Abstract
Regulatory T cells (Tregs) are critical mediators of immune tolerance, yet their involvement in the autoimmune disease systemic lupus erythematosus (SLE) is incompletely understood. We analyzed CD4+ T cell subpopulations with Treg-related phenotypes and their association with disease activity in peripheral blood (PB) and tissues of patients with SLE. In detail, we quantified subpopulations regarding CD25, FOXP3, CD62L, CCR6, CD27, CD45RA, and CD45RO expression in PB from 31 patients with SLE divided into two disease activity groups and 32 healthy controls using flow cytometry. CD4+ and FOXP3+ T cells in skin and kidney biopsies of patients with SLE were quantified by immunohistochemistry. CD4+CD25+/++FOXP3+ and CD4+CD25+CD45RA−/CD45RO+ T cell frequencies were significantly higher in PB from patients with active compared to inactive SLE. The fraction of CD4+CD25++FOXP3+ Tregs and CD4+CD25+CD45RA+/CD45RO− naïve Tregs was not significantly different between these groups. CD4+CD25++ Tregs from active SLE patients comprised significantly less CD27+ cells and more CCR6+ cells compared to patients with inactive SLE. The percentage of CD4+FOXP3+ T cells among inflammatory infiltrates in skin and kidney biopsies of SLE patients was not different from other inflammatory skin/kidney diseases. In conclusion, although CD4+FOXP3+ T cell frequencies in the inflamed tissues of SLE patients were comparable to other inflammatory diseases, distinct T cell subpopulations appeared misbalanced in PB of patients with active SLE. Here, cells phenotypically resembling activated T cells, but not Tregs, were increased compared to patients with inactive SLE. Within Tregs of patients with active SLE, markers related to Treg function and homing were altered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Immunologic self-tolerance, i.e., unresponsiveness to self-antigens, is an integral feature of the immune system and is maintained by various mechanisms of central and peripheral tolerance. One aspect of dominant peripheral tolerance is mediated through regulatory T cells (Tregs), which suppress other immune cells and play a critical role in inhibiting immune responses to self-antigens and, thus, autoimmune diseases (reviewed in [1]). Naturally occurring Tregs develop in the thymus as a subset of CD4+ T cells and were initially defined by expression of the IL-2 receptor α chain (CD25) [2]. In naïve mice, CD4+CD25+ Tregs constitute about 5 to 10 % of the entire peripheral CD4+ T cell population [1], while in humans, Tregs with potent suppressive activity are confined to the 1 to 2 % of CD4+ T cells that display high expression levels of CD25 (CD25++) (reviewed in [3]). Subsequently, expression of forkhead box P3 (FOXP3), a transcription factor also called Scurfin, was described as Treg lineage-defining factor (reviewed in [4]) and to date remains the best protein marker to determine Tregs. However, intermediate levels of FOXP3 are also transiently expressed by human-activated conventional T cells [4], demanding for additional markers and defined expression levels to delineate Tregs. Although expression of FOXP3 simultaneously with a high-level expression of CD25 identifies a largely pure Treg population, additional markers have been suggested to assure even better exclusion of activated conventional T cells, such as absence of CD49d [5] or CD127 [6] on Tregs; however, the latter does not enable detection of all Tregs [7, 8] and also includes FOXP3+ non-Tregs [9]. The best strategy so far to gate unequivocally on Tregs, outcompeting the use of CD127, was defined by Miyara et al. [9] who described that based on CD45RA in combination with expression levels of either CD25 or FOXP3, human bona fide Tregs (“fraction I” (“FrI”) naïve Tregs; “FrII” activated Tregs) can be distinguished from activated conventional T cells expressing low levels of FOXP3 (“FrIII” non-Tregs). Furthermore, it emerges that the Treg population is comprised of several subsets with increasing complexity: Treg subsets can be defined by functional activities such as expression of CD39 or CD27 [10–12]. Tissue-specific Treg subsets have been defined as well, interestingly, with Treg phenotypes mirroring effector T cell subsets based on lineage-defining transcription factors or chemokine receptors [13, 14]. Therefore, analysis of additional markers apart from CD25 and FOXP3, as well as analysis of Tregs in situ in tissues, may shed new light on the Treg compartment in disease stages.
Tregs are indispensable in the maintenance of immune tolerance, as exemplified by the fact that FOXP3 mutations in mice (scurfy FOXP3-mutant mouse strain or FOXP3 knockout mice) and men (IPEX syndrome) result in severe autoimmune diseases [1]. Due to the prominent role of Tregs in preventing autoimmune diseases, many studies investigated whether a dysregulation of the Treg population in peripheral blood (PB) can be related to the pathogenesis of human autoimmune diseases, with partially conflicting results (reviewed in [15]). Controversies may in part be caused by the lack of a unique marker for Tregs, appliance of different marker combinations and gating strategies, as well as disease stage and activity.
Systemic lupus erythematosus (SLE) is a complex autoimmune disease characterized by pathogenic autoantibodies and immune complexes, attributed to loss of immune tolerance primarily manifesting in deregulated B and T cell responses. Conflicting results have been published regarding Tregs in patients with SLE (reviewed in [16–18]). Early studies are complicated by the fact that CD25 expression alone is not sufficient to delineate Tregs particularly in patients with immune activation. Even considering only more recent studies that applied FOXP3 as a marker for Tregs, the results are conflicting: most studies reported reduced frequencies of Tregs in SLE patients [19–24], while others did not find alterations [25–27] or even increased numbers of Tregs [28, 29]. Resistance of target T cells to suppression by Tregs from SLE patients has also been suggested [26, 27]. Disease activity correlated with Treg numbers in several reports, yet many studies did not investigate such relationship. Only few studies have included CD45RA or CD45RO to determine above mentioned Treg fractions in SLE patients with partially contradictory results [9, 30, 31], and only one of these studies has distinguished patients based on disease activity [31]. Thus, there is a demand for studying these Treg fractions in additional SLE patient cohorts.
Due to the conflicting results, it remains unclear whether pathophysiological mechanisms of SLE might be connected to a defect in the Treg population. Notably, almost all studies have analyzed Tregs in PB of SLE patients, while studies of Tregs in the affected tissue of SLE patients are rare [21]. Investigating Treg subsets based on several markers and in different disease activity states as well as within inflamed tissues could lead to new insights into the pathogenesis of SLE. Indeed, a reduced number of CD4+FOXP3+ T cells in inflamed skin biopsy specimens from patients with cutaneous lupus erythematosus (CLE), a heterogeneous autoimmune disease characterized primarily by skin inflammation, was previously reported by our group [32].
In the present study, we investigated the frequency of CD4+FOXP3+ T cells in skin and kidney biopsies from patients with SLE compared to various other inflammatory diseases. Moreover, we analyzed the frequencies of CD4+CD25+/++FOXP3+ and CD4+CD25++FOXP3+ T cells in the PB of patients with SLE in relation to the SLE Disease Activity Index (SLEDAI). To gain a more detailed understanding of Treg subpopulations in SLE, we extended our analyses and included the measurement of FrI, II, and III T cells and we further studied markers related to Treg activity and tissue migration, namely CD62L, CCR6, and CD27. We show that the frequency of CD4+CD25+/++FOXP3+, CD4+CD25+CD45RA−/CD45RO+ T cells, and CCR6+ among CD4+CD25++ T cells was significantly higher in PB from patients with active compared to inactive SLE, while the fraction of CD4+CD25++FOXP3+ Tregs and CD4+CD25+CD45RA+/CD45RO− naïve Tregs was not significantly different. CD4+CD25++ Tregs from active SLE patients comprised significantly less CD27+ cells compared to inactive SLE. The percentage of FOXP3+CD4+ T cells among infiltrates in skin and kidney biopsies of SLE patients was not different from other inflammatory skin/kidney diseases.
Patients and methods
Patients
PB samples were obtained from 31 adult patients (five males and 26 females, mean ± SD age 41.9 ± 12.9 years) with the diagnosis of SLE according to the American College of Rheumatology criteria [33] (see Table 1). All SLE patients were diagnosed at the Department of Rheumatology, University of Heidelberg, Germany, and at the Municipal Hospital Karlsruhe, Germany. SLE patients were divided into two groups according to their disease activity using the SLEDAI, which is a validated index of SLE activity [34]. Thereby, inactive SLE was defined for patients with an SLEDAI ≤4 and active SLE for patients with an SLEDAI >4 respectively. Blood from 32 normal healthy donors (23 males and nine females, mean ± SD age 38.4 ± 11.0) was included as control.
Skin biopsies from five patients with SLE (five females, mean ± SD age 37.4 ± 9.6) were stained by immunohistochemistry. As control, results were compared with data from a previously published study [32] performed under the same conditions, including lesional skin biopsy specimens from patients with cutaneous lupus erythematosus, psoriasis, lichen planus, and atopic dermatitis.
Kidney biopsies from 11 patients with SLE (two males and nine females, mean ± SD age 38.6 ± 13.2 years) were stained by immunohistochemistry: one classified as II, two classified as III, and eight classified as IV according to the World Health Organization (WHO) classification for lupus nephritis [35]. As control, results were compared with kidney biopsy specimens from patients with chronic interstitial rejection (four males and one female, mean ± SD age 55.2 ± 20.2 years), nodular diabetic glomerulosclerosis (four males and one female, mean ± SD age 61.8 ± 7.3 years), polyomavirus infection of allogeneic renal transplants (four males and one female, mean ± SD age 52.6 ± 12.5 years), signs of focal florid interstitial rejection (two males and three females, mean ± SD age 58.8 ± 6.7 years), and interstitial nephritis (two males and three females, mean ± SD age 47.8 ± 8.3 years).
The study was approved by the local ethics committee of the University of Heidelberg, Heidelberg, Germany, and was conducted according to the Declaration of Helsinki.
Antibodies and reagents
For flow cytometry, antibodies against human CD4, CD25, CD45RO, CD45RA, CD62L, and CCR6 were obtained from BD PharMingen (Heidelberg, Germany); anti-CD27, anti-FOXP3 antibody (clone PCH101), and IgG2a isotype control antibody were obtained from eBioscience (San Diego, USA); anti-FOXP3 antibody (clone 259D) used for control was obtained from BioLegend (San Diego, USA). For immunohistochemical analysis, anti-CD4 antibody (clone 1F6; Novocastra, Newcastle, UK), anti-FOXP3 antibody (clone 236A/E7; a generous gift from Alison H. Banham, Nuffield Department of Clinical Laboratory Science, University of Oxford, Oxford, UK), and mouse IgG1 isotype control antibody (Dako, Glostrup, Denmark) were used.
Histologic and immunohistochemical analyses
At the time of initial presentation, biopsy specimens were obtained from patients with SLE. The biopsy specimens were fixed in formalin, and hematoxylin and eosin (H&E), CD4, FOXP3, and IgG1 isotype-matched control stainings were performed as described previously [36]. All specimens were analyzed by two independent investigators in a blinded manner. For each sample, the percentage of CD4+ and FOXP3+ cells in the infiltrate was calculated from three individual values obtained from three different areas using high power field (objective ×40).
Cell isolation and flow cytometry
PB was obtained from patients with SLE as well as from normal healthy donors and separated by Ficoll gradient centrifugation to obtain PB mononuclear cells (PBMCs). For multicolor flow cytometry, PBMCs were first stained with antibodies against surface molecules, followed by FOXP3 intracellular staining, if applicable. Intracellular FOXP3 staining was performed with the FOXP3 staining buffer set (ebioscience) according to the manufacturer’s recommendations modified as described previously [8, 32]. Acquisition was performed immediately on a FACSCanto II cytometer, and results were analyzed with FACSDiva software (BD Biosciences, San Jose, CA).
Statistical analysis
Data were analyzed in GraphPad Prism 6. Data are presented as individual values (each dot represents one subject) in addition to the median (median values are given and indicated by a line). Mann-Whitney U test was used to compare the percentage of the given cell population in PB of patients and controls as well as in biopsy specimens of kidney and skin. Univariate linear regression analysis was performed to determine the relationship between cell fractions and SLEDAI or age, respectively.
Principal component analysis (PCA, via singular value decomposition on the scaled and centered data matrix) was carried out with the programming language R. Multivariate linear regression (with following predictors: scaled age values, scaled SLEDAI values, and group variable defining normal healthy donor (NHD) or SLE patient) was performed in R.
P values <0.05 were considered significant and the following labels were used: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Results
Quantification of FOXP3+CD25+/++CD4+ T cells and FOXP3+CD25++CD4+ Tregs in PB of SLE patients
We determined CD4+FOXP3+ T cells in PB of patients with active SLE (SLEDAI higher than 4) or inactive SLE (SLEDAI less than or equal to 4) and NHD by flow cytometry. We analyzed coexpression of FOXP3 on CD4+ T cells with either high levels of CD25 (CD25++ “Treg”) or, alternatively, we considered all CD4+FOXP3+ cells expressing intermediate to high levels of CD25 (CD25+/++) (gating strategy, see Fig. 1a).
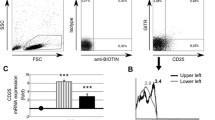
Analysis of FOXP3+CD25++ Tregs in human PB. Human PBMCs were purified from PB and stained for surface expression of CD4 and CD25. Cells were then fixed and stained intracellularly for FOXP3. NHD normal healthy donor; ≤4 inactive SLE patient with SLEDAI ≤4; >4 active SLE patient with SLEDAI >4. a Gating strategy: PBMCs (shown from a NHD) were gated for the lymphocyte population (left panel, gate P1), and from these, CD4+ T cells were gated (middle panel, gate P2). Within CD4+ T cells, FOXP3 and CD25 expression was determined (right); the whole of CD25-expressing FOXP3+ cells was defined as “CD25+/++” (gate P3). b Frequencies of CD25+/++FOXP3+ T cells (gate P3; left) or CD25++FOXP3+ Tregs (gate P4; right) were determined as in a. Median values are given and indicated as a line; each dot represents one subject. c Linear regression analysis of CD25+/++FOXP3+ or CD25++FOXP3+ cells within CD4+ cells in relation to the SLEDAI. d FOXP3 staining with the antibody clone PCH101 (used in other figures) was compared with clone 259D in the same blood samples of seven NHD and eight patients with SLE (each line connects samples from one individual); statistical analysis revealed no significant differences between both antibodies
The median percentage of CD25+/++FOXP3+ T cells of total CD4+ T cells was 11.7 % in patients with active SLE, which was significantly higher than in patients with inactive SLE (5.6 %) or in NHD (6.2 %) (Fig. 1b, left panel). The median percentage of CD25++FOXP3+ Tregs of total CD4+ T cells was 2.0 % in patients with active SLE, 1.7 % in patients with inactive SLE, and 2.3 % in NHD (Fig. 1b, right panel) with no significant differences between the three groups. When all patients were considered as one group, the median percentage of CD25++FOXP3+ Tregs was 2.0 % in SLE patients which was not significantly different from NHD (p = 0.34).
Next, we asked whether disease activity in SLE was related to the frequency of CD4+CD25++FOXP3+ Tregs or CD4+CD25+/++FOXP3+ T cells. Linear regression analysis was performed to determine the relationship between the frequency of these cells and SLEDAI. Despite a trend that higher SLEDAI coincided with higher CD4+CD25+/++FOXP3+ T cell levels, there was no significant linear relationship between the frequencies of either CD4+CD25+/++FOXP3+ T cells or CD4+CD25++FOXP3+ Tregs and SLEDAI (Fig. 1c).
The specificity of the antibody clone PCH101 to detect FOXP3+ cells has been discussed, particularly in relation to activated conventional T cells [37, 38]. To exclude any misinterpretation by using the PCH101 clone for FOXP3 detection, we compared two different anti-FOXP3 antibodies (clone PCH101 and clone 259D) for staining of NHD as well as SLE patient cells. Both clones yielded comparable results without significant differences regarding the frequency of CD4+CD25+/++FOXP3+ T cells or CD4+CD25++FOXP3+ Tregs both in NHD and in SLE patients (Fig. 1d).
Together, these results show that although there was no difference in the frequency of CD25++FOXP3+ Tregs in SLE patients, the frequency of CD25+/++FOXP3+ T cells was increased in patients with active SLE compared to patients with inactive SLE or healthy donors.
Quantification of CD45RA and CD45RO naïve/memory Treg subsets in PB of SLE patients and NHD
Because gating based on CD25 (or FOXP3) in combination with the naïve/memory markers CD45RA or CD45RO can distinguish resting/naïve Tregs (“FrI”), activated Tregs (“FrII”), and activated non-Treg cells (“FrIII”) [9], we evaluated these subsets in SLE patients. We applied two different gating strategies, using CD25 and either CD45RA or CD45RO (gating strategy, see Fig. 2a). We asked whether the groups could be distinguished by the fraction values: when performing principal component analysis considering all six fractions, we observed a partial separation of the groups (Fig. 2a). Thus, we next asked whether specific fractions are different in the groups and analyzed all fractions separately. Frequencies of FrI Tregs were significantly higher in patients with active SLE compared to NHD, whether gated via CD45RA or CD45RO (Fig. 2b). Further, both gating strategies revealed higher frequencies of FrIII non-Tregs in patients with active compared to inactive SLE. The frequencies of FrII Tregs were generally low and revealed significant differences only with one of the two gating strategies (Fig. 2b).
Analysis of “fraction I” naïve Tregs, “fraction II” activated Tregs, and “fraction III” activated non-Tregs in patients with SLE. a Gating strategy (pre-gated on lymphocytes and CD4+ cells, as in Fig. 1) to determine fraction (“Fr”) I, II, and III T cells based on CD45RA and CD25 staining (left panel) or based on CD45RO and CD25 staining (middle panel). The right panel shows principal component analysis (PCA) considering all six fractions, with different symbols representing NHD as well as SLE patients with SLEDAI ≤4 or >4 as indicated. b T cell fractions determined as in a for the different groups as indicated. In the left panel, FrI, FrII, and FrIII cells were determined based on CD45RA and in the right panel based on CD45RO. Each dot represents one individual and median values are given. c Linear regression analysis was performed to determine the relationship between FrIII (CD45RA−CD25+) T cells and the SLEDAI (left panel) or age (right panel). d Frequencies of CD45RO+ cells were determined within CD4+CD25+/++FOXP3+ T cells (left panel) or within CD4+CD25++FOXP3+ Tregs (right panel). Median values are indicated and each dot represents one individual (NHD or SLE patients as defined in a)
We next analyzed the relationship between Treg fractions and SLEDAI by linear regression. There was a positive linear relationship between FrIII T cells and SLEDAI when gated as CD45RA−CD25+ (Fig. 2c, left panel), in line with the results described above (Fig. 2b) in which active and inactive patients were categorized by SLEDAI. A similar trend, although not significant, was seen when FrIII T cells were defined as CD45RO+CD25+ (p = 0.0901; r 2 = 0.1437; data not shown). There was no significant linear relationship between either FrI or FrII Tregs and SLEDAI (data not shown). Because frequencies of CD45RA and CD45RO T cells might change with age, we tested whether age could be a confounding factor in our analysis, possibly explaining any differences of the respective T cell fractions in the different subject groups. Univariate linear regression revealed that none of the fractions was significantly dependent on age (data not shown). Of these, importantly, also the CD45RA−CD25+ (FrIII) T cell fraction was not significantly related to age in any of the groups or when considering all groups or all SLE patients together (Fig. 2c, right panel). Thus, age is unlikely to be the reason for differences in these T cell fractions between the different groups. To confirm the results from the univariate linear regression, we also performed multivariate linear regression in which we considered age, SLEDAI, and group as predictors. Here, SLEDAI had the strongest (positive) effect on FrIII values, while the effect of age was the weakest (data not shown), confirming that age is unlikely to explain the observed differences in FrIII cells.
We extended our analyses to include FOXP3 as a marker to further study whether Tregs in SLE patients are differentially activated compared to NHD. When studying the fraction of memory T cells (CD45RO+) only within FOXP3+CD25++ Tregs or within all FOXP3+ cells (CD25+/++), there were no significant differences in these subsets between NHD and SLE patients (Fig. 2d).
Effector and migratory phenotype of Tregs in PB of SLE patients compared to healthy controls
In addition to determining the frequency of CD4+CD25++ Tregs, we evaluated whether the phenotype of Tregs regarding effector molecules and homing markers was altered in SLE patients.
CD27 is a receptor for the costimulatory molecule CD70 and belongs to the TNF receptor family. The CD27/CD70 pathway provides stimulatory signals for T and B cell activation and was suggested to play a role in the pathogenesis of autoimmune disease. High CD27 expression was also suggested to be a hallmark of Tregs at sites of inflammation, because CD27 expression was high in Tregs (but not activated effector T cells) in inflamed synovial tissue of juvenile idiopathic arthritis patients [11] as well as in the skin during human cutaneous antigen-specific memory responses [39]. Notably, CD27 was described to classify a highly suppressive Treg subset [12]. We found that the median percentage of CD27+ cells among CD4+CD25++ Tregs was significantly lower in patients with active SLE (93.9 %) compared to patients with inactive SLE (98.8 %) (Fig. 3a). In normal healthy donors, the median percentage was 97.8 %. This reduction seemed to be specific for the CD4+CD25++ Treg population, since the median percentage of CD27+ cells among CD4+ T cells was not significantly different between the groups (Fig. 3b).
Analysis of Treg activation and homing markers in SLE patients compared to healthy controls. PBMCs were stained for surface expression of CD4 and CD25 (as in Fig. 1) in addition to the indicated proteins. Frequencies of CD27, CCR6, or CD62L expressing cells were determined within CD4+CD25++ Tregs or within total CD4+ cells as indicated. Median values are given and depicted by the line; each dot represents one individual (NHD or SLE patients as defined in Fig. 1)
We next determined the activation status of Tregs based on memory and homing markers. CCR6 is a chemokine receptor mainly expressed by memory T cells, involved in B cell maturation and recruitment of T cells to inflammatory sites. CCR6 was found to be highly expressed in human skin-resident T cells [40], and a tissue-accumulating Treg subset termed “effector memory” Treg was characterized by high expression of CCR6 and CD45RO [41]. Further, CCR6 together with other markers (such as IL17 and RORγt or CLA) can be used to identify a subset of Tregs that phenotypically mirrors Th17 or Th22 cells, respectively [14]. We determined that the frequency of CCR6+ T cells among CD4+CD25++ Tregs was significantly lower in patients with inactive SLE when compared to patients with active disease or NHD (Fig. 3c). CD62L, known as L-selectin, is expressed by naïve and central memory cells to ensure homing to the secondary lymphoid organs, while it is downregulated on effector T cells and effector memory cells [42]. The majority of Tregs was demonstrated to express CD62L [43], which is needed for their migration under homeostatic conditions [42]. Skin-homing Tregs express high levels of CD62L [44], and presence of Tregs in the skin has functional relevance for resolving inflammation in several settings [42, 45]. When we determined the percentages of CD62L+ T cells within CD4+CD25++ Tregs, we did not observe differences between the three groups (NHD, SLE patients with active or inactive disease) (Fig. 3d).
Frequency of CD4+FOXP3+ T cells in kidney biopsies of patients with SLE compared to other diseases
In order to investigate a potential deregulation of Tregs particularly within inflamed tissue, we analyzed the frequency of CD4+ and FOXP3+ cells in a series of kidney biopsy specimens obtained from patients with SLE using immunohistochemistry. As control, we included kidney biopsy specimens from patients with chronic interstitial rejection, nodular diabetic glomerulosclerosis, polyomavirus infection of allogeneic renal transplants, signs of focal florid interstitial rejection, and interstitial nephritis. Representative stained serial sections from patients with SLE as well as from control diseases are shown in Fig. 4a. The median frequency of CD4+ cells, expressed as the percentage of the total renal infiltrate, was determined to be 45.2 % (range 15.0 to 61.0 %) in SLE patients and was not significantly different compared to any of the other diseases (Fig. 4b). As expected, the frequency of CD4+ T cells in interstitial nephritis biopsies was significantly higher than in specimens with signs of focal florid interstitial rejection and nodular diabetic glomerulosclerosis (Fig. 4b).
Frequency of T cells in the inflammatory infiltrate of kidney specimens from patients with SLE or control diseases. a Kidney biopsy specimens from patients with lupus nephritis (SLE), interstitial nephritis (IN), chronic interstitial rejection (CIR), polyomavirus infection of allogeneic renal transplants (PI), signs of focal florid interstitial rejection (FIR), and nodular diabetic glomerulosclerosis (NG) were stained with antibodies against CD4 (left panels, original magnification ×40) and FOXP3 (right panels, original magnification ×40). Photos represent examples of the counted sections. Frequency of CD4+ cells (b), FOXP3+ cells (c) as well as FOXP3+ of CD4+ cells (d) in the inflammatory infiltrate of kidney biopsies was quantified. Frequencies of counted sections in b–d are shown for individual patients with median values plotted as line
Similarly, the frequency of FOXP3+ cells was not significantly different between SLE patients and any of the control diseases, whether analyzed within the total renal infiltrate (Fig. 4c) or as FOXP3+ frequency of CD4+ cells (Fig. 4d). In patients with SLE, the median percentage of FOXP3+ cells was 5.8 % (range 1.2 to 19.6 %) of the total renal infiltrate and 16.7 % (range 2.0 to 44.8 %) of CD4+ cells, respectively.
Frequency of CD4+FOXP3+ T cells in the skin of patients with SLE compared to other inflammatory skin diseases
In the present study, we sought to determine the frequency of CD4+ and FOXP3+ cells in a series of lesional skin biopsy specimens from patients with SLE. Our group demonstrated previously that there is an organ-specific decrease of FOXP3+ T cells in the skin of CLE patients [32]. Examples of SLE patient sections stained with anti-FOXP3 or anti-CD4 antibodies are shown in Fig. 5a. As control, we included skin biopsy specimens from patients with CLE, psoriasis, lichen planus, and atopic dermatitis (data published in [32]).
Frequency of T cells in the inflammatory infiltrate of skin biopsy specimens from patients with SLE or control diseases. Skin biopsy specimens from patients with SLE or inflammatory control diseases (CLE = cutaneous lupus erythematosus, PSO = psoriasis, LP = lichen planus, and AD = atopic dermatitis) were stained with antibodies against CD4 and FOXP3. a Pictures of representative stainings of CD4+ (upper panel) and FOXP3+ cells (lower panel) in the dermal infiltrate of skin biopsy specimens from patients with SLE are shown (original magnification ×20 and ×40). b The frequency of CD4+ cells and c FOXP3+ cells was determined as percentage of the total lymphocyte infiltrate. d The frequency of FOXP3+ cells as fraction of CD4+ cells was determined. Parts of the data were previously published in [32]
The frequency of CD4+ cells, expressed as the median percentage of the total dermal infiltrate, was 52.9 % (range 46.3 to 68.5 %) in SLE patient biopsies with no statistically significant differences compared to the control diseases (Fig. 5b). The percentage of FOXP3+ cells in the inflammatory infiltrate of skin biopsy specimens from patients with SLE (median 15.0 %, range 11.9 to 22.4 %) was not changed compared to other diseases except significantly increased compared to the median percentage observed in biopsies from CLE patients (median 5.3 %, range 0.8 to 8.7 %), who had lower percentages compared to all other tested diseases as well (Fig. 5c). Additionally, we calculated the frequency of FOXP3+ cells in relation to the number of CD4+ cells. Again, the median percentage in patients with SLE (median 32.4 %, range 23.1 to 33.0 %) was significantly higher than in patients with CLE (median 9.6 %, range 1.3 to 18 %), but there were no statistically significant differences between frequencies in patients with SLE compared to those in the other control diseases (Fig. 5d).
Discussion
Several studies have focused on the question whether an alteration in the number and/or function of Tregs is associated with the pathogenic mechanisms of human autoimmune diseases, with conflicting results [15]. Analyzing Tregs by more recently described less ambiguous gating strategies in flow cytometry analysis, as well as studying the presence of Tregs directly in the inflamed tissue, may help to resolve these controversies and was the subject of this study.
In our flow cytometry approach to determine Tregs in peripheral blood, we defined only cells expressing high levels of CD25, which simultaneously express FOXP3 (CD25++FOXP3+), as pure Tregs. Alternatively, we gated on all CD25-expressing (CD25+/++) cells which includes activated conventional T cells. Furthermore, we used gating strategies based on CD25 in combination with CD45RA or CD45RO to distinguish naïve Tregs, activated Tregs, and FOXP3+ non-Tregs. Using all three strategies to define Tregs, we did neither find a decrease of Tregs in patients with SLE compared to NHD nor in SLE patients with active versus inactive disease. Instead, cells resembling activated non-Tregs (CD25+/++FOXP3+ and FrIII cells) were strongly increased in active SLE patients, underlining the importance of appropriate marker combinations and gatings to exclude those activated T cells from Treg analyses.
It is suggested that only highly CD25-expressing cells, ideally in combination with FOXP3, should be designated Tregs; nevertheless, studies are conflicting regarding the fraction of Tregs in SLE patients based on this definition, ranging from decreased, unchanged, to increased levels [16–18]. These controversies may be related to slightly different gating strategies or differences in the patient cohorts and treatments, although results are also divergent regarding the correlation of Treg numbers with SLE disease activity. While not apparent in our study, when Tselios et al. prospectively analyzed a large patient cohort, an inverse relationship of CD4+CD25++FOXP3+ Tregs with disease activity was evident [46]. Our data indicate that controversies in the literature regarding Treg numbers in SLE do not seem to be due to the usage of the disputed anti-FOXP3 antibody clone PCH101 [37, 38]. Because FOXP3 as well as CD25 are also expressed by effector T cells upon activation, setting the gates in a reproducible manner between laboratories is difficult, and a more confident strategy seems to be the inclusion of CD45RA or CD45RO suggested by Miyara et al. [9], which enables definition of Treg subsets as well as the exclusion of activated effector T cells. However, analyses of T cells in SLE patients based on this strategy are very limited to date. Based on CD45RA and FOXP3, Miyara et al. described an increase in FrI (naïve) Tregs and FrIII non-Tregs in active SLE patients compared to healthy donors along with a decrease in FrII (active) Tregs [9]. Pan et al. measured CD45RA and FOXP3 and also observed that patients with active SLE displayed enhanced FrI Treg and FrIII non-Treg frequencies compared to healthy donors as well as to patients with inactive disease, while FrII (active) Tregs were not different between the three groups [31]. Furthermore, only FrI Tregs correlated significantly with the SLE disease activity index. Kim et al. [30] did not find differences in Treg fractions when comparing SLE patients with healthy controls; however, they included patients with variable disease states. Taken together, three independent studies ([9, 31] and our study) determined increased frequencies of FrI (naïve) Tregs in active SLE patients compared to NHD, and both Pan et al. and our study revealed a higher percentage of FrIII non-Tregs in active compared to inactive SLE patients (the latter comparison was not made by Miyara et al. but the respective fraction was increased when comparing SLE patients with NHD). This increase in FrIII (FOXP3low) non-Tregs in SLE patients might contribute to the controversy in the field, underlining the importance of including CD45RA/RO as markers such as in the present study. Taken together, these studies seem to outline a promising strategy to define Tregs in a way that is more reproducible between different laboratories, and at least regarding naïve Tregs, these studies do not support the view of a Treg decrease in SLE that emerged from previous studies (summarized in [17, 18]). Considering FrII (active) Tregs, however, there are differences between these recent studies (the present study and [9, 31]), which might be due to different cohorts or technical issues considering the very low frequencies of these cells in general. Notably, within our study, we defined the fractions based on CD45RA and CD45RO in parallel (within the same PB samples for most samples) and observed divergent results regarding FrII Tregs, while the results for FrI and FrIII were independent of the gating strategy. Thus, we propose that using both CD45RA and CD45RO-based gating strategies in parallel may enhance the confidence of results and contribute to less controversy in the literature.
In patients with active compared to inactive SLE, we could furthermore observe an increased fraction of CCR6+ cells and decreased fraction of CD27+ cells among CD4+CD25++ Tregs, respectively, which may reflect alterations in Treg homing or functional activity. Future studies are needed to address the functional relevance of these findings, as well as the coexpression of these markers in different Treg subsets. The increased frequency of CCR6+CD4+CD25++ T cells may mediate an increased recruitment of these cells to the inflamed tissues [41, 47] and we therefore studied the frequency of Tregs in tissues as well.
Although extensive studies were carried out on Tregs in PB, we are aware of only limited analyses of Tregs in the affected tissue of SLE patients [21]. A reduced number of CD4+FOXP3+ T cells in inflamed skin biopsy specimens (but not in PB) from patients with CLE was previously described [32], and now we extended these studies to kidney and skin biopsies of SLE patients, compared to a range of control diseases. We observed that the percentage of CD4+FOXP3+ T cells among infiltrates in skin and kidney biopsies of SLE patients was not different from the one in other inflammatory skin/kidney diseases. Notably, the frequency of CD4+FOXP3+ T cells in the skin was decreased in CLE patients also compared to SLE patients, highlighting the importance for studies in the different disease subtypes. To our knowledge, our study represents the most differentiated study so far to analyze Tregs in the tissue of SLE patients, and our results do not reveal altered Treg numbers in the affected tissue of SLE patients. However, inherent limitations of these studies are the lack of “negative controls” due to unavailability of inflamed tissues in healthy donors, as well as restricted possibilities (compared to flow cytometry) in the study of Treg subsets based on marker combinations or expression levels.
It should be considered that nearly all patients in the current study were receiving systemic medication, such as corticosteroids, antimalarials, and/or methotrexate, which may influence the number of T cells. Most studies that analyzed the effects of treatment have suggested an increase in Treg numbers particularly after treatment with steroids but also other treatments such as rituximab [19, 24, 48, 49]. Another study revealed, however, that the increase in Treg numbers during SLE disease remission was independent of the type of treatment regimen and likely an epiphenomenon of disease remission [50]. Nevertheless, our results regarding FrI Treg and FrIII T cell frequencies are in line with two other reports which studied untreated patients [9, 31].
In conclusion, our study reveals an increase of T cells reflecting activated effector T cell phenotypes (CD4+CD25+/++FOXP3+, CD4+CD25+CD45RA−/CD45RO+) in PB of active SLE patients, while total Treg numbers (CD4+CD25++FOXP3+, CD4+CD25+CD45RA+/CD45RO−) were unchanged. We also revealed that Treg numbers in different inflamed tissues of SLE patients were unchanged compared to several control diseases. At the same time, specific Treg subsets based on a range of different markers appeared misbalanced in SLE patients: within CD4+CD25++ Tregs of patients with active SLE, markers related to Treg function (CD27) and homing (CCR6) were altered, which may contribute to disease pathogenesis. These findings highlight the importance of studying Tregs on the subpopulation level, and additional studies in larger and independent patient cohorts will be important to decipher the role of Tregs in this disease and to ultimately improve targeted SLE therapy in the future.
References
Sakaguchi S. Regulatory T cells: history and perspective. Methods Mol Biol. 2011;707:3–17.
Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64.
Shevach EM. Certified professionals: CD4(+)CD25(+) suppressor T cells. J Exp Med. 2001;193:F41–6.
Ramsdell F, Ziegler SF. FOXP3 and scurfy: how it all began. Nat. Rev. Immunol. 2014;14:343–9.
Kleinewietfeld M, Starke M, Di Mitri D, Borsellino G, Battistini L, Rotzschke O, et al. CD49d provides access to “untouched” human Foxp3+ Treg free of contaminating effector cells. Blood. 2009;113:827–36.
Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–700.
Simonetta F, Chiali A, Cordier C, Urrutia A, Girault I, Bloquet S, et al. Increased CD127 expression on activated FOXP3+CD4+ regulatory T cells. Eur J Immunol. 2010;40:2528–38.
Klein S, Kretz CC, Krammer PH, Kuhn A. CD127(low/−) and FoxP3(+) expression levels characterize different regulatory T-cell populations in human peripheral blood. J Invest Dermatol. 2010;130:492–9.
Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911.
Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65.
Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, et al. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med. 2005;201:1793–803.
Koenen HJ, Fasse E, Joosten I. CD27/CFSE-based ex vivo selection of highly suppressive alloantigen-specific human regulatory T cells. J Immunol. 2005;174:7573–83.
Pesenacker AM, Broady R, Levings MK. Control of tissue-localized immune responses by human regulatory T cells. Eur J Immunol. 2015;45:333–43.
Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood. 2012;119:4430–40.
Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–59.
Kuhn A, Beissert S, Krammer PH. CD4+CD25+ regulatory T cells in human lupus erythematosus. Arch Dermatol Res. 2009;301:71–81.
Miyara M, Gorochov G, Ehrenstein M, Musset L, Sakaguchi S, Amoura Z. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun Rev. 2011;10:744–55.
Ohl K, Tenbrock K. Regulatory T cells in systemic lupus erythematosus. Eur J Immunol. 2014;10:1–12.
Lyssuk EY, Torgashina AV, Soloviev SK, Nassonov EL, Bykovskaia SN. Reduced number and function of CD4+CD25highFoxP3+ regulatory T cells in patients with systemic lupus erythematosus. Adv Exp Med Biol. 2007;601:113–9.
Bonelli M, Savitskaya A, von Dalwigk K, Steiner CW, Aletaha D, Smolen JS, et al. Quantitative and qualitative deficiencies of regulatory T cells in patients with systemic lupus erythematosus (SLE). Int Immunol. 2008;20:861–8.
Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, et al. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol. 2005;175:8392–400.
Barath S, Soltesz P, Kiss E, Aleksza M, Zeher M, Szegedi G, et al. The severity of systemic lupus erythematosus negatively correlates with the increasing number of CD4+CD25(high)FoxP3+ regulatory T cells during repeated plasmapheresis treatments of patients. Autoimmunity. 2007;40:521–8.
Suen JL, Li HT, Jong YJ, Chiang BL, Yen JH. Altered homeostasis of CD4+ FoxP3+ regulatory T-cell subpopulations in systemic lupus erythematosus. Immunology. 2009;127:196–205.
Lee H-Y, Hong Y-K, Yun H-J, Kim Y-M, Kim J-R, Yoo W-H. Altered frequency and migration capacity of CD4+ CD25+ regulatory T cells in systemic lupus erythematosus. Rheumatology (Oxford). 2008;47:789–94.
Alvarado-Sanchez B, Hernandez-Castro B, Portales-Perez D, Baranda L, Layseca-Espinosa E, Abud-Mendoza C, et al. Regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2006;27:110–8.
Vargas-Rojas MI, Crispín JC, Richaud-Patin Y, Alcocer-Varela J. Quantitative and qualitative normal regulatory T cells are not capable of inducing suppression in SLE patients due to T-cell resistance. Lupus. 2008;17:289–94.
Venigalla RK, Tretter T, Krienke S, Max R, Eckstein V, Blank N, et al. Reduced CD4+,CD25- T cell sensitivity to the suppressive function of CD4+,CD25high,CD127−/low regulatory T cells in patients with active systemic lupus erythematosus. Arthritis Rheum. 2008;58:2120–30.
Lin S-C, Chen K-H, Lin C-H, Kuo C-C, Ling Q-D, Chan C-H. The quantitative analysis of peripheral blood FOXP3-expressing T cells in systemic lupus erythematosus and rheumatoid arthritis patients. Eur J Clin Investig. 2007;37:987–96.
Yan B, Ye S, Chen G, Kuang M, Shen N, Chen S. Dysfunctional CD4+,CD25+ regulatory T cells in untreated active systemic lupus erythematosus secondary to interferon-alpha-producing antigen-presenting cells. Arthritis Rheum. 2008;58:801–12.
Kim J-R, Chae J-N, Kim S-H, Ha J-S. Subpopulations of regulatory T cells in rheumatoid arthritis, systemic lupus erythematosus, and Behcet’s disease. J Korean Med Sci. 2012;27:1009.
Pan X, Yuan X, Zheng Y, Wang W, Shan J, Lin F, et al. Increased CD45RA+ FoxP3(low) regulatory T cells with impaired suppressive function in patients with systemic lupus erythematosus. PLoS One. 2012;7:e34662.
Franz B, Fritzsching B, Riehl A, Oberle N, Klemke CD, Sykora J, et al. Low number of regulatory T cells in skin lesions of patients with cutaneous lupus erythematosus. Arthritis Rheum. 2007;56:1910–20.
Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40.
Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521–30.
Klemke CD, Fritzsching B, Franz B, Kleinmann EV, Oberle N, Poenitz N, et al. Paucity of FOXP3+ cells in skin and peripheral blood distinguishes Sezary syndrome from other cutaneous T-cell lymphomas. Leukemia. 2006;20:1123–9.
Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+ FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–90.
Pillai V, Karandikar NJ. Attack on the clones? Human FOXP3 detection by PCH101, 236A/E7, 206D, and 259D reveals 259D as the outlier with lower sensitivity. Blood. 2008;111:463–4.
Vukmanovic-stejic M, Agius E, Booth N, Dunne PJ, Lacy KE, Reed JR, et al. The kinetics of CD4+ Foxp3+ T cell accumulation during a human cutaneous antigen-specific memory response in vivo. J Clin. 2008;118:3639–50.
Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K-I, Dowgiert RK, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–9.
Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rötzschke O, Falk K. CCR6 expression defines regulatory effector/memory-like cells within the CD25+ CD4+ T-cell subset. Blood. 2005;105:2877–86.
Grailer JJ, Kodera M, Steeber DA. L-selectin: role in regulating homeostasis and cutaneous inflammation. J Dermatol Sci. 2009;56:141–7.
Huehn J, Siegmund K, Lehmann JCU, Siewert C, Haubold U, Feuerer M, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–13.
Clark RA, Kupper TS. IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood. 2007;109:194–202.
Rodriguez RS, Pauli ML, Neuhaus IM, Yu SS, Arron ST, Harris HW, et al. Memory regulatory T cells reside in human skin. J Clin Invest. 2014;124:1027–36.
Tselios K, Sarantopoulos A, Gkougkourelas I, Boura P. CD4+ CD25highFOXP3+ T regulatory cells as a biomarker of disease activity in systemic lupus erythematosus: a prospective study. Clin Exp Rheumatol. 2014;32:630–9.
Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181:8391–401.
Zhang B, Zhang X, Tang F, Zhu L, Liu Y. Reduction of forkhead box P3 levels in CD4+CD25high T cells in patients with new-onset systemic lupus erythematosus. Clin Exp Immunol. 2008;153:182–7.
Vallerskog T, Gunnarsson I, Widhe M, Risselada A, Klareskog L, van Vollenhoven R, et al. Treatment with rituximab affects both the cellular and the humoral arm of the immune system in patients with SLE. Clin Immunol. 2007;122:62–74.
Tselios K, Sarantopoulos A, Gkougkourelas I, Boura P. The influence of therapy on CD4+CD25(high)FOXP3+ regulatory T cells in systemic lupus erythematosus patients: a prospective study. Scand J Rheumatol. 2015;44:29–35.
Acknowledgements
We thank Astrid Riehl, Christine Stumpf, Raphael Klembt, and Sabine Herzog, former members of the Division of Immunogenetics, Tumor Immunology Program, German Cancer Research Center Heidelberg, Germany, for their support in the FACS analysis, help in immunohistochemistry, and for support in the preparation of the manuscript, respectively. We are also indebted to Professor Martin Glombitza, MD, Municipal Hospital Karlsruhe, Department of Nephrology and Rheumatology, for providing us with blood samples of patients with SLE, and Dr. Ferdinand Toberer, MD, Professor Wolfgang Hartschuh, MD, and Professor Alexander Enk, MD, University of Heidelberg, Department of Dermatology, for providing us with skin biopsies of patients with SLE. We also thank Aysche Landmann, Division of Immunogenetics, Tumor Immunology Program, German Cancer Research Center Heidelberg, Germany, for copy-editing the manuscript.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the local ethics committee of the University of Heidelberg, Heidelberg, Germany, and was conducted according to the Declaration of Helsinki.
Conflict of interest
The authors have no conflicting financial interests.
Additional information
Angelika Schmidt and Cosima C. Rieger are shared first authors.
Rights and permissions
About this article
Cite this article
Schmidt, A., Rieger, C.C., Venigalla, R.K. et al. Analysis of FOXP3+ regulatory T cell subpopulations in peripheral blood and tissue of patients with systemic lupus erythematosus. Immunol Res 65, 551–563 (2017). https://doi.org/10.1007/s12026-017-8904-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-017-8904-4