ages. This was both due to ethical reasons and also hardness in obtaining blood samples from children at several months of age.
In conclusion, the results of this study have presented the similarities between healthy adults and healthy children in terms of mitogen-stimulated lymphocyte proliferation. Here, the usage of healthy adult blood samples for comparison with pediatric patient values will be possible. A conclusion that comes out alternatively is that this study revealed the necessity and importance of forfeiting attention to the age group of healthy controls in terms of proliferation. An increasing number of studies will use proliferation tests in the near future, and an answer to the healthy control issue arose from this study.
Data availability
The data related with this study is available upon request from the corresponding author.
References
Lyons AB, Blake SJ, Doherty KV. Flow cytometric analysis of cell division by dilution of CFSE and related dyes. Curr Protoc Cytom. 2013;Chapter 9:Unit9 11. https://doi.org/10.1002/0471142956.cy0911s64.
Marits P, Wikstrom AC, Popadic D, Winqvist O, Thunberg S. Evaluation of T and B lymphocyte function in clinical practice using a flow cytometry based proliferation assay. Clin Immunol. 2014;153(2):332–42. https://doi.org/10.1016/j.clim.2014.05.010.
Kervevan J, Chakrabarti LA. Role of CD4+ T cells in the control of viral infections: recent advances and open questions. Int J Mol Sci 2021;22(2):523. https://doi.org/10.3390/ijms22020523.
Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557–69. https://doi.org/10.1182/blood-2008-05-078154.
Nowell PC. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Can Res. 1960;20:462–6.
Cost KM, Fineman D, Steger S. A flow cytometry-based screening assay for lymphocyte proliferation. Clin Immunol Newsl. 1993;13(7):82–5. https://doi.org/10.1016/0197-1859(93)90012-9.
Fulcher D, Wong S. Carboxyfluorescein succinimidyl ester-based proliferative assays for assessment of T cell function in the diagnostic laboratory. Immunol Cell Biol. 1999;77(6):559–64. https://doi.org/10.1046/j.1440-1711.1999.00870.x.
Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc. 2007;2(9):2049–56. https://doi.org/10.1038/nprot.2007.296.
Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171(1):131–7. https://doi.org/10.1016/0022-1759(94)90236-4.
Deniz G, Erten G, Kucuksezer UC, Kocacik D, Karagiannidis C, Aktas E, et al. Regulatory NK cells suppress antigen-specific T cell responses. J Immunol. 2008;180(2):850–7. https://doi.org/10.4049/jimmunol.180.2.850.
Kucuksezer UC, Palomares O, Ruckert B, Jartti T, Puhakka T, Nandy A, et al. Triggering of specific Toll-like receptors and proinflammatory cytokines breaks allergen-specific T-cell tolerance in human tonsils and peripheral blood. J Allergy Clin Immunol. 2013;131(3):875–85. https://doi.org/10.1016/j.jaci.2012.10.051.
Kucuksezer UC, Zekiroglu E, Kasapoglu P, Adin-Cinar S, Aktas-Cetin E, Deniz G. A stimulatory role of ozone exposure on human natural killer cells. Immunol Invest. 2014;43(1):1–12. https://doi.org/10.3109/08820139.2013.810240.
Palomares O, Ruckert B, Jartti T, Kucuksezer UC, Puhakka T, Gomez E, et al. Induction and maintenance of allergen-specific FOXP3+ Treg cells in human tonsils as potential first-line organs of oral tolerance. J Allergy Clin Immunol. 2012;129(2):510–20. https://doi.org/10.1016/j.jaci.2011.09.031.
Ekici Y, Yilmaz A, Kucuksezer UC, Gazioglu SB, Yamalioglu ZD, Gurol AO, et al. Combined evaluation of proliferation and apoptosis to calculate IC50 of VPA-induced PANC-1 cells and assessing its effect on the Wnt signaling pathway. Med Oncol. 2021;38(9):109. https://doi.org/10.1007/s12032-021-01560-4.
Jaye DL, Bray RA, Gebel HM, Harris WA, Waller EK. Translational applications of flow cytometry in clinical practice. J Immunol. 2012;188(10):4715–9. https://doi.org/10.4049/jimmunol.1290017.
McKinnon KM. Flow Cytometry: An Overview. Curr Protoc Immunol. 2018;120(5):1–5. https://doi.org/10.1002/cpim.40.
Azarsiz E, Karaca N, Ergun B, Durmuscan M, Kutukculer N, Aksu G. In vitro T lymphocyte proliferation by carboxyfluorescein diacetate succinimidyl ester method is helpful in diagnosing and managing primary immunodeficiencies. J Clin Lab Anal. 2018;32(1):e22216. https://doi.org/10.1002/jcla.22216.
De Stefano A, Boldt A, Schmiedel L, Sack U, Kentouche K. Flow cytometry as an important tool in the diagnosis of immunodeficiencies demonstrated in a patient with ataxia-telangiectasia. Lab Med. 2016;40(4):255–61. https://doi.org/10.1515/labmed-2016-0018.
Stone KD, Feldman HA, Huisman C, Howlett C, Jabara HH, Bonilla FA. Analysis of in vitro lymphocyte proliferation as a screening tool for cellular immunodeficiency. Clin Immunol. 2009;131(1):41–9. https://doi.org/10.1016/j.clim.2008.11.003.
Bonilla FA. Interpretation of lymphocyte proliferation tests. Ann Allergy Asthma Immunol. 2008;101(1):101–4. https://doi.org/10.1016/S1081-1206(10)60842-3.
Sallakci N, Tahrali I, Kucuksezer UC, Cetin EA, Gul A, Deniz G. Effect of different cytokines in combination with IL-15 on the expression of activating receptors in NK cells of patients with Behcet’s disease. Immunol Res. 2022;70(5):654–66. https://doi.org/10.1007/s12026-022-09298-5.
Tahrali I, Akdeniz N, Yilmaz V, Kucuksezer UC, Oktelik FB, Ozdemir O et al. The modulatory action of C-Vx substance on the immune system in COVID-19. Emerg Microbes Infect. 2022:1–28. https://doi.org/10.1080/22221751.2022.2125347.
Gelmez MY, Oktelik FB, Tahrali I, Yilmaz V, Kucuksezer UC, Akdeniz N, et al. Immune modulation as a consequence of SARS-CoV-2 infection. Front Immunol. 2022;13:954391. https://doi.org/10.3389/fimmu.2022.954391.
Choi P, Reiser H. IL-4: role in disease and regulation of production. Clin Exp Immunol. 1998;113(3):317–9. https://doi.org/10.1046/j.1365-2249.1998.00690.x.
Van Belle K, Herman J, Boon L, Waer M, Sprangers B, Louat T. Comparative in vitro immune stimulation analysis of primary human B cells and B cell lines. J Immunol Res. 2016;2016:5281823. https://doi.org/10.1155/2016/5281823.
Hui CW, Wu WC, Leung SO. Interleukins 4 and 21 protect anti-IgM induced cell death in Ramos B cells: implication for autoimmune diseases. Front Immunol. 2022;13:919854. https://doi.org/10.3389/fimmu.2022.919854.
Grossmann A, Ledbetter JA, Rabinovitch PS. Reduced proliferation in T lymphocytes in aged humans is predominantly in the CD8+ subset, and is unrelated to defects in transmembrane signaling which are predominantly in the CD4+ subset. Exp Cell Res. 1989;180(2):367–82. https://doi.org/10.1016/0014-4827(89)90064-5.
Song L, Kim YH, Chopra RK, Proust JJ, Nagel JE, Nordin AA, et al. Age-related effects in T cell activation and proliferation. Exp Gerontol. 1993;28(4–5):313–21. https://doi.org/10.1016/0531-5565(93)90058-l.
Kovaiou RD, Weiskirchner I, Keller M, Pfister G, Cioca DP, Grubeck-Loebenstein B. Age-related differences in phenotype and function of CD4+ T cells are due to a phenotypic shift from naive to memory effector CD4+ T cells. Int Immunol. 2005;17(10):1359–66. https://doi.org/10.1093/intimm/dxh314.
Acknowledgements
The authors like to thank Mr. Abdullah Yilmaz for his important assistance during the laboratory works.
Funding
This present work was supported by the Research Fund of Istanbul University. Project No. 29901.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Zakya Shoub Elshari carried out the experiments, collected the data, and contributed in the initial draft of the manuscript. Serdar Nepesov, Ayca Kiykim, and Yildiz Camcioglu coordinated the establishment of donor groups and obtained the blood samples and also contributed in finalization of the manuscript. Ilhan Tahrali contributed in experiments, data analysis, and construction of graphics. Gunnur Deniz contributed in experiment design, discussion of data, and also proofreading. Umut Can Kucuksezer contributed in acquisition of adult samples, design and conduction of experiments, statistical analysis, graphics design, finalization of the manuscript, and proofreading.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the local ethical committee of Istanbul University, Istanbul Faculty of Medicine (date: 23–06-2017/ no:817). The written informed consents for participation in this study were obtained from all of the participants or from their parents.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elshari, Z.S., Nepesov, S., Tahrali, I. et al. Comparison of mitogen-induced proliferation in child and adult healthy groups by flow cytometry revealed similarities. Immunol Res 71, 51–59 (2023). https://doi.org/10.1007/s12026-022-09328-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-022-09328-2