Abstract
Inflicted shaking trauma can cause injury in infants, but exact injury mechanisms remain unclear. Controversy exists, particularly in courts, whether additional causes such as impact are required to produce injuries found in cases of (suspected) shaking. Publication rates of studies on animal and biomechanical models of inflicted head injury by shaking trauma (IHI-ST) in infants continue rising. Dissention on the topic, combined with its legal relevance, makes maintaining an up-to-date, clear and accessible overview of the current knowledge-base on IHI-ST essential. The current work reviews recent (2017–2023) studies using models of IHI-ST, serving as an update to two previously published reviews. A systematic review was conducted in Scopus and PubMed for articles using animal, physical and mathematical models for IHI-ST. Using the PRISMA methodology, two researchers independently screened the publications. Two, five, and ten publications were included on animal, physical, and mathematical models of IHI-ST, respectively. Both animal model studies used rodents. It is unknown to what degree these can accurately represent IHI-ST. Physical models were used mostly to investigate gross head-kinematics during shaking. Most mathematical models were used to study local effects on the eye and the head’s internal structures. All injury thresholds and material properties used were based on scaled adult or animal data. Shaking motions used as inputs for animal, physical and mathematical models were mostly greatly simplified. Future research should focus on using more accurate shaking inputs for models, and on developing or and validating accurate injury thresholds applicable for shaking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflicted head injury (IHI), also referred to as abusive head trauma (AHT), is diagnosed 14–41 times a year per 100.000 infants < 1 year old [1,2,3,4,5]. The most prevalent causes of IHI in infants are blunt force trauma shaking trauma, or a combination of both [8]. Shaking trauma (ST) is caused by repetitive acceleration/deceleration of an infant’s head, usually by forcefully moving the torso back and forth in the sagittal plane. Injuries associated with inflicted head injury by shaking trauma (IHI-ST) include subdural hematomas, diffuse axonal injury, retinal hemorrhage, and cervical spinal cord injury [9, 10]. However, these can also result from other medical conditions or accidents, so no combination of injuries is considered pathognomonic for IHI-ST. Concluding that a case of head injury resulted from shaking follows an exhaustive medical work-up that considers the exclusion of alternative causes (e.g. no signs of blunt force head-trauma), and statements from (alleged) perpetrators and witnesses. Although generally accepted among physicians [11], and despite medical and biomechanical evidence [12, 13], some still deny that shaking alone can result in serious injury and lethal outcomes. In addition, some question whether an adult human is strong enough to generate the accelerations observed in studies of IHI-ST using small animals and experimental or mathematical models. This reluctance is due partly to the fact that concluding ‘shaking’ as a cause of head injury is based on excluding other causes, and partly to the lack of consensus on mechanisms of shaking-induced injuries [6, 7]. Additionally, shaking incidents with injuries have hardly ever been observed.
For obvious ethical reasons, conducting experiments on living infants to investigate IHI-ST is impossible. Instead, various modeling approaches have been adopted. Juvenile animal models have been used to study (injury) outcomes resulting from ST, focusing mostly on intracranial and retinal tissue damage. Physical models, usually mechanical surrogates, have been used to experimentally record kinematics and dynamics of shaking. Finally, mathematical models of IHI-ST in which (part of) an infant is represented by sets of constitutive mechanical and material equations, have been used to study shaking-biomechanics. These mathematical models can be subdivided into rigid body models (RBMs) and finite element models (FEMs). In RBMs, an infant is represented by an assembly of rigid segments, interconnected by joints [14]. Displacements or forces can be applied to segments to simulate shaking input, with resulting model kinematics being the output. In FEMs, modeled structures are subdivided into small parts (“elements”) with (visco-)elastic properties. FEMs have been used to study IHI-ST at tissue level, by studying local loading and deformation of internal structures during shaking [15].
Vester et al. [6] and Van Zandwijk et al. [7] conducted a two-part systematic review of literature on animal models and mathematical/physical models of IHI-ST, respectively, identifying several gaps in the literature. First of all, Vester et al. [6] concluded that large randomized animal studies of IHI-ST were lacking in literature. They found only three publications [16,17,18] using an animal model they considered representative of IHI-ST in human infants, all on one study using lambs. Additionally, Vester et al. [6] found nine studies using piglets, though they considered these difficult to translate to IHI-ST, as the “shaking” perturbations applied were non-cyclic and limited to purely rotational movements. Van Zandwijk et al. [7] identified a number of physical, RBM and FEM models of IHI-ST. They found that most studies using physical models, and some using RBMs, concluded that IHI-ST cannot induce injury, whereas FEM studies mostly suggested the opposite. The main gap Van Zandwijk et al. [7] identified was that virtually all included studies used injury thresholds derived from scaled non-infant data, often from studies on impact injuries, making the applicability of those thresholds to IHI-ST questionable.
-
1.
The number of hits on the search query used in Vester et al. [6] and Van Zandwijk et al. [7] has continued rising since their last included study from 2016 (Fig. 1), illustrating the growing relevance. Therefore, the current review serves as an update to both parts of the systematic review by Vester et al. [6] and Van Zandwijk et al. [7], with the aim to:identify recent (2017–2023) findings and innovations from animal, mathematical and physical models that can contribute to understanding injury mechanisms underlying IHI-ST, by identifying characteristics of:
-
(a)
model inputs (shaking kinematics),
-
(b)
model properties (animal characteristics; material and joint properties), and
-
(c)
model outputs (injury outcomes, injury thresholds applied);
-
(a)
-
2.
Systematically present these models of IHI-ST to enable comparison between models, and create an updated accessible overview for legal and medical practitioners.
-
3.
Find which abovementioned gaps in IHI-ST modeling identified by Vester et al. [6] and Van Zandwijk et al. [7], if any, have been addressed, which remain, and to identify new gaps.
Methods
Database search
A systematic literature search was conducted in PubMed and Scopus for articles from January 1st, 2017, to October 1st, 2023. The search queries used—identical to those in Vester et al. [6] and Van Zandwijk et al. [7]—were constructed to find studies using animal, mathematical or physical models related to IHI-ST (Fig. 2, Appendix). Articles in English, German, French and Dutch were included. Search results from PubMed and Scopus were combined and de-duplicated.
Article selection
Relevant articles were selected using the PRISMA methodology. The relevance of each paper was assessed on title, abstract, and full-text, in that order. Animal, physical and mathematical models were assessed separately. For studies on animal models, inclusion required acceleration/deceleration trauma and a standardized method of inducing trauma. Studies were excluded if these were a secondary source only, studied impact trauma only, used adult animals only, or a trauma mechanism was absent. For physical and mathematical modeling, inclusion required cyclic acceleration/deceleration and use of a physical or mathematical model. Studies were excluded for being a secondary source only, investigating isolated trauma without shaking, modeling adult anatomy only, or not applying sagittal shaking.
Authors AJL and KH performed the selection process independently. In case of disagreement, consensus was sought, including the article if none was reached. References of included articles were screened for additional articles (backward snowballing), which then went through the selection process above. Articles included in the previous reviews were excluded.
Data extraction
Data were extracted from the full texts of all included articles by author KH using pre-designed data extraction sheets, identical to those in the previous reviews [6, 7]. For the animal modeling studies, features such as study design, animal characteristics, trauma mechanism and input dynamics, as well as cerebral, ocular, and spinal cord outcomes were recorded. Information extracted from physical or mathematical modeling studies included model description, model inputs, dynamic and kinematic response, applied injury criteria, and stated conclusions.
Data structuring
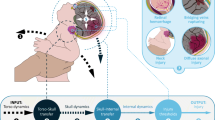
The same data-organization approach was used as in the original reviews [6, 7]. To organize data from articles on mathematical and physical models of IHI-ST, Vester et al. [6] and Van Zandwijk et al. [7] subdivided the event of IHI-ST into seven steps (Fig. 3). Their “7-step description” describes IHI-ST as a biomechanical chain of events, viewing the infant as a system of interconnected elements, with distinct geometries and material properties. In alternating order, each step is either a system state of the modeled infant (steps 1, 3, 5 and 7), or a transfer function describing the effect of the dynamics of one part on another (steps 2, 4 and 6). The goal of using the 7-step structure, is that an overview emerges of which steps of an IHI-ST event have been investigated, how these contribute to our understanding of IHI-ST, and where gaps in our understanding lie. The 7 steps are as follows:
-
1.
Torso dynamics: Shaking motion applied to the infant’s torso serves as system input
-
2.
Torso-Skull transfer: Torso motion is transferred from the torso, through the neck, to the head.
-
3.
Skull-dynamics: Motion transferred to the head results in the dynamics of the skull.
-
4.
Skull-Internal transfer: Movement of the skull is transferred to the cranial contents.
-
5.
Internal dynamics: Motion transferred to the cranial contents results in the head’s internal dynamics, including loading and deformation of brain matter, eyes, bridging veins and other intracranial elements.
-
6.
Injury thresholds: Internal dynamics may cause thresholds for material damage of internal elements to be exceeded.
-
7.
Output injury: Exceeding injury thresholds results in the output injury presence.
Results
Search results & quality assessment
1725 articles remained after the initial search, successive backward snowballing, and deduplication. After full-text assessment, 2 results on animal, 5 on physical and 10 on mathematical models were included. See the PRISMA flowchart in Fig. 4. for an overview of the screening process and reasons for full-text exclusions. Publications included for animal models were assessed on quality using CASP: both were prospective studies of low quality.
Data extraction results
The complete data extraction tables from the current study are included as online supplementary materials. The data extraction tables from Vester et al. [6] and Van Zandwijk et al. [7], and all tables they included in-text, were also updated and made available.
Animal model results
Two animal studies were identified, both in rodents (Table 1) [19, 20]. Daniel et al. conducted experiments with rats, comparing rhythmic oscillatory high-duration shaking, to violent high-intensity shaking [19]: 15 min of shaking at 1 Hz for five consecutive days, against 6 s of shaking at 3.3 Hz, respectively, each repeated 10 consecutive times with 6 s breaks. The body was fixated in a tube, allowing free head/neck movement. The tube was mounted to an oscillating plate, inducing mostly sagittal flexion/extension. They found extra-axial hemorrhage in 10% of the low intensity/high duration group, and in 40% of the animals exposed to high intensity/low duration shaking (Table 1).
Wang et al. conducted shaking experiments with mice [20] immobilized at thoracic level, with their head fixed in a holder that rotated to 90 degree sagittal flexion/extension and allowed no out-of-plane movement. The mice were divided into 4 groups, undergoing 30, 60, 80 or 100 shaking cycles consecutively, at a frequency of 3 Hz (Table 1). The authors found subdural hematomas, subarachnoid hemorrhage, brain swelling, diffuse gliosis, neuronal degeneration and retinal hemorrhage in injured mice.
Physical and mathematical model results
The results of the data extraction from the included studies on mathematical models were divided into the abovementioned 7-steps description of the IHI-ST biomechanical chain of events described in Fig. 3. Tables 2 and 3 describe which of the 7 steps were addressed in the studies on physical and mathematical models, respectively. The values of kinematic parameters reported in these studies can be found in Tables 4 and 5.
Step 1
Physical models
In all but one of the studies using physical models, an infant-size doll was shaken [21,22,23,24]. In each, volunteers were instructed to hold the surrogate’s torso and shake it, with [21, 23] or without [22, 24] explicit instructions on shaking direction and intensity. Shaking frequencies attained were 3.5–6 Hz (Table 4).
Mathematical models
In three studies using RBMs of IHI-ST [25,26,27], kinematic constraints prescribed the motion of the modeled torso. Reimann [25] prescribed a rotational sinusoidal (4 Hz) movement to the torso, resulting in head rotation about an axis in the neck. In Lintern et al. [26] a range of sinusoidal movements (1.6–2.5 Hz) were applied to the elbow and shoulder joints of a RBM of an adult with its hands rigidly attached to the infant torso. The resulting shaking frequencies were 0.5 to 40 Hz.
None of the reviewed FEM models included the torso in their model. Song et al. [28] and Suh et al. [29] used parameters from an experiment using a physical surrogate shaking experiment from literature to determine the input (2.2 Hz) for their FEM model of the eye. However, it was not elaborated whether a complete description of motion with respect to the inertial reference frame was used, or whether simplified boundary conditions were used that may not capture the effects of the head’s movements (Table 5).
Step 2
Physical models
Measured head kinematics in studies using dolls are dependent on its neck properties, forming the torso-head coupling. Glowinski et al. [23] used the commercial RealCareTM Shaken Baby simulator, typically used for educational purposes. Nadarasa et al. [22], Stray-Pedersen et al. [21] and Schiks et al. [24] used the Q0 commercial crash-test dummy, representing a new-born. Most studies agree quantitative data on infant neck properties is lacking, making it hard to assess surrogates’ biofidelity.
Mathematical models
Lintern et al. [26] used their RBM in a Monte Carlo analysis to investigate the effect of neck joint properties on resulting head velocities and accelerations. The authors concluded that head kinematics were highly dependent on shaking frequency, but relatively insensitive to properties of the infant model, including its neck joint properties. Reimann [25] modeled the infant neck in their RBM as a frictionless hinge and estimated they were of similar magnitude to accelerations that produce concussions.
Step 3
Physical models
In all doll studies [21,22,23,24], head kinematics were recorded using accelerometers mounted on and/or inside the doll’s head, sometimes combined with video-recordings or marker-based motion capture.
In the only non-doll study found, by Pasquesi and Margulies [30], the physical model consisted of a post-mortem lamb skull and brain, mounted to a mechanical linkage and shaken. Note that this study was not included among animal model studies, since inclusion of animal models required induced trauma, while here it was only a physical model to study brain kinematics during shaking (see Step 5).
Mathematical models
In the RBM studies by Lintern et al. [26] and Reimann [25] linear velocities and peak linear accelerations of the head were reported as output. Pasquesi and Margulies [30] and Pasquesi et al. [31] used angular accelerations from animal experiments [32, 33] as input for their FEM of a piglet’s head. Schiks et al. implemented a model that took the spatiotemporal variation of the center of rotation of the head during shaking into account [24]. The authors compared head acceleration outcomes when using their model, compared to models with a fixed rotation center, and found that their model strongly improves the replication of accelerations found in shaking experiments.
Step 4
Physical models
None of the included studies using doll surrogates modeled the head’s internal anatomy.
Mathematical models
Daboin and Saboori [27] was the only RBM study including internal transfer in the head, using a brain and skull interconnected by elastic elements. They investigated relative brain-skull displacements, during linear sinusoidal displacements of the torso. They concluded that resonance may cause oscillatory effects in linear and angular relative skull-brain displacements.
In Pasquesi et al. [31] and Pasquesi and Margulies [30], an anatomically detailed FEM of a neonatal porcine head was used to model IHI-ST. Both studies used an identical model that included the skull, brain, falx and bridging veins.
Step 5
Physical models
In the only physical modeling study that investigated the internal dynamics of the head, Pasquesi and Margulies [30], measured relative brain-skull displacement during shaking. They transected a post-mortem lamb skull and brain, marked the cut-surface of the brain in order to track it, and rigidly attached the skull’s cut-surface to a translucent plate, which was then shaken. Average maximum brain-skull displacements of 0.76 ± 0.38 mm were recorded. When applying two consecutive rotations, the second produced higher displacements.
Mathematical models
All FEM studies included in the current review investigated the dynamics of the internal structures of the head [22, 28,29,30,31, 34]. The stresses and strains on the modeled structures are estimated using the model, and compared to their material failure thresholds (Step 6).
Step 6
To relate kinematic and dynamic outcomes from physical and mathematical models to injury outcomes, threshold values are used. Injury thresholds based on actual infant data are scarce, as material characterizations of infant biological material is hardly available. Consequently, scaled adult or animal data is used. Applied thresholds from studies using physical and mathematical models can be found in Tables 6 and 7, respectively.
Physical models
None of the studies using physical models extensively analyzed their outcomes based on injury thresholds (Table 6).
Mathematical models
Lintern et al. [26] and Reimann [25] both compare the peak linear head accelerations obtained using their RBMs, to concussion thresholds scaled from primate data from literature (Table 7).
Several FEM studies compared stresses in the eye during shaking to retinal adhesive force or retinal separation pressure [22, 28, 29, 34]. Song et al. [28], Suh et al. [29] and Nadarasa et al. [22] used 3D eye-models, consisting of several layers including the sclera, retina and vitreous. Song et al. [28] based this retinal detachment threshold on their own experiments on sheep and monkey eyes, whereas Suh et al. [29] and Nadarasa et al. [22] used literature values [35, 36]. In all three, exceeding of injury thresholds suggested RH would occur. Umstead et al. [34] also used a FEM to investigate RH in shaking by modeling a 2D slice of the retina. By comparing stress/strain in the modeled vessel walls during shaking to retinal vein material thresholds, they investigated likelihoods of RH occurring in shaking (Table 7).
Pasquesi and Margulies [30] and Pasquesi et al. [31] investigated the possibility of extra-axial hemorrhage resulting from bridging vein rupture in IHI-ST, using a FEM of a piglet’s head. Predicted bridging vein strains were compared to a maximum stretch ratio (determined experimentally in piglets). Pasquesi and Margulies [30] attempted to create a threshold for the number of failed bridging vein elements that was predictive of finding extra axial hemorrhage in piglets, by comparing it to their FEM piglet model of IHI-ST (Table 7).
Step 7
As noted previously by Van Zandwijk et al. [7], the conclusions drawn concerning the likelihood of injury resulting from shaking in studies using models of IHI-ST can be divided into these categories:
-
Threshold comparison: outputs obtained from modeled IHI-ST are compared to injury thresholds to assess the likeliness of specific injuries occurring in IHI-ST.
-
Comparison with other activities: outputs obtained from modeled shaking are compared to outputs when simulating other events (e.g. falls or every-day activities), to assess the comparative likelihood of injury occurring in shaking.
-
Qualitative conclusions: qualitative opinions on the likelihood of injury resulting from IHI-ST based on study outcomes, without comparison to injury thresholds or to other activities. Instead focusing on comparison to outcomes reported in other studies, validation of a model, or recommendation of future research.
Tables 8 and 9 provide an overview of the conclusions drawn in the literature included in the current review, as well as methodological points of attention when assessing the conclusions of these papers.
Physical
In included physical modeling studies, conclusive statements made were all either qualitative or based on comparison to impacts.
Mathematical
In all but one RBM study, conclusive statements were also qualitative or based on comparison to impacts, and none of these suggest that shaking alone cannot produce injuries associated with IHI-ST [24,25,26,27]. Only Lintern et al. [26] based their conclusion on a comparison to injury thresholds, determining that either shaking alone cannot cause injuries AHT, or the applied concussive injury threshold estimates are incorrect. Five of the included FEM studies based their conclusions on comparison to material failure thresholds of specific structures (bridging veins and retina) [22, 28,29,30,31]. In general, FEM studies suggested that shaking could result in these material thresholds being exceeded, and thus that injuries typically associated with shaking are reflected by material failure predicted by these models.
Discussion
Animal models
Two studies on animal models of IHI-ST were identified in the current review, both in rodents [19, 20]. Vester et al. [6] found that all but one of their included studies used shaking movements not representative of infant shaking (significant restrictions or non-cyclic). Conversely, the two included in the current review did use cyclic shaking motions to induce injury [19, 20], one of which unconstrained (Daniel et al. [19]). The duration of shaking applied by Wang et al. [19] (10–33 s) was within the range of durations that have been recorded in physical surrogate shaking experiments (5–53 s). [21] Conversely, Daniel et al. subjected animals to shaking for 5 consecutive days, 10 times a day, for up to 15 min each [19]. This seems unrealistically often and long in situations of infant abuse. Both studies found injuries that are typically associated with IHI-ST such as extra-axial hemorrhages [19, 20] and retinal hemorrhages [19]. However, it is unknown how well mice/rat anatomy represents mechanisms underlying IHI-ST in human infants. The shaking frequencies used in Daniel et al. [19] (1–3.3 Hz) and Wang et al. [19] (3 Hz) are within the range of shaking frequencies found in experiments using infant surrogates, but differences in size and mass between humans and rodents make estimating whether this accurately models IHI-ST in humans difficult.
The articles included by Vester et al. [6] came from only two research groups [32, 33, 35, 37,38,39,40,41,42,43] [16,17,18]. In the current follow-up review, no new publications fitting this topic by these groups were found. In Vester et al. [6] four articles on rodents [44,45,46,47] were ‘excluded for their inconclusive reporting of methodology and results’. For the current review, it was decided for completeness’ sake to also add the data from those previously excluded studies to the combined data extraction table from the current and previous reviews (online supplementary materials).
Physical & mathematical models
Physical and RBM models of IHI-ST focused almost exclusively on linear and rotational accelerations of the head as a whole during simulated shaking (Steps 1–3 in Fig. 3). FEMs tend to concentrate on studying local effects of shaking events on tissues of brain or eye structures (Steps 4–7 in Fig. 3). In general, there has been little development on the validation and verification of the applicability of current models of IHI-ST to human infants over the past 6 years.
Most studies using mathematical models of IHI-ST used simplified shaking kinematics as input for their models, e.g. imposing purely sinusoidal rotations around a fixed axis or purely translational movements. Schiks et al. showed that implementing a moving, rather than fixed, center of rotation in their model strongly improves the replication of experimental shaking kinematics, and indicated that existing studies should hence be critically viewed and redone [24]. Daboin and Saboori [27], Song et al. [28] and Suh et al. [29] did use input data from surrogate shaking experiments, though it is unclear whether a complete description of motion of the surrogate with respect to the inertial frame was used, or whether simplified pure rotational or translational movements were used, as input kinematics were incompletely described.
Van Zandwijk et al. [7] found that most studies up to 2017 using physical models and RBMs of IHI-ST used concussion thresholds to estimate whether recorded head accelerations were hazardous. Van Zandwijk et al. [7] suggested (later supported by Schiks et al. [48]) that these impact-based injury thresholds might be unsuitable. This is because in impact, injuries result from single high-intensity, short-duration force peaks, whereas in shaking, lower intensity, longer duration forces are exerted repeatedly. In the current follow-up review, while still being applied in some simplified RBM studies, most statements on injury outcomes were not based on concussion thresholds as a criterion, but mostly on comparison of kinematics between different shaking inputs, or on tissue failure thresholds.
Injury thresholds used in studies using FEMs to investigate IHI-ST were all based on tissue material failure thresholds, such as retinal adhesive force or maximum bridging vein stretch-ratios. Using material properties as a measure for injury thresholds makes these thresholds applicable independent of the trauma mechanisms, in contrast to context specific thresholds such as concussion thresholds. However, injury thresholds and material properties used in all the reviewed studies were derived from non-infant data, using either scaled adult or (scaled) animal data. It remains unknown to what degree these scaled thresholds and material properties reflect those of actual infants [48]. So while obtaining data on tissue properties and injury criteria from actual infants remains a difficult task due to both ethical reasons and a lack of available research material, the contribution of such research to the field would be considerable.
Key points
-
1.
Both identified animal model of IHI-ST were rodents. Due to significant differences in anatomy and scale it is unknown how well these represent the mechanisms underlying IHI-ST in infants.
-
2.
Studies using physical and mathematical models of IHI-ST using injury thresholds derived these from adult or animal data. Obtaining data on infant tissue properties would be valuable to future research to determine valid injury thresholds.
-
3.
Input shaking-kinematics used in mathematical models of IHI-ST were mostly highly simplified, which likely negatively affected the accuracy of resulting head kinematics, and therefore potentially the accuracy of outcomes regarding injury mechanisms.
-
4.
Most included FEM studies suggested that IHI-ST can result in injuries such as RH or torn bridging veins. Most studies using physical models or RBMs tentatively suggested that shaking can result in injury.
Data and materials
The full data extraction results from this review are included in an Online Resource.
References
Talvik I, et al. Inflicted traumatic brain injury (ITBI) or shaken baby syndrome (SBS) in Estonia. Acta Paediatr. 2007;95(7):799–804. https://doi.org/10.1111/j.1651-2227.2006.tb02343.x.
Niederkrotenthaler T, Xu L, Parks SE, Sugerman DE. Descriptive factors of abusive head trauma in young children–United States, 2000–2009. Child Abuse Negl. 2013;37(7):446–55. https://doi.org/10.1016/j.chiabu.2013.02.002.
Keenan HT, Runyan DK, Marshall SW, Nocera MA, Merten DF, Sinal SH. A population-based study of inflicted traumatic brain injury in young children. JAMA. 2003;290(5):621–6. https://doi.org/10.1001/jama.290.5.621.
Fanconi M, Lips U. Shaken baby syndrome in Switzerland: results of a prospective follow-up study, 2002–2007. Eur J Pediatr. 2010;169(8):1023–8. https://doi.org/10.1007/s00431-010-1175-x.
Ellingson KD, Leventhal JM, Weiss HB. Using hospital discharge data to track inflicted traumatic brain injury. Am J Prev Med. 2008;34(4 Suppl):S157–62. https://doi.org/10.1016/j.amepre.2007.12.021.
Vester MEM, Bilo RAC, Loeve AJ, van Rijn RR, Van Zandwijk JP. Modeling of inflicted head injury by shaking trauma in children: what can we learn? Part I: A systematic review of animal models. Forensic Sci Med Pathol. 2019;15(3):408–22. https://doi.org/10.1007/s12024-019-0082-3.
Van Zandwijk JP, Vester MEM, Bilo RA, van Rijn RR, Loeve AJ. Modeling of inflicted head injury by shaking trauma in children: what can we learn? Part II: A systematic review of mathematical and physical models. Forensic Sci Med Pathol. 2019;15(3):423–36. https://doi.org/10.1007/s12024-019-00093-7.
Christian CW, Block R, Committee on Child Abuse and Neglect. Abusive head trauma in infants and children. Pediatrics. 2009;123(5):1409–11. https://doi.org/10.1542/peds.2009-0408.
Choudhary AK, et al. Consensus statement on abusive head trauma in infants and young children. Pediatr Radiol. 2018;48(8):1048–65. https://doi.org/10.1007/s00247-018-4149-1.
Herman BE, Makoroff KL, Corneli HM. Abusive head trauma. Pediatr Emerg Care. 2011;27(1):65–9. https://doi.org/10.1097/pec.0b013e31820349db.
Narang SK, Estrada C, Greenberg S, Lindberg D. Acceptance of shaken baby syndrome and abusive head trauma as medical diagnoses. J Pediatr. 2016;177:273–8. https://doi.org/10.1016/j.jpeds.2016.06.036.
Adamsbaum C, Grabar S, Mejean N, Rey-Salmon C. Abusive head trauma: judicial admissions highlight violent and repetitive shaking. Pediatrics. 2010;126(3):546–55. https://doi.org/10.1542/peds.2009-3647.
Greeley CS. Abusive head trauma: a review of the evidence base. AJR Am J Roentgenol. 2015;204(5):967–73. https://doi.org/10.2214/AJR.14.14191.
Vallery H, Schwab AL. Advanced Dynamics. Delft: Delft University of Technology (in English); 2017.
Zienkiewicz OC, Taylor RL, Zhu JZ. The finite element method : its basis and fundamentals, Seventh edition. ed. Oxford, UK; Butterworth-Heinemann. 2013. [Online]. Available: http://www.books24x7.com/marc.asp?bookid=56579
Finnie JW, et al. Neuropathological changes in a lamb model of non-accidental head injury (the shaken baby syndrome). J Clin Neurosci. 2012;19(8):1159–64. https://doi.org/10.1016/j.jocn.2011.12.019.
Finnie JW, Blumbergs PC, Manavis J, Vink R. Pattern of cerebrospinal immediate early gene c-fos expression in an ovine model of non-accidental head injury. J Clin Neurosci. 2013;20(12):1759–61. https://doi.org/10.1016/j.jocn.2013.03.010.
Finnie JW, Manavis J, Blumbergs PC. Diffuse neuronal perikaryal amyloid precursor protein immunoreactivity in an ovine model of non-accidental head injury (the shaken baby syndrome). J Clin Neurosci. 2010;17(2):237–40. https://doi.org/10.1016/j.jocn.2009.07.001.
Daniel SDÁ, et al. Comparative study of brain damage and oxidative stress using two animal models of the shaken baby syndrome. Exp Gerontol. 2022;166:111874. https://doi.org/10.1016/j.exger.2022.111874.
Wang G, et al. Pathophysiological and behavioral deficits in developing mice following rotational acceleration-deceleration traumatic brain injury. DNM Dis Models Mech. 2018;11(1):dmm030387. https://doi.org/10.1242/dmm.030387.
Stray-Pedersen A, Strisland F, Rognum TO, Schiks LAH, Loeve AJ. Violent infant surrogate shaking: Continuous high-magnitude centripetal force and abrupt shift in tangential acceleration may explain high risk of subdural hemorrhage. Neurotrauma Rep (in eng). 2021;2(1):224–31. https://doi.org/10.1089/neur.2021.0013.
Nadarasa J, Deck C, Meyer F, Bourdet N, Raul JS, Willinger R. Development of a finite-element eye model to investigate retinal hemorrhages in shaken baby syndrome. Biomech Model Mechanobiol. 2018;17(2):517–30. https://doi.org/10.1007/s10237-017-0975-6.
Glowinski S, Majdanik S, Glowinska A, Majdanik E. Trauma in a shaken infant? A case study. Aggress Violent Behav. 2021;56:101515. https://doi.org/10.1016/j.avb.2020.101515.
Schiks LAH, Dankelman J, Loeve AJ. Inflicted head-injury by shaking-trauma in infants: the importance of spatiotemporal variations of the head’s rotation center. Sci Rep (in English). 2023;13(1):15226. https://doi.org/10.1038/s41598-023-42373-x.
Reimann RJ. Fundamental limits of shaking a baby. J Forensic Sci. 2018;63(6):1864–6. https://doi.org/10.1111/1556-4029.13777.
Lintern TO, Nash MP, Kelly P, Bloomfield FH, Taberner AJ, Nielsen PMF. Probabilistic description of infant head kinematics in abusive head trauma. Comput Methods Biomech Biomed Eng. 2017;20(16):1633–42. https://doi.org/10.1080/10255842.2017.1403593.
Daboin J, Saboori P. Effect of shaking at or near resonance of a simple head model on skull/brain connectors. 2021;5. https://doi.org/10.1115/IMECE2021-69054. [Online]. Available: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85124431309&doi=10.1115%2fIMECE2021-69054&partnerID=40&md5=05b03dcd3a5b6e739619c674b481478e, https://asmedigitalcollection.asme.org/IMECE/proceedings-abstract/IMECE2021/85598/V005T05A055/1132682.
Song HH, Thoreson WB, Dong P, Shokrollahi Y, Gu L, Suh DW. Exploring the vitreoretinal interface: a key instigator of unique retinal hemorrhage patterns in pediatric head trauma. Kor J Ophthalmol. 2022;36(3):253–63. https://doi.org/10.3341/kjo.2021.0133.
Suh DW, Song HH, Mozafari H, Thoreson WB. Determining the tractional forces on vitreoretinal interface using a computer simulation model in abusive head trauma. Am J Ophthalmol. 2021;223:396–404. https://doi.org/10.1016/j.ajo.2020.06.020.
Pasquesi SA, Margulies SS. Measurement and finite element model validation of immature porcine brain-skull displacement during rapid sagittal head rotations. Front Bioeng Biotechnol (in English). 2018;6(FEB):16. https://doi.org/10.3389/fbioe.2018.00016.
Pasquesi SA, Seidi M, Hajiaghamemar M, Margulies SS. Predictions of neonatal porcine bridging vein rupture and extra-axial hemorrhage during rapid head rotations. J Mech Behav Biomed Mater. 2020;106:103740. https://doi.org/10.1016/j.jmbbm.2020.103740.
Coats B, et al. Cyclic head rotations produce modest brain injury in infant piglets. J Neurotrauma. 2017;34(1):235–47. https://doi.org/10.1089/neu.2015.4352.
Raghupathi R, Margulies SS. Traumatic axonal injury after closed head injury in the neonatal pig. J Neurotrauma. 2002;19(7):843–53. https://doi.org/10.1089/08977150260190438.
Umstead C, Barhorst A, Kasemsri T, Mitchell K. Modeling hypertension as a contributor to retinal hemorrhaging from abusive head trauma. J Healthc Eng. 2020;2020:4714927. https://doi.org/10.1155/2020/4714927.
Coats B, Binenbaum G, Peiffer RL, Forbes BJ, Margulies SS. Ocular hemorrhages in neonatal porcine eyes from single, rapid rotational events. Invest Ophthalmol Vis Sci (in English). 2010;51(9):4792–7. https://doi.org/10.1167/iovs.10-5211.
Kita M, Marmor M.F. Retinal adhesive force in living rabbit, cat, and monkey eyes: Normative data and enhancement by mannitol and acetazolamide. Invest Ophthalmol Vis Sci (in English). 1992;33(6):1879–82. Available: https://www.scopus.com/inward/record.uri?eid=2-s2.0-0026704265&partnerID=40&md5=9cddcf8c4af4b57e1910e488f18fe9b1.
Eucker S. Effect of head rotation direction on closed head injury in neonatal piglets. Dissertations available from ProQuest. 2009.
Eucker SA, Smith C, Ralston J, Friess SH, Margulies SS. Physiological and histopathological responses following closed rotational head injury depend on direction of head motion. Exp Neurol (in English). 2011;227(1):79–88. https://doi.org/10.1016/j.expneurol.2010.09.015.
Friess SH, et al. Neurobehavioral functional deficits following closed head injury in the neonatal pig. Exp Neurol (in English). 2007;204(1):234–43. https://doi.org/10.1016/j.expneurol.2006.10.010.
Friess SH, et al. Repeated traumatic brain injury affects composite cognitive function in piglets. J Neurotrauma (in English). 2009;26(7):1111–21. https://doi.org/10.1089/neu.2008.0845.
Ibrahim NG, Margulies SS. Biomechanics of the toddler head during low-height falls: An anthropomorphic dummy analysis - Laboratory investigation. J Neursurg Pediatr (in English). 2010;6(1):57–68. https://doi.org/10.3171/2010.3.PEDS09357.
Naim MY, et al. Folic acid enhances early functional recovery in a piglet model of pediatric head injury. Dev Neurosci (in English). 2011;32(5–6):466–79. https://doi.org/10.1159/000322448.
Raghupathi R, Mehr MF, Helfaer MA, Margulies SS. Traumatic axonal injury is exacerbated following repetitive closed head injury in the neonatal pig. J Neurotrauma (in English). 2004;21(3):307–16. https://doi.org/10.1089/089771504322972095.
Bonnier C, Mesples B, Carpentier S, Henin D, Gressens P. Delayed white matter injury in a murine model of shaken baby syndrome. Brain Pathol. 2002;12(3):320–8. https://doi.org/10.1111/j.1750-3639.2002.tb00446.x.
Bonnier C, Mesples B, Gressens P. Animal models of shaken baby syndrome: revisiting the pathophysiology of this devastating injury. Pediatr Rehabil. 2004;7(3):165–71. https://doi.org/10.1080/13638490410001703325.
Smith SL, Andrus PK, Gleason DD, Hall ED. Infant rat model of the shaken baby syndrome: preliminary characterization and evidence for the role of free radicals in cortical hemorrhaging and progressive neuronal degeneration. J Neurotrauma. 1998;15(9):693–705. https://doi.org/10.1089/neu.1998.15.693.
Smith SL, Hall ED. Tirilazad widens the therapeutic window for riluzole-induced attenuation of progressive cortical degeneration in an infant rat model of the shaken baby syndrome. J Neurotrauma. 1998;15(9):707–19. https://doi.org/10.1089/neu.1998.15.707.
Schiks LAH, Dankelman J, Loeve AJ. Thresholds for the assessment of inflicted head injury by shaking trauma in infants: a systematic review. Forensic Sci Intvol. 2020;306:110060. https://doi.org/10.1016/j.forsciint.2019.110060.
Pasquesi SA, Margulies SS. Failure and fatigue properties of immature human and porcine parasagittal bridging veins. Ann Biomed Eng. 2017;45(8):1877–89. https://doi.org/10.1007/s10439-017-1833-5.
Duhaime AC, Gennarelli TA, Thibault LE, Bruce DA, Margulies SS, Wiser R. The shaken baby syndrome. A clinical, pathological, and biomechanical study. J Neurosurg. 1987;66(3):409–15. https://doi.org/10.3171/jns.1987.66.3.0409.
Thibault KL, Margulies SS. Age-dependent material properties of the porcine cerebrum: effect on pediatric inertial head injury criteria. J Biomech. 1998;31(12):1119–26. https://doi.org/10.1016/s0021-9290(98)00122-5.
Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973;48(10):757–67. https://doi.org/10.1136/adc.48.10.757.
Ommaya AK, Goldsmith W, Thibault L. Biomechanics and neuropathology of adult and paediatric head injury. Br J Neurosurg. 2002;16(3):220–42. https://doi.org/10.1080/02688690220148824.
Goldsmith W, Plunkett J. A biomechanical analysis of the causes of traumatic brain injury in infants and children. Am J Forensic Med Pathol. 2004;25(2):89–100. https://doi.org/10.1097/01.paf.0000127407.28071.63.
Liu X, Wang L, Wang C, Sun G, Liu S, Fan Y. Mechanism of traumatic retinal detachment in blunt impact: a finite element study. J Biomech. 2013;46(7):1321–7. https://doi.org/10.1016/j.jbiomech.2013.02.006.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Kim Hutchinson: Conceptualization, methodology, data collection, analysis and interpretation of results, writing – original draft, visualization. Jan Peter Van Zandwijk: Conceptualization, methodology, writing – review & editing. Marloes E.M Vester: Conceptualization, methodology, writing – review & editing. Ajay Seth: Writing – review & editing, supervision. Rob A. C. Bilo: Conceptualization, methodology, writing – review & editing. Rick R. Van Rijn: Conceptualization, methodology, writing – review & editing. Arjo J. Loeve: Conceptualization, methodology, data collection, writing – review & editing, visualization, supervision. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval and informed consent
Not applicable to this systematic review.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hutchinson, K., van Zandwijk, J.P., Vester, M.E.M. et al. Modeling of inflicted head injury by shaking trauma in children: what can we learn?. Forensic Sci Med Pathol (2024). https://doi.org/10.1007/s12024-023-00765-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s12024-023-00765-5