Abstract
The term “cocoon syndrome” defines a sclerosing encapsulating peritonitis (SEP) that involves a chronic fibrotic inflammatory reaction of the parietal peritoneum and of the viscera leading to a complete sclerosis. The cocoon that is formed causes an incarceration of the intestinal loops with severe complications leading to high mortality. We are presenting the case of a 15-year-old young man that underwent surgery for appendectomy and that was evaluated for having a regular abdominal state. During the post-surgery period, however, several episodes of intestinal occlusion required further surgical interventions leading to a right hemicolectomy. The presence of a fibrotic-adhesive ligneous peritonitis with blended intestinal loops, severely thickened walls, and intestinal scaring stenosis was observed during his second surgical operation. A stenosis of the colostomy led to a worsening of the vital signs of the young man with the onset of a cardiac failure and subsequent decease. Macroscopic autopsy examination and histological analysis confirmed the severe obstructive adhesive encapsulating abdominal context allowing to trace back the cause of death to a cocoon syndrome. Since no predisposing factor could be found, we hypothesized that this case could be characterized by an excessive peritoneal reactivity due to surgical appendectomy. Cocoon syndrome is a rare pathology, and its microscopic features are seldomly observed and could be underestimated. We present a directly observed case with a very substantial macroscopic and microscopic context.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Case report
A Chinese 15-year-old young man suffering from epigastric and hypochondrium pain underwent appendectomy surgery with evidence of an inflamed appendix without other abdominal pathologies or adherences. A regular laparotomy intervention and post-surgery progresses led to discharge of the patient. Twenty-three days after, severe abdominal pain forced the adolescent to a new visit at the emergency room where an intestinal therapy-resistant obstruction was diagnosed. During a median laparotomy, an extensive serous effusion and a mechanic intestinal obstruction due to an encapsulating fibrous mass incorporating the loops could be observed with stenosis of the sigmoid intestine. Omentum-parietal and colon-parietal adhesions were lysed with a right hemicolectomy. Microscopic examination on surgical specimens highlighted a ligneous fibrous-adhesive ileum-colon peritonitis with non-necrotizing granulomatous chronic inflammation. Biomarkers and tumoral markers as carcinoembryonic antigen (CEA), carbohydrate antigen (Ca 19-9), and α-fetoprotein (AFP) were normal. Culturing of the peritoneal liquid sampled during surgery resulted to be negative.
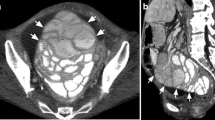
After 10 days, severe peristaltic pain emerged associated to a globose and non-treatable abdomen. Abdominal x-rays (RX) highlighted the bloating of intestinal loops with air-fluid levels. Computed tomography (CT) scan with contrast medium showed a peritoneum markedly thickened and proximal loops of the small intestine severely dilated. Abnormalities in the contour and caliber of the loops were also observed which appeared tangled in bowel loops and encapsulated within a thick pouch. These findings made it necessary a new surgical operation. A further median xiphoid-pubic laparotomy was assessed leading to a intraoperative diagnosis of fibrotic-adhesive ligneous peritonitis with blended intestinal loops, thickened walls, scarring stenosis, and fibrous-sclerotic inflammation of a part of the descending colon. A colostomy was performed after removal of the visceral-parietal and visceral-visceral adhesions. Routine laboratory tests revealed a hemoglobin of 13.1 g/dL; a total leukocyte count of 10,600 cells/mL; and normal serological tests, protein electrophoresis, and urine tests. Mantoux testing, research for fecal parasites (Amoeba and Giardia), blood cultures, IgE immunoglobulin assay (relative to soy, brown rice, peas, peanuts, hazelnuts and almonds), autoantibodies (anti-nuclei, anti-mitochondrial, anti-smooth muscle, anti-microsomal, anti-DNA, anti-extractable nuclear antigens) and complement testing, cytological examination of the peritoneal lavage fluid, and anti-cytomegalovirus antibody dosing were performed, all of which resulted normal. In a few days, a stenosis of the colostomy associated with abdomen closed to feces, non-functioning preternatural anus, and repeated episodes of biliary vomiting took place. Pathologically increased values of hepatic necrosis indices were recorded, as in aspartate aminotransferase (GOT), alanine aminotransferase (GPT), ɣ-glutamyl transferase (GGT), and both total and direct bilirubin, with the onset of jaundice. A worsening of the vital parameters took place with the onset of cardio-circulatory failure, gasping, and death. The inquiring magistrate ordered a judicial autopsy examination 2 days after the event to establish the cause of death.
At autopsy examination, a regularly conformed brain, congested lungs with bilateral subpleural petechiae, citrine pleural effusion (120 cc to the right, 135 cc to the left), and a normal heart (weight 225 g) could be noted.
During examination of the abdomen, autopsy highlighted tenacious peritoneal-visceral adhesions, connecting the parietal peritoneum to the underlying visceral mass in the absence of liquid effusion. It was also noted a tenacious fibrotic mass engulfing the intestine (Fig. 1) uniting the intestinal loops to each other and to the omentum and with the parietal peritoneum that appeared thickened with a maximum thickness of 5 cm; intestinal walls, markedly compressed, showed a diffused stenosed lumen (Fig. 2). Overall, it was impossible to untangle the intestinal skein. The liver (weight of 1085 g) appeared yellowish with an irregular surface due to multiple diffuse adhesions of yellowish-brown color; the spleen and kidneys turned out as normal.
Microscopic examination of the mesenterial small intestine documented marked proliferation of collagen fibers with sclerosis in the subserous adipose tissue and thickening of the serous tissue (Fig. 3). Interstitial fibrous shoots were also observed that connected the adjacent intestinal loops and, in the context of sclerosis, focal lymphocyte inflammatory infiltrates and rare capillaries were present (Fig. 4), as well as non-caseous granulomas and rare giant cells (Fig. 5). Occasional aggregates of foamy cytoplasm macrophages, focal areas of fibrin, and serous cellular debris in the serous site were also found in subserous adipose tissue. At last, the cause of death was identified in an obstructive, progressive, and encapsulating adhesional abdominal event, also known as cocoon syndrome.
Microscopic view on the left (H&E, 50×) of a marked fibrous proliferation arranged in occasionally parallel bundles that replaces mesenteric fat and infiltrates up to the villi, with a detail in the oval; on the right (H&E, 100×), extensive fibrous proliferation with a detail (H&E, 400×) of fibroblasts that are sometimes enclosed in acellular and scar sclerotic areas
Microscopic views on the left (H&E, 50×) of peritoneal fibrosis infiltrating and encasing abdominal adipose tissue; the higher magnification detail (H&E, 400×) shows the presence of fibroblasts in the context of the marked fibrosis. On the right (H&E, 50×), the transition zone of adipose and muscle tissue with the presence of focal inflammatory infiltrates shown in the rectangle and visible in the higher magnification detail (H&E, 400×)
Microscopic view on the left (H&E, 100×) of the marked and compact peritoneal fibrosis infiltrating up to the abdominal muscles, encompassing them; the detail in the rectangle is shown on the right at higher magnification (H&E, 400×) with evidence of multinucleated giant cells in the context of the widespread and recent fibrosis with abundant fibroblasts
Discussion
By cocoon syndrome, we mean a sclerosing encapsulating peritonitis (SEP) (also described as sclerosing obstructive peritonitis, encapsulating peritonitis, obliterative adhesive peritonitis) including several heterogeneous morbid conditions [1, 2]. The disease was first described in 1978 [3] in adolescent of Asian ethnicity (Chinese and Malay) aged between 13 and 18 years, all with intestinal occlusion symptoms [4]. It is mainly found in Asia [5], and occasionally in other parts of the world: Africa [6], Europe [7], Australia [8], and America [9].
It is possible to classify SEPs into a primary or idiopathic form and a secondary form [10] even though etiology remains unknown [11]. In the first case, recent studies [10, 12] have identified possible predisposing factors (mostly immune and genetic, to date not fully clarified), while for the secondary form, with greater incidence, numerous predisposing conditions have been identified such as the use of drugs and substances [13], previous abdominal surgery and medical procedures [14, 15], infections [16], inflammatory [17], and autoimmune diseases [18], as well as other pathologies [19, 20].
Regardless of the triggering cause, the forms of SEP are characterized by the appearance of a chronic fibrotic inflammatory reaction of the parietal and visceral peritoneum [21], leading to complete sclerosis. The thickened peritoneum encapsulates, binds, and forces the intestinal loops of the small intestine, to different degrees, into a single and extensive sclerotic fibrous membrane [9, 22]. It gives an encapsulating cocoon appearance, which can partially or completely involve the remaining abdominal viscera as well. The cocoon is characterized by an abnormal thickening of the peritoneal membrane varying from several hundreds to over 4000 µm [23]. It seems to develop from the connective tissue and is classified into three types, in relation to the degree of intestinal incorporation: type 1 when it incorporates part of the small intestine; type 2 when it incorporates the intestine in its totality; and type 3, when it extends incorporating also other organs [24]. Histologically, it consists of a thick layer of fibrous tissue, focally vascularized and with focal areas of lymphoplasmacellular infiltrates arranged on the intestinal peritoneum, with mild fibrinous deposition [23]. This may result in severe complications such as peritonitis and intestinal occlusion [25] with high mortality [26]. Being a rare pathology and lacking of specific symptoms, it is very difficult to diagnose, and is often randomly detected in abdominal surgeries, performed for other indications [27]. However, its early identification allows to treat those affected conservatively, while in advanced cases with extensive intestinal obstruction [10], although surgery is considered the gold standard therapeutic approach, the results are often unsatisfactory. In fact, postoperative intestinal obstructions, new surgical adhesions [12], and secondary damage, such as necrosis, intestinal perforations, enterocutaneous fistulas, and sepsis, may occur [28]. On this topic, in fact, a scientific debate with a lack of consensus on the indications, the optimal timing of execution, and the method of intervention [10] is still undergoing.
Not only the therapeutic approach but also the diagnostic framework of cocoon syndrome requires attention, being necessary an accurate differential diagnosis with other pathologies, first of all those granulomatous, such as tuberculosis and sarcoidosis. In fact, they too are able to determine peritoneal resentment [29]. As for tuberculosis, it can be located in the abdominal area and the area most commonly involved is the ileocecal region. This is a more frequent finding in tropical regions [30], although rare to observe [31]. Tuberculosis when localized to the peritoneum may result in peritoneal adhesions and fibrosis similar to those observed in cocoon syndrome. In fact, a membranous structure surrounding the small intestine whose loops are partially or completely trapped inside it can be observed, with possible involvement of lymph nodes and visceral organs such as the pancreas and liver [32]. In these cases, the mortality is very high. The diagnosis of abdominal tuberculosis associated with cocoon syndrome is often complicated by the difficult detection of the microbiological agent within the voluminous fibrotic mass. However, the Mantoux skin test and the detection of caseous granulomas on histological examination are of great diagnostic importance [30]. If abdominal tuberculosis, in addition to causing cocoon syndrome, also presents a pulmonary localization, the microbiological examination for the search for the tuberculous bacillus on the sputum is decisive [33]. Finally, an association between female genital pelvic inflammatory disease and cocoon syndrome-like picture has also been described in the literature [34].
With regard to sarcoidosis, a unique association of sclerosing peritonitis has been described in the literature with the presence on histological examination of non-caseous epithelioid granulomas found in a context of peritoneal fibrosis [35].
A significant aspect to underline concerns the fact that the correct diagnosis and interpretation of cocoon syndrome can have great relevance even in pathological-forensic areas. First of all, it is a pathology that can have a subtle onset and a relapsing course and be chronically progressive and worsening especially in patients with a history of previous abdominal trauma and repeated peritoneal surgeries (postoperative traumatization). On the basis of this evidence in the literature, any forensic judgments on the causal link and on possible profiles of medical malpractice [36, 37] should be formulated. Another forensic reality concerns the possible relationship between cocoon syndrome and the battered person syndrome especially in children and women. In fact, severe abdominal trauma consisting of ruptures of the small intestine, colon, and viscera such as the pancreas, with shock, biliary vomiting, and unexplainable peritonitis can result from mistreatment and physical abuse [38]. Pictures similar to cocoon syndrome can also be observed in different contexts such as body packing. It is an illicit activity of smuggling drugs into rubber or plastic containers introduced into the gastrointestinal tract of carriers. Ingestion of these foreign bodies can expose to numerous risks including respiratory failure, acute intoxication in case of container breakage, and death. As for the abdomen specifically, intestinal obstructions may arise with slowing down of the passage of foreign bodies and their accumulation, as well as intestinal perforations of different severity and peritonitis characteristics [39, 40]. In fact, in general, intra-abdominal fibrogenic foreign bodies can induce encapsulating fibrotic reactions very similar to cocoon syndrome [41]. In the fibrous tissue surrounding foreign bodies, the main constituent cells are fibroblasts, macrophages, and vascular cells, which lead to the accumulation of extracellular matrix with subsequent tissue remodeling [42].
Finally, cocoon syndrome, being able to have a subtle and pauci-symptomatic course, can result in unexpected and sudden death. Therefore, those who die for this cause is frequent to come to the attention of a forensic pathologist. It is therefore essential that the suspicion emerges that these may be less common pathological forms, which require appropriate differential laboratory diagnoses. In particular, cocoon syndrome is a very rare finding and, in this context, the significant images we reported also aim to provide illustrations that can help forensic pathologists to know to be able to recognize truly unusual macroscopic pictures.
In the current case, clinical and forensic findings documented an extensive chronic inflammatory reaction of the peritoneum, resulting in fibrous adhesions, with modest inflammatory cellular component and rare granulomatous formations. This evidence has made it possible to make a diagnosis of abdominal cocoon syndrome. Regarding the mode of onset, a tumultuous evolution of the pathology was observed immediately after the appendectomy surgery, during which the abdominal findings were completely normal except for an inflamed appendix. However, in a few weeks, there was a situation of massive incarceration of the intestinal loops by a fibrotic reaction of the peritoneum, not resolved even by two further surgeries. Given the absence of predisposing factors, we hypothesized that it was a case characterized by abnormal peritoneal reactivity following appendectomy surgery. Laparotomic surgical accesses could have been the trigger of the very rapidly evolving sclerogenic process, characterized by increasingly severe relapses following subsequent surgical interventions, until the death of the patient.
Data availability
All the data have been reported in the manuscript.
Code availability
Not applicable
Change history
14 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12024-023-00604-7
References
Chew MH, Sophian H, Chan G, Ong HS, Wong WK. A problem encapsulated: the rare peritoneal encapsulation syndrome. Singap Med J. 2006;47:808–10. PMID: 16924364.
Dutranoy JC, Molle G. Sclerosing peritonitis. J Chir (Paris). 2005;142:78–84. https://doi.org/10.1016/s0021-7697(05)80854-3.
Foo KT, Ng KC, Rauff A, Foong WC, Sinniah R. Unusual small intestinal obstruction in adolescent girls: the abdominal cocoon. Br J Surg. 1978;65:427–30. https://doi.org/10.1002/bjs.1800650617.
Yeniay L, Karaca CA, Calışkan C, Fırat O, Ersin SM, Akgün E. Abdominal cocoon syndrome as a rare cause of mechanical bowel obstruction: report of two cases. Ulus Travma Acil Cerrahi Derg. 2011;17:557–60. https://doi.org/10.5505/tjtes.2011.39018.
Masuda C, Fujii Y, Kamiya T, Miyamoto M, Nakahara K, Hattori S, et al. Idiopathic sclerosing peritonitis in a man. Intern Med. 1993;32:552–5. https://doi.org/10.2169/internalmedicine.32.552.
Marinho A, Adelusi B. The abdominal cocoon - case report. Br J Obstet Gynecol. 1980;87:249–50. https://doi.org/10.1111/j.1471-0528.1980.tb04529.x.
Garosi G, Di Paolo N. Peritoneal sclerosis: one or two nosological entities? Semin Dial. 2000;13:297–308. https://doi.org/10.1046/j.1525-139x.2000.00080.x.
Le C, Pitcher ME, Jones T. The abdominal cocoon. Aust NZ J Surg. 1996;66:777. https://doi.org/10.1111/j.1445-2197.1996.tb00744.x.
Cambria RP, Shamberger RC. Small bowel obstruction caused by the abdominal cocoon syndrome: possible association with the LeVeen shunt. Surgery.1984;95:501-3. PMID: 6710346.
Akbulut S. Accurate definition and management of idiopathic sclerosing encapsulating peritonitis. World J Gastroenterol. 2015;21:675–87. https://doi.org/10.3748/wjg.v21.i2.675.
Machado NO. Sclerosing encapsulating Peritonitis: review. Sultan Qaboos Univ Med J. 2016;16:e142-51. https://doi.org/10.18295/squmj.2016.16.02.003.
Li N, Zhu W, Li Y, Gong J, Gu L, Li M, et al. Surgical treatment and perioperative management of idiopathic abdominal cocoon: single-center review of 65 cases. World J Surg. 2014;38:1860–7. https://doi.org/10.1007/s00268-014-2458-6.
Brown P, Baddeley H, Read AE, Davies JD, McGarry J. Sclerosing peritonitis, an unusual reaction to a beta-adrenergic-blocking drug (practolol). Lancet. 1974;21:2:1477–81. https://doi.org/10.1016/s0140-6736(74)90218-9.
Nomoto Y, Kawaguchi Y, Kubo H, Hirano H, Sakai S, Kurokawa K. Sclerosing encapsulating peritonitis in patients undergoing continuous ambulatory peritoneal dialysis: a report of the japanese sclerosing encapsulating Peritonitis Study Group. Am J Kidney Dis. 1996;28:420–7. https://doi.org/10.1016/s0272-6386(96)90501-6.
Veech RL, Gitomer WL. The medical and metabolic consequences of administration of sodium acetate. Adv Enzyme Regul. 1988;27:313–43. https://doi.org/10.1016/0065-2571(88)90024-6.
Puppala R, Sripathi S, Kadavigere R, Koteshwar P, Singh J. Abdominal cocoon secondary to disseminated tuberculosis. BMJ Case Rep. 2014;19:1–3. https://doi.org/10.1136/bcr-2013-202568.
Kayastha K, Mirza B. Abdominal cocoon simulating acute appendicitis. APSP J Case Rep. 2012;3:8. PMID: 22953302.
Yavuz R, Akbulut S, Babur M, Demircan F. Intestinal obstruction due to idiopathic sclerosing encapsulating peritonitis: a Case Report. Iran Red Crescent Med J. 2015;31:17:e21934. https://doi.org/10.5812/ircmj.17(5)2015.21934.
Shah J, Kumar A, Singh H, Agarwala R, Sharma V, Rana SS. Cocoon carcinomatosa: an unusual cause of intestinal obstruction. Drug Discov Ther. 2017;13:51–3. https://doi.org/10.5582/ddt.2017.01003.
Wang YZ, King H, Diebold A. Cocoon formation in patients with midgut neuroendocrine tumors: a rare and unrecognized final pathway. Pancreas. 2013;42:944–8. https://doi.org/10.1097/MPA.0b013e318287ce77.
Dequanter D, Lefebvre JC, De Pauw L, Nortier J, Kinnaert P. Sclerosing peritonitis: report of three cases. Acta Chir Belg. 2003;103:408–11. https://doi.org/10.1080/00015458.2003.11679454.
Al-Abassi AA, Emad M. Abdominal cocoon. an unusual cause of intestinal obstruction. Saudi Med J. 2004;25:1482-5. PMID: 15494828.
Di Paolo N, Garosi G. Peritoneal sclerosis. J Nephrol. 1999;12:347–61. PMID: 10626824.
Tannoury JN, Abboud BN. Idiopathic sclerosing encapsulating peritonitis: abdominal cocoon. World J Gastroenterol. 2012;18:1999–2004. https://doi.org/10.3748/wjg.v18.i17.1999.
Samarasam I, Mathew G, Sitaram V, Perakath B, Rao A, Nair A. The abdominal cocoon and an effective technique of surgical management. Trop Gastroenterol. 2005;26:51–3. PMID: 15974242.
Kumar M, Deb M, Parshad R. Abdominal cocoon: report of a case. Surg Today. 2000;30:950–3. https://doi.org/10.1007/s005950070053.
Fei X, Yang HR, Yu PF, Sheng HB, Gu GL. Idiopathic abdominal cocoon syndrome with unilateral abdominal cryptorchidism and greater omentum hypoplasia in a young case of small bowel obstruction. World J Gastroenterol. 2016;22:4958–62. https://doi.org/10.3748/wjg.v22.i20.4958.
Carcano G, Rovera F, Boni L, Dionigi G, Uccella L, Dionigi R. Idiopathic sclerosing encapsulating peritonitis: a case report. Chir Ital. 2003;55:605-8. PMID: 12938612.
Chorti A, Panidis S, Konstantinidis D, Cheva A, Papavramidis T, Michalopoulos A, Paramythiotis D. Abdominal cocoon syndrome: rare cause of intestinal obstruction-case report and systematic review of literature. Med (Baltim). 2022;101:e29837. https://doi.org/10.1097/MD.0000000000029837.
Sharma V, Singh H, Mandavdhare HS. Tubercular abdominal cocoon: systematic review of an uncommon form of tuberculosis. Surg Infect (Larchmt). 2017;18:736–41. https://doi.org/10.1089/sur.2017.110.
Singh AU, Subedi SS, Yadav TN, Gautam S, Pandit N. Abdominal cocoon syndrome with military tuberculosis. Clin J Gastroenterol. 2021;14:577–80. https://doi.org/10.1007/s12328-021-01344-3.
Sharma MP, Bhatia V. Abdominal tuberculosis. Indian J Med Res. 2004;120:305–15. PMID: 15520484.
Sharma V, Mandavdhare HS, Rana SS, Singh H, Kumar A, Gupta R. Role of conservative management in tubercular abdominal cocoon: a case series. Infection. 2017;45:601–6. https://doi.org/10.1007/s15010-017-1012-5.
Lalloo S, Krishna D, Maharajh J. Case report: abdominal cocoon associated with tuberculous pelvic inflammatory disease. Br J Radiol. 2002;75:174–6. https://doi.org/10.1259/bjr.75.890.750174.
Ngô Y, Messing B, Marteau P, Nouël O, Pasquiou A, Lavergne A, Rambaud JC. Peritoneal sarcoidosis. An unrecognized cause of sclerosing peritonitis. Dig Dis Sci. 1992;37:1776–80. https://doi.org/10.1007/BF01299875.
Awe JA. Abdominal cocoon syndrome (idiopathic sclerosing encapsulating peritonitis): how easy is its diagnosis preoperatively? A case report. Case Rep Surg. 2013;2013:604061. https://doi.org/10.1155/2013/604061.
Kirshtein B, Mizrahi S, Sinelnikov I, Lantsberg L. Abdominal cocoon as a rare cause of small bowel obstruction in an elderly man: report of a case and review of the literature. Indian J Surg. 2011;73:73–5. https://doi.org/10.1007/s12262-010-0200-7.
Ogata M, Tsuganezawa O. An isolated perforation of the jejunum caused by child abuse. A case report. Am J Forensic Med Pathol. 1995;16:17–20. https://doi.org/10.1097/00000433-199503000-00003.
Hutchins KD, Pierre-Louis PJ, Zaretski L, Williams AW, Lin RL, Natarajan GA. Heroin body packing: three fatal cases of intestinal perforation. J Forensic Sci. 2000;45:42–7. PMID: 10641918.
Henebiens M, van Geloven AA, Gouma DJ. Diagnostiek en behandeling bij ‘body-packer’-syndroom [Diagnosis and treatment of ‘body packer’ syndrome]. Ned Tijdschr Geneeskd. 2007;151:1868–73. Dutch. PMID: 17902559.
Araújo JAB, Martines JADS, Martines BMR, da Silva AF, Lovisolo SM, de Castro CC. Idiopathic sclerosing encapsulating peritonitis: an uncommon cause of intestinal obstruction. Autops Case Rep. 2012;2:51–6. https://doi.org/10.4322/acr.2012.026.
Katou F, Ohtani H, Nagura H, Motegi K. Procollagen-positive fibroblasts predominantly express fibrogenic growth factors and their receptors in human encapsulation process against foreign body. J Pathol. 1998;186:201–8. https://doi.org/10.1002/(SICI)1096-9896(1998100)186:2%3c201::AID-PATH157%3e3.0.CO;2-C.
Author information
Authors and Affiliations
Contributions
TS and GG equally contributed to this work. They devised the project and the main conceptual idea of the article, collected data, drafted the manuscript, and performed literature research. BM contributed to the sample collection, investigation, and methodology. ZR contributed to analysis interpretation, literature research, and editing. GA, guarantor of the project and directed the study, devised the main conceptual idea of the article.
Corresponding author
Ethics declarations
Ethics approval
This study was performed from data from human cadavers. This article does not contain any studies with (living) human participants or animals performed by any of the Authors. The subject involved in this study underwent a judicial autopsy at the Institute of Legal Medicine of Milan in order to identify the cause of death. Data collecting, sampling and subsequent forensic analysis were authorized by the public prosecutor. Therefore data were acquired as part of a forensic judicial investigation and in accordance to Italian Police Mortuary Regulation. Consequently, in accordance with Italian law, ethical approval is not required in these cases. Publication of data is allowed when the case has been closed, but the anonymity of the subject must be guaranteed.
Consent to participate
The authors declared that all the investigations were carried out accordingly to the Italian Law.
Consent for publication
All the authors agree for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tambuzzi Stefano and Gentile Guendalina are the first co-author.
Authors have realized that on Pubmed the citation string of their is incorrect and names have been inverted with surnames. Currently the citation string is as follows: Stefano T, Guendalina G, Michele B, Riccardo Z, Andrea G. A forensic case of abdominal cocoon syndrome. Forensic Sci Med Pathol. 2022 Dec 2. doi: 10.1007/s12024-022-00562-6. Epub ahead of print. PMID: 36459388. But it is wrong. Here is the correct first and last names: Stefano (first name) Tambuzzi (surname) Guendalina (first name) Gentile (surname) Michele (first name) Boracchi (surname) Riccardo (first name) Zoja (surname) Andrea (first name) Gentilomo (surname)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tambuzzi, S., Gentile, G., Boracchi, M. et al. A forensic case of abdominal cocoon syndrome. Forensic Sci Med Pathol 19, 273–279 (2023). https://doi.org/10.1007/s12024-022-00562-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12024-022-00562-6