Abstract
Contrasting results have been reported about the prevalence of thyroid autoimmunity (AT) and dysfunction (TD) in patients with psoriatic arthritis (PsA). In this study, we pointed to evaluate the incidence of new cases of clinical and subclinical TD in a broad group of patients with PsA versus a control group, matched by age and gender belonging to the same geographic area. PsA patients with TD were excluded firstly, and new cases of thyroid disorders were evaluated in 97 PsA patients and 97 matched controls, who had comparable iodine intake (median follow-up of 74 months in PsA versus 92 in controls). A raised rate of new cases of hypothyroidism, TD, positive antithyroid peroxidase (AbTPO) antibodies, and appearance of a small hypoechoic thyroid pattern in PsA, especially in female gender, compared to controls has been evidenced. Risk factors in female gender for the development of TD are thyroid-stimulating hormone (TSH) within the normal range but at the higher limit, positive AbTPO, and small thyroid volume. To sum up, thyroid function follow-up and suitable treatments should be performed regularly in female patients at high risk (TSH within the normal range but at the higher limit, positive AbTPO, hypoechoic and small thyroid).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriatic arthritis (PsA) has been shown associated with thyroid autoimmune disorders and hypothyroidism.
A higher prevalence of thyroid antimicrosomal antibodies positivity has been shown in 42 PsA patients in a study by Bianchi and coworkers [1]. Moreover, we have demonstrated [2] a higher prevalence of thyroid autoimmunity (positive antithyroid peroxidase (AbTPO) antibodies, thyroid hypoechogenicity) in PsA patients than in controls. However, other studies reported contrasting results [3–5]. For example, a study reported thyroid autoimmunity in patients with psoriasis was no different from that found in healthy individuals [4]. The main aim of this study was to evaluate the incidence of new cases of clinical and subclinical thyroid dysfunction (TD) in a wide group of patients with PsA versus an age- and sex-matched control group from the same geographic area.
Methods
A thyroid evaluation was conducted in 132 patients with PsA consecutively referred to the Rheumatology Unit and the Internal Medicine of the University of Pisa (1995–2012). The diagnosis of PsA was done according to Vasey and Espinoza criteria [6].
Disease activity was evaluated by ACR joint count, serum C-reactive protein, Health Assessment Questionnaire, morning stiffness duration, and presence of spinal and nocturnal pain [7].
The criteria of exclusion from the study were (1) PsA patients who had previous treatment with radiotherapy in the regions of neck or mediastinum regions, or with biologics; (2) PsA patients having clinical or subclinical hypothyroidism, clinical, or subclinical hyperthyroidism (or Graves’ disease). Ninety-seven patients with PsA without TD had the eligibility criteria for the longitudinal study. Patients were additionally assessed one or more times, at least 1 year after the first evaluation, and subsequently every year.
The follow-up interval (median) from the initial evaluation was 74 months (range 13–171 months).
The 97 eligible PsA patients were matched by age and gender, one-to-one, with controls (without TD), from the same geographic context (North-West Tuscany), having a comparable iodine intake (that is a risk factor for thyroid autoimmune diseases outcome).
Urinary iodine excretion [8] was evaluated in 32 patients with PsA and in 35 control subjects, and it was not significantly different (median, 91.0 μg/L; interquartile range (IR), 43.3–161.2 μg/L and median, 94.0 μg/L; IR, 37.2–157.4 μg/L, respectively; not significant (NS)).
Controls were selected (by random selection and assignment) from more than 2000 subjects extracted from a survey of thyroid disorders (population-based), firstly evaluated in 1994 and then again (with a complete thyroid re-evaluation) in 2002–2003. The follow-up interval (median) from the initial evaluation was 92 months (range 84–119 months).
PsA and control subjects were reassessed by (1) physical examination; (2) thyroid ultrasonography [9]; and (3) serum-free thyroxine (FT4), free triiodothyronine (FT3) (AMERLEX-MAB FT4/FT3 Kit; Amersham, UK), thyroid-stimulating hormone (TSH) (normal limits 0.3–3.6 μU/mL) (DiaSorin, USA), AbTPO and AbTg antibodies (ICN Pharmaceuticals, USA; positivity >100 IU/mL) (each dosage was done in triplicate).
In the case of an appearance of TD in the follow-up of PsA patients, they were treated properly and excluded from the study.
Institutional ethic committee approved the study, and subjects taking part to it gave their informed consent.
Normally distributed variables were compared by ANOVA, or mean group values by Mann-Whitney U test, while categorical variables were evaluated by χ2 test. Age, gender, smoking, thyroid hypoechogenicity, TSH, positive AbTg or AbTPO, and thyroid volume (at the initial evaluation) were included as variables (independent) in a logistic regression analysis in PsA subjects and hypothyroidism (dependent variable) at last evaluation.
Results
The basal thyroid status of PsA patients participating to the study and matched controls is described in Table 1.
Slightly higher, but significant, TSH, AbTg, and AbTPO were reported in PsA patients. The prevalence of indices of thyroid autoimmunity, such as subjects with positive AbTPO, AbTg, thyroid volume <6 mL, or a thyroid hypoechoic pattern, were more elevated, even if not significantly, in the PsA group with respect to control subjects.
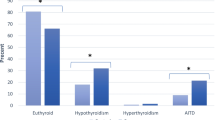
At the last evaluation (upon a median of 74 and 92 months, respectively, in patients with PsA and controls, P < 0.01, ANOVA) TSH, AbTPO, and AbTg levels were significantly more elevated in the PsA patients than in controls (Table 2). Subclinical hypothyroidism was more frequent (P < 0.05) in PsA patients with respect to control subjects, while subclinical hyperthyroidism was more prevalent in PsA without reaching the statistical significance. Overall, prevalence of TDs (clinical or subclinical hypo- and hyperthyroidism) was more common (P < 0.05) in PsA, such as the one of subjects showing a thyroid hypoechoic pattern, AbTPO-positivity, and volume <6 mL.
In Table 3, we have shown the prevalence and the incidence of new cases of thyroid disorders. TD, subclinical hypothyroidism, hypothyroidism, a thyroid hypoechoic pattern, AbTPO-positivity, thyroid autoimmunity, or a small thyroid (<6 mL) were more frequent (P < 0.05) in the PsA group.
All PsA patients with subclinical hypothyroidism showed polyarticular involvement (P < 0.05) and a longer duration of the disease than other PsA patients (years 18 ± 17 versus 9 ± 9, P = 0.005). PsA disease activity was not related with the presence of any kind of thyroid disorders.
At the conclusion of the follow-up, in PsA patients, there was a significant association among hypothyroidism, positive AbTPO (P < 0.014), a small thyroid (<6 mL) (P < 0.009), and hypoechogenicity (P < 0.021) (all by χ2), but not with any other thyroid parameter. Furthermore, clinical parameters of PsA patients and AbTg, AbTPO, or other studied parameters, were not associated.
The number of euthyroid patients with PsA and control subjects who were “thyroid autoimmunity–negative” (“clean”) at entry was, respectively, 78 and 86. The number of new cases of subclinical hypothyroidism or hyperthyroidism in these clean patients with PsA were four and zero, respectively (4 of 78 (5.1%)), whereas in clean control subjects, they were zero and zero, respectively (0 of 86 (0%)); the difference was statistically significant (Pearson P = 0.033, Mantel-Hanszel P = 0.034; Fisher’s exact test, P = 0.049) (Table 4). No other dysfunctions were observed in the clean patients.
In PsA, the logistic regression analysis (considering gender, age, TSH, AbTg-positivity, AbTPO-positivity, thyroid volume, thyroid hypoechogenicity (presence/absence), (all at the first examination) as independent variables, and hypothyroidism (at last visit) as the dependent variable) evidenced that the onset of hypothyroidism was linked to gender (female) (coefficient, 0.901; Exp(coef), 2.423; 95% lower, 1.304; 95% upper, 4.89; P = 0.01), initial normal TSH but at the higher limit (coefficient, 0.876; Exp(coef), 2.264; 95% lower, 1.126; 95% upper, 4.07; P = 0.036), and AbTPO-positivity (coefficient, 0.871; Exp(coef), 2.012; 95% lower, 1.095; 95% upper, 4.176; P = 0.027) (Table 5).
No association was found among thyroid dysfunctions or autoimmunity and PsA disease activity.
In controls, the logistic regression analysis (considering gender, age, TSH, AbTg-positivity, AbTPO-positivity, thyroid volume, thyroid hypoechogenicity (presence/absence), (all at the first examination) as independent variables, and hypothyroidism (at last visit) as the dependent variable) did not show any significant statistical association.
Discussion
In this study, we demonstrate a higher incidence of new cases of AbTPO-positivity, hypothyroidism, TD, hypoechoic thyroid pattern, and thyroid autoimmunity in PsA patients versus control subjects despite a significantly longer follow-up in the control group. A polyarticular involvement and longer disease duration have been shown in the PsA patients with subclinical hypothyroidism, too.
Interestingly, it was seen a similar incidence of new cases of AbTPO and hypothyroidism in controls as the one evidenced in other epidemiological studies [10–12]; for this reason, control group is not biased versus a low prevalence of AT [13].
As reported by other papers and by our preceding transversal study [2, 14], mean TSH level, a small thyroid volume, a thyroid hypoechoic pattern, and antithyroid antibodies were significantly more elevated in PsA patients than in controls.
The logistic regression analysis demonstrated in PsA that the onset of hypothyroidism was associated with the female gender, TSH within the normal range but at the higher limit, AbTPO-positivity, and small thyroid volume, as shown in the general population [10–12].
At least in part, the significant differences in the baseline autoimmune thyroid parameters between PsA patients and controls could be the reason of the more elevated incidence of hypothyroidism in PsA patients.
It is well known that autoimmune disorders are associated [15], although its pathogenetic basis is not known [16, 17].
In PsA, a Th1 immune predominance has been shown in the first phases of the disease [18]. CXCL10 (the prototype Th1 chemokine) is determinant in the pathogenesis of psoriasis and PsA. CXCR3 and CXCL10 were demonstrated in keratinocytes and the infiltrate from active dermal psoriatic plaques; an effective therapy of active plaques reduced CXCL10 expression in plaques. High circulating CXCL10 levels have been observed in psoriasis, with a Th1 immune predominance in the first phases of the disease; while CXCL10 levels decline in long lasting psoriasis [18]. Moreover, circulating CXCL10 levels in psoriatic patients are higher (P < 0.05) in the presence of AT [19, 20]. On the basis of the above mentioned observations, it has been speculated that CXCL10 may be a good circulating marker to control psoriasis activity and/or progression, and selective and potent CXCR3 or CXCL10 antagonists have been assessed in autoimmune diseases, suggesting their potential use also in this disease [18].
The common above reported Th1 immune predominance profile in the initial phases of both PsA and AT, under the influence of environmental and genetic conditions, may be the pathogenetic basis of the onset of autoimmune phenomena that involve various organs in the same person [21–24].
To sum up, an elevated incidence of new cases of TD, hypothyroidism, positive AbTPO, and appearance of a hypoechoic and small thyroid have been reported in PsA patients, particularly in females, versus controls. These data suggest that PsA patients are at risk for the development of new thyroid autoimmune disorders and consequently thyroid dysfunctions. In fact, risk factors in females for the development of TD are TSH within the normal range but at the higher limit, a small thyroid volume, or positive AbTPO.
We suggest that approximately every year, thyroid function (and suitable treatments) should be assessed in female patients with a major risk (TSH within the normal range but at the higher limit, positive AbTPO, a small or hypoechoic thyroid).
References
Bianchi G, Marchesini G, Zoli M, Falasconi MC, Iervese T, Vecchi F, et al. Thyroid involvement in chronic inflammatory rheumatological disorders. Clin Rheumatol. 1993;12:479–84.
Antonelli A, Delle Sedie A, Fallahi P, Ferrari SM, Maccheroni M, Ferrannini E, et al. High prevalence of thyroid autoimmunity and hypothyroidism in patients with psoriatic arthritis. J Rheumatol. 2006;33:2026–8.
Soy M, Guldiken S, Arikan E, Altun BU, Tugrul A. Frequency of rheumatic diseases in patients with autoimmune thyroid disease. Rheumatol Int. 2007;27:575–7.
Gul U, Gonul M, Kaya I, Aslan E. Autoimmune thyroid disorders in patients with psoriasis. Eur J Dermatol. 2009;19:221–3.
Unsal E, Oren O, Salar K, Makay B, Abaci A, Ozhan B, et al. The frequency of autoimmune thyroid disorders in juvenile idiopathic arthritis. Turk J Pediatr. 2008;50:462–5.
Vasey FB, Espinoza LR. Psoriatic arthropathy. In: Calin A, editor. Spondyloarthropathies. Orlando: Grune & Stratton, Inc; 1984. p. 151–85.
Kavanaugh A, Cassell S. The assessment of disease activity and outcomes in psoriatic arthritis. Clin Exp Rheumatol. 2005;23(Suppl 39):S142–7.
Antonelli A, Fallahi P, Ferrari SM, Mancusi C, Giuggioli D, Colaci M, et al. Incidence of thyroid disorders in systemic sclerosis: results from a longitudinal follow-up. J Clin Endocrinol Metab. 2013;98:E1198–202.
Antonelli A, Ferri C, Fallahi P, Cazzato M, Ferrari SM, Sebastiani M, et al. Clinical and subclinical autoimmune thyroid disorders in systemic sclerosis. Eur J Endocrinol. 2007;156:431–7.
Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol. 1995;43:55–68.
Roos A, Links TP, de Jong-van den Berg LT, Gans RO, Wolffenbuttel BH, Bakker SJ. Thyroid peroxidase antibodies, levels of thyroid stimulating hormone and development of hypothyroidism in euthyroid subjects. Eur J Intern Med. 2010;21:555–9.
Li Y, Teng D, Shan Z, Teng X, Guan H, Yu X, et al. Antithyroperoxidase and antithyroglobulin antibodies in a five-year follow-up survey of populations with different iodine intakes. J Clin Endocrinol Metab. 2008;93:1751–7.
Aghini-Lombardi F, Antonangeli L, Martino E, Vitti P, Maccherini D, Leoli F, et al. The spectrum of thyroid disorders in an iodine-deficient community: the Pescopagano survey. Clin Endocrinol Metab. 1999;84:561–6.
Antonelli A, Ferri C, Fallahi P, Ferrari SM, Frascerra S, Carpi A, et al. Alpha-chemokine CXCL10 and beta-chemokine CCL2 serum levels in patients with hepatitis C-associated cryoglobulinemia in the presence or absence of autoimmune thyroiditis. Metabolism. 2008;57:1270–1.
Antonelli A, Fallahi P, Mosca M, Ferrari SM, Ruffilli I, Corti A, et al. Prevalence of thyroid dysfunctions in systemic lupus erythematosus. Metabolism. 2010;59:896–900.
Miyadera H, Tokunaga K. Associations of human leukocyte antigens with autoimmune diseases: challenges in identifying the mechanism. J Hum Genet. 2015;60:697–702.
Cho JH, Feldman M. Heterogeneity of autoimmune diseases: pathophysiologic insights from genetics and implications for new therapies. Nat Med. 2015;21:730–8.
Ferrari SM, Ruffilli I, Colaci M, Antonelli A, Ferri C, Fallahi P. CXCL10 in psoriasis. Adv Med Sci. 2015;60:349–54.
Antonelli A, Fallahi P, Delle Sedie A, Ferrari SM, Maccheroni M, Bombardieri S, et al. High values of Th1 (CXCL10) and Th2 (CCL2) chemokines in patients with psoriatic arthritis. Clin Exp Rheumatol. 2009;27:22–7.
Antonelli A, Fallahi P, Delle Sedie A, Ferrari SM, Maccheroni M, Bombardieri S, et al. High values of alpha (CXCL10) and beta (CCL2) circulating chemokines in patients with psoriatic arthritis, in presence or absence of autoimmune thyroiditis. Autoimmunity. 2008;41:537–4.
Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Human Th1 dichotomy: origin, phenotype and biologic activities. Immunology. 2014.
Antonelli A, Ferrari SM, Frascerra S, Pupilli C, Mancusi C, Metelli MR, et al. CXCL9 and CXCL11 chemokines modulation by peroxisome proliferator-activated receptor-alpha agonists secretion in Graves' and normal thyrocytes. J Clin Endocrinol Metab. 2010;95:E413–20.
Antonelli A, Fallahi P, Ferrari SM, Pupilli C, d'Annunzio G, Lorini R, et al. Serum Th1 (CXCL10) and Th2 (CCL2) chemokine levels in children with newly diagnosed Type 1 diabetes: a longitudinal study. Diabet Med. 2008;25:1349–53.
Fallahi P, Ferrari SM, Ruffilli I, Elia G, Giuggioli D, Colaci M, et al. Incidence of thyroid disorders in mixed cryoglobulinemia: results from a longitudinal follow-up. Autoimmun Rev. 2016;15:747–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Nothing to declare.
Conflicts of interest
The authors declare that they have no conflict of interest.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Fallahi, P., Ferrari, S.M., Ruffilli, I. et al. Increased incidence of autoimmune thyroid disorders in patients with psoriatic arthritis: a longitudinal follow-up study. Immunol Res 65, 681–686 (2017). https://doi.org/10.1007/s12026-017-8900-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-017-8900-8