Abstract
We have investigated the in vitro immunogenicity and in vivo prophylactic and therapeutic potential of lambda (λ) phage particles displaying the E75 peptide (derived from HER2 protein) in an implantable TUBO breast tumor model of BALB/c mice. The mice were immunized with the E75-displaying phage (λF7-gpD::E75) every 2-week intervals over a 6-week period, and the generated immune responses were studied. Results showed in vitro induction of immune responses by the λF7 (gpD::E75) construct compared to the control λF7 and buffer groups. In the in vivo prophylactic study, all the control and vaccinated mice groups developed tumors. However, in the therapeutic experiments, we observed a significant difference in tumor size at days 14–36 for mice immunized with λF7 (gpD::E75) compared to control groups (P < 0.05). Moreover, the survival time prolonged in mice immunized with λF7 (gpD::E75). The discrepancy between the results obtained from the in vitro and in vivo studies may have been a result of the induction of Foxp3 CD4+CD25+ which has been previously reported to hamper effective T cell functionality. In conclusion, we observed a significant immune stimulatory response in the in vitro study, while in vivo, the vaccine was not able to exert significant tumor inhibitory effects. We suggest that the presence of Foxp3+ CD4+CD25+ cells may have impaired the anti-tumor response in mice challenged in vivo with the TUBO xenograft tumor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tumor-associated antigens (TAAs) are presented by different human tumors. TAAs have been identified as self-antigens and are able to induce immune responses against the tumor tissue [1]. The greatest problem in developing an efficient cancer vaccine is the low immunogenicity and insufficient response of immune system against the TAA. Exploitation and use of adjuvants and new delivery systems are promising approaches for enhancement of the immunogenicity of peptide vaccines [2, 3]. Peptides derived from phages can be loaded on MHC I and MHC II. Due to this feature, phage displaying foreign T cell epitopes is able to prime strong T-helper-dependent cytotoxic T cell responses [4]. Furthermore, the excellent safety profile, ease of production and cost-effectiveness of manufacturing, and their intrinsic adjuvant properties make bacteriophage-derived nanoparticles suitable candidates for phage-based vaccine discoveries [5]. Since the discovery of the phage display technique, the system has been used widely for numerous medicinal applications. Several studies have shown that displayed peptides on the phage surface were able to elicit immune responses in different settings [6,7,8]. Initial studies in this field were conducted using lytic phages such as phages T4 and T7, and temperate phage λ, as these phages were suitable candidates for the design of fusion vectors for display of immune stimulant peptides [9]. Bacteriophage λ has a linear dsDNA genome harbored within an icosahedral capsid comprised of major capsid proteins gpE and gpD. The gpE is responsible for forming the initial phage prohead while the gpD is crucial for phage head morphology and stabilization [10, 11]. The gpD is ideal for foreign peptide presentation and this is due to its ability to fuse peptides to both N- and C-termini [12, 13] and its high decoration capacity (up to 420 molecules) per phage particle [11].
In this study, we used a TUBO tumor model of BALB/c mice to investigate the immunogenicity and anti-tumor effects of λ phage nanoparticles displaying the E75 peptide, derived from rat HER2 molecule.
The intact functional gpD protein is necessary for insertion and compaction of the full-length λ genome into the phage capsid. It also provides structural integrity for the phage. Phage λF7 encodes an amber stop codon within its gpD gene precluding its growth on a non-suppressor strain of Escherichia coli. It therefore must be propagated on amber suppressor mutant strains, and based on the suppressor strain, different amino acids may insert into the peptide chain at an encountered stop signal. SupD (serU132), SupF (tyrT5888), and SupE (glnV44) derivative strains of E. coli K-12 (W3101) code for serine, tyrosine, and glutamine in place of the amber stop signal [14]. We utilized the bacteriophage λ capsid protein gpD for efficient display of E75 peptide on the surface of the phage. The gpD::E75 was provided from a temperature-inducible expression plasmid (pGPD::E75) regulated by the temperature-sensitive repressor CI857 on the plasmid pPL451 in tandem with the amber suppressor strain (W3101 SupE).
Materials and methods
Ethics statement
All animal experiments conducted in this study were approved by the Institutional Ethical Committee and Research Advisory Committee of Mashhad University of Medical Sciences. This was based on the National Specific Ethical Guidelines for Biomedical Research issued by the Ministry of Health and Medicinal Education (MOHME) of Iran in 2005.
Mice and cell lines
Female BALB/c mice were purchased from the Pasteur Institute of Iran (Karaj, Iran). Mice used for experiments were between 4 and 6 weeks old. TUBO cell line is a HER2/neu-overexpressing cell line generated from a spontaneous mammary gland tumor from BALB-neuT transgenic mice [15]. The cell line was kindly provided by professor Pier-Luigi Lollini (University of Bologna, Italy). It was cultured in a DMEM medium supplemented with 20% FCS (fetal cow serum).
Strains and plasmids
All strains, phages, and plasmids are presented in Table 1. Plasmid pGPD::E75 was constructed based on plasmid pPL451-gpD from previous studies [14]. The construction and cloning of plasmid pGPD::E75 were based on the protocols described previously [14]. The gpD::E75 sequence designed creates a C-terminal E75 translational fusion with λ D capsid gene, separated by an in-frame short linker encoding 17 amino acids (ACTAGCGGTTCCGGTTCTGGTTCCGGTTCTGGTTCCGGTTCTGGCGGTACC).
The expression of gpD::E75 from the multi-copy plasmid (pGPD::E75) was governed by a temperature-sensitive λ CI857 repressor.
Phage amplification and purification
Cultures of E. coli strains SupE, SupE (pGPD::E75), and BB4 Sup + (SupE, SupF double suppressor) were grown in Luria Bertani (LB) broth at 37 °C overnight. Phage lysates were titered before each step of plating and after amplification and purification by standard viability assays on fresh BB4 E. coli cells. This strain has been reported to repeatedly generate the highest titers of λF7 phage particles [14]. Dilutions of primary lysates (1:10) were prepared in 1 mL of TN buffer (0.01 M Tris–HCl and 0.1 M NaCl, pH 7.8). Lysate dilutions were added to 0.5 mL of 1 × 108 cells and then added to 5 mL of LB top agar (0.7 g agar per 100 mL of LB broth). Plates were incubated overnight at 37 °C to obtain complete lysis of the bacterial lawns. The lysates were generated by adding 10 mL of TN buffer to the plate and incubating overnight at 4 °C. The top agar solution was transferred to a pre-chilled 50-mL conical tube (Frogga Bio), mixed, and centrifuged (Sigma 3-30 K, Sigma, Germany) at 10,000 RPM for 20 min at 4 °C [18, 19]. DNAse and RNAse (Cinaclon, Iran) were added to the lysate to degrade the released bacterial nucleic acids. The phage particles were precipitated from the culture supernatant by the addition of 1 M NaCl and 10% polyethylene glycol (PEG)-8000 (Scharlau, Scharlab S. L., Spain) using a standard protocol [18, 19]. The resultant pellet of phage particles was resuspended in TN buffer and 0.02 volume of chloroform was added, vortexed gently for 30 s, and centrifuged at 4300 RPM for 15 min at 4 °C to remove cellular debris and residual PEG. The aqueous phase containing phage particles was removed and filtered through a sterile 0.45-μm syringe filter (BD Biosciences, India) and kept at 4 °C for further use.
Removal of bacterial endotoxin from phage particles
The bacterial endotoxin (LPS) was removed from phage particles based on a method for removal of endotoxin from protein solutions by phase separation using Triton X-114 as described by Aida et al. [20].
Immunization schedule
Female BALB/c mice (4–6 weeks old) were administered subcutaneously with either 100 μL of 107 PFU/mL of λ F7 phage particles (phage particle control) or E75 peptide-displaying phage particles (test group) three times on days 0, 14, and 28. Similar amounts of TN buffer were also administered to another group as a control for buffer. Two weeks after the last booster, serum and spleens of mice were collected for immunological assays.
Determination of IFN-γ and IL-4 concentrations in serum
IFN-γ and IL-4 concentrations were determined by enzyme-linked immunosorbent assay (ELISA) using kits commercially available from eBioscience (affymetrix). Two weeks after the last booster, mice sera were collected and the concentrations of IFN-γ and IL-4 were determined according to standard curves generated by reference concentration of cytokine at 450 nm detected by using a Synergy H4 microplate reader (BioTek, USA). The lower thresholds of sensitivity of the IFN-γ and IL-4 assays were 15 and 4 pg/mL, respectively.
Flow cytometric assay
Evaluation of CD8+ T cells and Foxp3 CD4+ CD25+ was carried out on isolated splenocytes of immunized mice 2 weeks after the last booster according to the manufacture’s instruction (Cytofix/CytopermTMPlus Fix-ation/Permeabilization, BD Biosciences, California, USA) and analyzed by the FACS Calibur (BD Biosciences).
In vitro CTL activity assay
An in vitro cytotoxic T lymphocytes (CTL) assay was performed using ex vivo expanded splenocytes as described previously [21]. Two weeks after the final injection, splenocytes (effector cells) were harvested from immunized and control mice and transferred to U-bottom plates in triplicate wells. TUBO tumor cells (target cells) were resuspended in a DMEM medium containing FBS (20%). Calcein acetoxymethyl (calcein AM; Invitrogen/Molecular Probes) (12.5 μM) was added to the TUBO cells and incubated for 1 h at 37 °C in the dark. The non-internalized calcein-AM was removed by washing with DMEM 20% FBS medium. The CAM-loaded target cells (1.2 × 105 cells/well) were co-cultured with various numbers of effector splenocytes. Plates were incubated for 4 h at 37 °C while rotating at 1600 RPM for 5 min. The supernatant was transferred to a 96-well dark plate. Fluorescence intensity in the supernatant was measured using a Synergy H4 microplate reader (BioTek USA) with excitation at 485 nm and emission at 538 nm. The percentage of specific lysis was calculated by the following formula: ([(release by CTL − release by targets alone) / (release by 2% Triton X-100 − release by targets alone)] × 100%). Culture medium and 2% Triton X-100 were added to the wells to determine the minimum and maximum release by target cells, respectively.
Prophylactic and therapeutic models of TUBO challenge
BALB/c mice (five animals per group) were vaccinated as described in the “Immunization schedule” section. After 14 days of receiving different formulations, mice were challenged (s.c) with 5 × 105 TUBO cells. Mice were observed regularly to monitor survival and tumor growth. In the therapeutic experiment, BALB/c mice (five animals per group) were first challenged (s.c) with 5 × 105 TUBO cells. When the tumors were palpable, immunotherapy was initiated. Tumor volume (mm3) was obtained by measuring two diameters, including the largest diameter for each tumor, and calculated using the formula: length × (width)2 × 0.52.
The time to reach endpoint (TTE) obtained from the exponential regression of the tumor growth curve and the percentage of tumor growth delay (% TGD) were calculated based on the difference between the mean TTE of treatment group (T) and the mean TTE of the control group (C) (% TGD = [(T − C)/C] × 100]) [22].
Statistical analysis
All statistical analyses were performed using Graph Pad Prism 6 Software. Survival data was analyzed by the log-rank test. Tukey’s post test was applied to determine the statistical significance of differences among various groups with P values < 0.05 considered significant.
Results
Phage displaying E75 peptide stimulated secretion of IL-4 and IFN-γ cytokines
The sera of immunized mice (three animals per group) were collected 14 days after the last booster and assayed for IL-4 and IFN-γ by ELISA (Fig. 1). Mice stimulated with λF7 (gpD::E75) secreted a significantly higher level of both IL-4 and IFN-γ compared to λF7 and buffer groups (P < 0.0001).
Secretion of IL-4 and IFN-γ cytokines against phages expressing E75 peptide. BALB/c mice were immunized with λF7 (gpD::E75) every 2 weeks for three times. Blood samples were collected 14 days after the last booster and the concentration of IL-4 and IFN-γ cytokines was determined using ELISA. Mice immunized with λF7 (gpD::E75) showed significantly higher levels of IL-4 and IFN-γ cytokines compared to λF7 and buffer groups. Data represent mean ± SD (n = 3). ****(P < 0.0001)
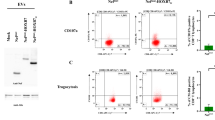
Determination of CD8+ T cells and Foxp3 CD4+CD25+ Treg cells
As shown in Fig. 2a, the percentage of CD8+ T cells was significantly enhanced in mice immunized with λF7 (gpD::E75) compared to buffer (P < 0.05) and λF7 groups (P < 0.01). In addition, Foxp3 in CD25+/CD4+ cells was significantly increased in vaccinated mice with λF7 (gpD::E75) compared to buffer and λF7 groups (P < 0.01) (Fig. 2b).
Flow cytometry assay with splenocytes of immunized mice after 2 weeks of last booster. a Percentage of CD8+ T cells significantly increased in λF7 (gpD::E75) group compared to buffer *( P < 0.05) and λF7 **(P < 0.01). b Foxp3 geometric mean in CD4+ CD25+ gated cells. The Foxp3 CD4+ CD25+ in λF7 (gpD::E75) group was significantly higher **(P < 0.01) compared to λF7 and buffer groups
Cytotoxicity assay
The CTL activity of spleen cells from vaccinated mice was assessed using rHER2/neu-expressing TUBO tumor cells in vitro (Fig. 3). TUBO cells (target cells) were exposed to different ratios of spleen cells (effector cells) (at 20/1 and 10/1 ratios). The CTL activity of immunized mice with λF7 (gpD::E75) was significantly higher than in the control groups that received λF7 and buffer [ratio 20/1 (P < 0.0001) and 10/1 (P < 0.05)].
In vitro CTL activity of isolated splenocytes induced by various formulations. Calcein AM-loaded rHER2/neu-expressing TUBO tumor cells were co-incubated with isolated splenocytes. The dye released from the lysed TUBO cells was measured spectrofluorimetrically. The mice immunized with λF7 (gpD::E75) showed significantly higher CTL activity compared to λF7 and buffer groups at E/T (effector/target cells) ratios of 20/1 ****(P < 0.0001) and 10/1 *(P < 0.05). Data represent mean ± SD (n = 3)
Effects of prophylactic vaccination against TUBO tumor in BALB/c mice
To evaluate whether the CTL responses induced by phages displaying the E75 peptide were potent enough to inhibit tumor growth, BALB/c mice (five per group) were vaccinated three times over 2-week intervals; after which, they were challenged (s.c) with 5 × 105 TUBO cells. Results showed that both the control and vaccinated mice developed tumors, indicating that the challenging experiments using phages displaying E75 did not confer a significant protective effect against the tumor challenge (Fig. 4).
Prophylactic immunization of BALB/c mice with λF7 (gpD::E75). Vaccination with different groups was performed three times with 2-week intervals. Fourteen days after the last booster, the mice were challenged s.c with 5 × 105 TUBO cells. Phages expressing E75 were not able to protect mice against the tumor challenge. Data represent mean ± SEM (n = 5)
Effects of therapeutic vaccination against TUBO in BALB/c mice
The therapeutic effects of λ phages expressing E75 peptide were evaluated in BALB/c mice. Mice were injected with the TUBO cell line at a concentration of 5 × 105, and when the tumors were palpable, the vaccine challenge was started. As shown in Fig. 5a, a significant difference in the tumor size was seen at days 14–36 between mice immunized with λF7 (gpD::E75) compared to the buffer control group (P < 0.05). This was associated with a prolonged survival time for mice immunized with λF7 (gpD::E75) compared to the buffer group (P < 0.05) (Fig. 5b and Table 2).
Therapeutic immunization with different formulations in BALB/c mice. a Tumor growth curves. Mice immunized with λF7 (gpD::E75) showed a significant difference at days 14–36 compared to the buffer group * P < 0.05. b Survival curve. The test group showed a difference with the buffer *(P < 0.05). Data represent mean ± SEM (n = 5)
Discussion
In recent years, phage nanoparticles displaying tumor antigen-derived peptides have attracted great interest as new vehicles for cancer vaccine delivery. This is due to the phage vector advantages including its intrinsic adjuvant activity, safety profile, high multivalent display potential, and its ease of construction and manufacturing [5]. The displayed peptides on the phage particles are accessible and can elicit immune responses in different animal systems. For example, in one study, a peptide corresponding to the reverse transcriptase of the human immunodeficiency virus (HIV-1) was displayed on a filamentous phage and elicited a specific CTL response in mice [23]. Bastien et al. reported a protective immune response against respiratory syncytial virus (RSV) in mice immunized with recombinant phage displaying a B cell epitope of human RSV [6]. The immunization of mice with phages displaying a CTL epitope from melanoma antigen (MAGE161–169) was shown to trigger specific CTL responses, leading to protective and therapeutic effects against melanoma in mice [24]. Vaccination of BALB/c mice with chimeric T7 phage nanoparticles displaying p66 epitope imparted prophylactic and therapeutic anti-tumor responses against rat HER2-positive TUBO tumors [25].
In the current study, we employed λF7 phage particles displaying the E75 peptide as a fusion to the gpD coat protein of the phage (gpD::E75) fusion. We aimed to investigate the immunogenicity and anti-tumor effects of the phage nanoparticles against an implantable TUBO cancer model evaluating both its prophylactic and therapeutic potential. The E75 peptide is an HLA-A2-binding epitope derived from HER2/neu (p369-377) and has been previously used as a vaccine against metastatic breast cancer provoking CTL responses in patients [26]. Here, we report the successful in vitro induction of tumor-specific CD8+ T cells and CTL activity immune responses in mice vaccinated with λF7 (gpD::E75). Despite the general assumption that induction of potent tumor-specific CD8+ T cells is sufficient to control tumor growth, we found that effective tumor growth inhibition did not occur in mice bearing TUBO tumor in prophylactic experiments, where a significant difference in tumor size was only observed in the treatment group compared to buffer in a therapeutic study. It has been previously suggested that induction of anti-tumor activity in the circulation may be insufficient and the direct presence of functional T cells in a tumor site is necessary. The evidence suggests that immune tolerance may take place within a tumor [27], which could be conferred by suppressive dendritic cells, expression of indoleamine 2, 3 dioxygenase [28], and by regulatory T cells.
Regulatory T cells have been shown to strongly impede effective T cell functionality [29]. We observed a significant increase in the level of Foxp3+ CD25+ CD4+ in mice receiving phage nanoparticles expressing E75 peptide compared to the buffer and control groups. The expression of Foxp3 in CD25+/CD4+ T cells is known as an indicating marker of regulatory T cells [29,30,31]. Speiser et al. reported the induction of melanoma-specific CD8+ following vaccination with a melanoma A peptide in the circulation. However, a clinical benefit was not achieved due to the increased regulatory T cell activity in the tumor site [32]. Antony et al. showed that regulatory T cells can impair anti-tumor T cell responses [33] via a TGF-beta-dependent mechanism in a mouse model [34] promoting cancer growth. It was reported that inhibition of Foxp3 by P60 peptide led to improvement of the efficacy of vaccination of mice with a cytotoxic T cell epitope AH1 from CT26 tumor cells [35]. Similarly, depletion of Foxp3 Treg improved the efficacy of vaccination against established ovalbumin (OVA)-expressing B16 melanoma [36]. Therefore, we suggest that in a similar manner to those studies, in our study, the induction of tumor regulatory T cells may have prevented the expected benefit of successful vaccination which may consequently impair anti-tumor response in vivo.
In summary, the results of this study demonstrated that although vaccination with phage nanoparticles expressing E75 peptide led to induction of specific CD8+ T cells in vitro, the immunization process was not sufficient to eradicate the implantable TUBO tumor in BALB/c mice. We suggest that the presence of Foxp3+ CD4+CD25+ may have impaired the anti-tumor response in mice challenged in vivo with the TUBO tumor. While practical limitations did not allow us to examine the effects of Treg cells directly in the tumor site, the evaluation of the effect of these cells in the tumor may improve our information and support the data of this study.
References
Vergati M, Intrivici C, Huen NY, Schlom J, Tsang KY. Strategies for cancer vaccine development. J Biomed Biotechnol. 2010;1–13. https://doi.org/10.1155/2010/596432.
Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462(7272):449–60.
Bramwell VW, Perrie Y. Particulate delivery systems for vaccines. Crit Rev Ther Drug Carrier Syst. 2005;22(2):151–214.
Prisco A, De Berardinis P. Filamentous bacteriophage fd as an antigen delivery system in vaccination. Int J Mol Sci. 2012;13(4):5179–94.
Plummer EM, Manchester M. Viral nanoparticles and virus-like particles: platforms for contemporary vaccine design. Wiley Interdiscip Rev: Nanomedicine Nanobiotechnology. 2011;3(2):174–96.
Bastien N, Trudel M, Simard C. Protective immune responses induced by the immunization of mice with a recombinant bacteriophage displaying an epitope of the human respiratory syncytial virus. Virology. 1997;234(1):118–22.
Magliani W, Polonelli L, Conti S, Salati A, Rocca PF, Cusumano V, et al. Neonatal mouse immunity against group B streptococcal infection by maternal vaccination with recombinant anti-idiotypes. Nat Med. 1998;4(6):705–9.
Manoutcharian K, Terrazas LI, Gevorkian G, Acero G, Petrossian P, Rodriguez M, et al. Phage-displayed T-cell epitope grafted into immunoglobulin heavy-chain complementarity-determining regions: an effective vaccine design tested in murine cysticercosis. Infect Immun. 1999;67(9):4764–70.
Garufi G, Minenkova O, Passo CL, Pernice I, Felici F. Display libraries on bacteriophage lambda capsid. Biotechnol Annu Rev. 2005;11:153–90.
Lankes H, Zanghi C, Santos K, Capella C, Duke C, Dewhurst S. In vivo gene delivery and expression by bacteriophage lambda vectors. J Appl Microbiol. 2007;102(5):1337–49.
Mikawa YG, Maruyama IN, Brenner S. Surface display of proteins on bacteriophage λ heads. J Mol Biol. 1996;262(1):21–30.
Vílchez S, Jacoby J, Ellar DJ. Display of biologically functional insecticidal toxin on the surface of λ phage. Appl Environ Microbiol. 2004;70(11):6587–94.
Dokland T, Murialdo H. Structural transitions during maturation of bacteriophage lambda capsids. J Mol Biol. 1993;233(4):682–94.
Nicastro J, Sheldon K, El-zarkout FA, Sokolenko S, Aucoin MG, Slavcev R. Construction and analysis of a genetically tuneable lytic phage display system. Appl Microbiol Biotechnol. 2013;97(17):7791–804.
Rovero S, Amici A, Di Carlo E, Bei R, Nanni P, Quaglino E, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol. 2000;165(9):5133–42.
Bachmann BJ. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972;36(4):525.
Sokolenko S, Nicastro J, Slavcev R, Aucoin MG. Graphical analysis of flow cytometer data for characterizing controlled fluorescent protein display on λ phage. Cytometry Part A. 2012;81(12):1031–9.
Yamamoto KR, Alberts BM, Benzinger R, Lawhorne L, Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970;40(3):734–44.
Sheldon K. Two dimensional genetic approach to the development of a controllable lytic phage display system. University of Waterloo, ON, Canada, Ph.D.Thesis, 2013. https://uwspace.uwaterloo.ca/bitstream/handle/10012/7371/Sheldon_Katlyn.pdf?sequence=1. Accessed 8 Oct 2017.
Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990;132(2):191–5.
Jalali SA, Sankian M, Tavakkol-Afshari J, Jaafari MR. Induction of tumor-specific immunity by multi-epitope rat HER2/neu-derived peptides encapsulated in LPD nanoparticles. Nanomedicine. 2012;8(5):692–701.
Schluep T, Hwang J, Cheng J, Heidel JD, Bartlett DW, Hollister B, et al. Preclinical efficacy of the camptothecin-polymer conjugate IT-101 in multiple cancer models. Clin Cancer Res. 2006;12(5):1606–14.
De Berardinis P, Sartorius R, Fanutti C, Perham RN, Del Pozzo G, Guardiola J. Phage display of peptide epitopes from HIV-1 elicits strong cytolytic responses. Nat Biotechnol. 2000;18(8):873–6.
Fang J, Wang G, Yang Q, Song J, Wang Y, Wang L. The potential of phage display virions expressing malignant tumor specific antigen MAGE-A1 epitope in murine model. Vaccine. 2005;23(40):4860–6.
Pouyanfard S, Bamdad T, Hashemi H, Bandehpour M, Kazemi B. Induction of protective anti-CTL epitope responses against HER-2-positive breast cancer based on multivalent T7 phage nanoparticles. PLoS One. 2012;7(11):e49539.
Mittendorf EA, Holmes JP, Ponniah S, Peoples GE. The E75 HER2/neu peptide vaccine. Cancer Immunol Immunother. 2008;57(10):1511–21.
Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–74.
Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2, 3-dioxygenase. Nat Med. 2003;9(10):1269–74.
Sakaguchi S. Naturally arising Foxp3-expressing CD25+ CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345.
Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–61.
Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–6.
Appay V, Jandus C, Voelter V, Reynard S, Coupland SE, Rimoldi D, et al. New generation vaccine induces effective melanoma-specific CD8+ T cells in the circulation but not in the tumor site. J Immunol. 2006;177(3):1670–8.
Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174(5):2591–601.
Chen M-L, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-β signals in vivo. Proc Natl Acad Sci U S A. 2005;102(2):419–24.
Casares N, Rudilla F, Arribillaga L, Llopiz D, Riezu-Boj JI, Lozano T, et al. A peptide inhibitor of FOXP3 impairs regulatory T cell activity and improves vaccine efficacy in mice. J Immunol. 2010;185(9):5150–9.
Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MW, Ngiow SF, et al. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 2010;70(20):7788–99.
Funding
This work was financially supported by Mashhad University of Medical Sciences (MUMS) to JB and NSERC to RS and JN.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics statement
All animal experiments conducted in this study were approved by the Institutional Ethical Committee and Research Advisory Committee of Mashhad University of Medical Sciences. This was based on the National Specific Ethical Guidelines for Biomedical Research issued by the Ministry of Health and Medicinal Education (MOHME) of Iran in 2005.
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Arab, A., Nicastro, J., Slavcev, R. et al. Lambda phage nanoparticles displaying HER2-derived E75 peptide induce effective E75-CD8+ T response. Immunol Res 66, 200–206 (2018). https://doi.org/10.1007/s12026-017-8969-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-017-8969-0