Abstract
We report an unusual case of a 15-month old previously healthy girl who died of pneumococcal septicemia in the background of visceral heterotaxy with polysplenia. Heterotaxy can also present with asplenia whereas polysplenia cases usually present with functional asplenia. Of particular note, this girl received the 13-valent pneumococcal conjugate vaccine as recommended by the Centers for Disease Control and Prevention in the routine pediatric immunization schedule used in the USA and Canada. Unfortunately, although the strain causing death (serotype 22F) is not contained in Prevnar 13®, it is in the 23-valent pneumococcal polysaccharide vaccine (e.g. Pneumovax 23®), currently suggested only for immunocompromised children age 2 with either functional or anatomic asplenia. This syndrome has the potential of being diagnosed prenatally. The intent of our case report is to raise awareness of the syndrome, highlight that heterotaxy patients with polysplenia are at danger for infections with encapsulated organism, such as pneumococcus, meningococcus, and Haemophilus influenza amongst others due to functional asplenia, recommend the 23-valent pneumococcal polysaccharide vaccine for these children before age two for the outlined reasons, and illustrate that with early diagnosis of the heterotaxy syndrome, and early diagnosis and treatment of septic complications, the morbidity or death of young children with heterotaxy syndrome can likely be reduced or prevented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
We report a case of a 15-month old child who died suddenly and unexpectedly of Streptococcus pneumoniae sepsis. Autopsy revealed the incidental finding of visceral heterotaxy with polysplenia. Pneumococcus serotype (22F) was isolated from lung and spleen tissue. This girl had received a vaccine currently suggested for infants in the USA and Canada to prevent pneumococcal infections (PCV-13; e.g. Prevnar 13®), but serotype 22F is only part of the PPSV23 vaccine (e.g. Pneumovax 23®) recommended for older children and adults with immune compromise. Awareness of the syndrome and associated risks as well as right treatment with possible early diagnosis in utero will aid in preventing infections from possibly fatal encapsulated organisms in this susceptible patient group.
Case report
History
The decedent was a 15-month old female child who was previously healthy and fully immunized. She lived with her parents and a 3-year old sibling in a small town in Southern Ontario and was seen regularly by a Pediatrician. In early June 2015, she developed a fever and mild upper respiratory symptoms. Her parents took her to the local hospital where she was assessed and sent home with instructions for fever control. Her condition remained the same during the next day, however, in the early morning of the following day, the girl looked very ill with signs of respiratory distress. Her parents returned her to the local hospital, but she became unconscious in the car about 5 min from the hospital. Aggressive cardiopulmonary resuscitation was unsuccessful.
Pathological, microbiological and imaging findings
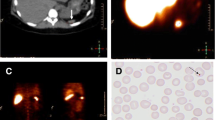
At autopsy, the decedent was a well-nourished and normally developed 15-month old girl with appropriate growth parameters. Internal examination revealed visceral heterotaxy involving the heart, lungs, liver, spleen and abdominal organs (see Figs. 1, 2 and 3). The lungs showed three lobes on the left side and two lobes on the right without evidence of pneumonia or other abnormalities. The heart was enlarged (93 g; normal for age: 45–48 g) and showed evidence of dextrocardia (see Figs. 1 and 2) and situs inversus with a morphologic right atrium present on the left side of the heart. There were no septal defects. Concordant atrioventricular and ventriculoarterial connections were observed. Regional non-compaction was present within the apical third of left ventricular myocardium consistent with focal left ventricular non-compaction cardiomyopathy. A morphologically normal liver was in the “mirror image” position on the left. The splenic tissue consisted of six splenules (nodules) which coalesced to form the organ with a combined weight normal for age (23 g; see Fig. 3). The intestines were normally arranged. The remainder of the internal exam was unremarkable.
Gross anatomy of the thoracic and abdominal cavity. The heart is located in the right chest (dextrocardia) and the lungs and liver show situs inversus (the multiple splenules detected in this girl are not shown as these are located posteriorly at the level of the kidneys (see Fig. 3)
Microscopic examination showed features consistent with sepsis. The heart demonstrated foci of incipient neutrophilic myocarditis in the right ventricle. The lungs showed patchy edema and intra-alveolar hemorrhage. The kidneys revealed marked and extensive microvascular thrombosis represented by fibrin microthrombi involving most of the glomeruli capillary loops associated with abundant cocci bacteria (see Fig. 4). Within the adrenals, there were incipient acute medullary hemorrhages. The thymus showed moderate to severe acute stress effects. The upper respiratory tract showed mild to moderate mononuclear cell infiltrates within the mucosa without evidence of acute inflammation. Within the intestines, there were scattered peri-vascular acute hemorrhages of the lamina propria and sub-mucosa in the colon. The splenules showed unremarkable splenic pulp.
Streptococcus pneumoniae serotype 22F was grown from lung and spleen tissue. Virologic studies revealed rhinovirus DNA in the nasopharynx.
Postmortem whole body computed tomography and magnetic resonance imaging showed visceral heterotaxy with the heart being positioned in the right chest with its apex pointing towards the right flank (see Fig. 1), a bilobed right and trilobed left lung, a liver situated on the left side of the body and a spleen composed of six small nodules (so-called splenules) within the right upper quadrant of the abdomen (see Fig. 3).
Discussion
Heterotaxy syndrome is a rare and sporadic disorder, but also may be familial [1]. This syndrome presents with a broad variety of clinical pictures, including complex malformations of abdominal organs, especially splenic abnormalities [2, 3]. Heterotaxy is defined as an incomplete lateralization disorder that results in any arrangement of the viscera across the left-right axis that differs from complete situs solitus or complete situs inversus in utero [4]. Due to failure of the developing embryo to establish normal left-right asymmetry, the thoraco-abdominal organs and vessels are usually malpositioned, leading to complex congenital heart disease and extracardiac defects involving midline-associated structures. The spleen is almost always affected, and there is syndromic clustering of the malformations corresponding to the type of splenic abnormality present [5].
Noncompaction cardiomyopathy (NCCM) has been reported in association with heterotaxy [6, 7]. Noncompaction of ventricular myocardium is a rare congenital cardiomyopathy. It is usually associated with congenital left or right-ventricular outflow tract obstruction, and rarely detected as an isolated anomaly [1]. NCCM may occur alone or associated with other congenital defects and results from failure in the process of compaction of myocardial fibers, resulting in the persistence of trabeculations and deep recesses that communicate with the ventricular cavity [8]. This case demonstrated regional noncompaction in the apex of the left heart.
A large number of patients with heterotaxy syndrome develop sepsis, with both polysplenia and asplenia, despite appropriately prescribed antibiotic prophylaxis [9]. Pediatric pneumococcal immunization was introduced in 2000 and is crucial in visceral heterotaxy patients, as conjugated pneumococcal vaccination has successfully prevented pneumococcal sepsis [9]. The 13-valent pneumococcal conjugate vaccine (PCV13; e.g. Prevnar 13®) is now routinely included in the pediatric vaccination schedule in the USA and Canada at 2 months, 4 months and 12 months age and targets 13 different Streptococcus pneumoniae strains [10]. The 23-valent pneumococcal polysaccharide vaccine (PPSV23; e.g. Pneumovax 23®) includes 23 strains. The vaccine antigens cover approximately 87% of studied bacteremic cases and most penicillin-resistant serotypes and is currently recommended for all adults 65 years or older and people 2 to 64 years old who are considered at high risk of pneumococcal disease, which would include children with functional or anatomic asplenia [9, 11, 12].
The risk of overwhelming sepsis is highest in young infants with functional or anatomic asplenia and perhaps decreases with age, though systematic data are not available [13, 14]. The fatality rates of overwhelming sepsis are high (40–50%) despite the use of antibiotics, and some of the reported patients were receiving prophylactic antibiotics as well. This suggests that sepsis should be recognized earlier and underscores the need for patient education [15], early diagnosis and likely change in recommendations in the use of pneumococcal vaccination in patients with visceral heterotaxy, as the predisposition to sepsis in functionally and anatomically asplenic individuals has been well-documented [16,17,18,19,20,21]. Antibiotic prophylaxis and immunization against Haemophilus influenzae and Streptococcus pneumoniae are critical adjuncts in the prevention of sepsis in patients with functional asplenia [22, 23]. In this regard, our patient was vaccinated against pneumococcus by routine childhood vaccination, but the pneumococcus strain which was responsible for her sepsis was not included in the 13-valent pneumococcus conjugate vaccine Prevnar 13®. It is covered in the 23-valent pneumococcus vaccine Pneumovax 23®, which is currently only recommended for children with functional asplenia after age 2. This girl was 15-months old and her heterotaxy syndrome was undiagnosed.
Key points
-
1.
Heterotaxy syndrome is a rare syndrome which can present with asplenia or polysplenia.
-
2.
Heterotaxy syndrome features anatomical or functional asplenia (polysplenia subset).
-
3.
Heterotaxy syndrome is associated with an increased risk of infection with polysaccharide encapsulated bacteria, such as Haemophilus influenzae type b (Hib), Streptococcus pneumoniae (pneumococcus), Neisseria meningitidis (meningococcus), Group B streptococcus (GBS), Klebsiella pneumonia, and Salmonella typhi.
-
4.
We suggest vaccination with the 23-valent pneumococcal polysaccharide vaccine (e.g. Pneumovax 23®), currently recommended only for immunocompromised children age 2 and older with either functional or anatomic asplenia, in lieu of the 13-valent pneumococcal conjugate vaccine routinely given at 2 months, 4 months and 12 months.
References
Wessels MW, De Graaf BM, Cohen-Overbeek TE, Spitaels SE, de Groot-de Laat LE, Ten Cate FJ, et al. A new syndrome with noncompaction cardiomyopathy, bradycardia, pulmonary stenosis, atrial septal defect and heterotaxy with suggestive linkage to chromosome 6p. Hum Genet. 2008;122:595–603.
Phoon CK, Neill CA. Asplenia syndrome: insight into embryology through an analysis of cardiac and extracardiac anomalies. Am J Cardiol. 1994;73:581–7.
Tatar IG, Ergun O, Altunoglu H, Tatar T. Heterotaxia associated with polysplenia. BMJ Case Rep. 2014;2014.
Bowers PN, Brueckner M, Yost HJ. The genetics of left-right development and heterotaxia. Semin Perinatol. 1996;20:577–88.
Bartram U, Wirbelauer J, Speer CP. Heterotaxy syndrome -- asplenia and polysplenia as indicators of visceral malposition and complex congenital heart disease. Biol Neonate. 2005;88:278–90.
Celik M, Amasyali B, Celik T, Iyisoy A. Heterotaxy syndrome: noncompaction ventricular myocardium and cardiac conduction disturbance. J Cardiovasc Med (Hagerstown). 2012;13:403–5.
Goncalves LF, Souto FM, Faro FN, Mendonca Rde C, Oliveira JL, Sousa AC. Dextrocardia with situs inversus associated with non-compaction cardiomyopathy. Arq Bras Cardiol. 2013;101:e33–6.
Oechslin E, Jenni R. Left ventricular non-compaction revisited: a distinct phenotype with genetic heterogeneity? Eur Heart J. 2011;32:1446–56.
Prendiville TW, Barton LL, Thompson WR, Fink DL, Holmes KW. Heterotaxy syndrome: defining contemporary disease trends. Pediatr Cardiol. 2010;31:1052–8.
Pneumococcal Vaccines (PCV13 and PPSV23). Available from http://www.immunize.org/askexperts/experts_pneumococcal_vaccines.asp.
Pneumococcal Vaccination. Available from http://www.cdc.gov/pneumococcal/vaccination.html.
Nuorti JP, Whitney CG, Centers for Disease Control and Prevention. Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1–18.
Kothari SS. Non-cardiac issues in patients with heterotaxy syndrome. Ann Pediatr Cardiol. 2014;7:187–92.
Melles DC, de Marie S. Prevention of infections in hyposplenic and asplenic patients: an update. Neth J Med. 2004;62:45–52.
Chiu SN, Shao PL, Wang JK, Chen HC, Lin MT, Chang LY, et al. Severe bacterial infection in patients with heterotaxy syndrome. J Pediatr. 2014;164:99–104e1.
Biggar WD, Ramirez RA, Rose V. Congenital asplenia: immunologic assessment and a clinical review of eight surviving patients. Pediatrics. 1981;67:548–51.
Dyke MP, Martin RP, Berry PJ. Septicaemia and adrenal haemorrhage in congenital asplenia. Arch Dis Child. 1991;66:636–7.
Nagel BH, Williams H, Stewart L, Paul J, Stumper O. Splenic state in surviving patients with visceral heterotaxy. Cardiol Young. 2005;15:469–73.
Schutze GE, Mason EO Jr, Barson WJ, Kim KS, Wald ER, Givner LB, et al. Invasive pneumococcal infections in children with asplenia. Pediatr Infect Dis J. 2002;21:278–82.
Waldman JD, Rosenthal A, Smith AL, Shurin S, Nadas AS. Sepsis and congenital asplenia. J Pediatr. 1977;90:555–9.
Wu MH, Wang JK, Lue HC. Sudden death in patients with right isomerism (asplenism) after palliation. J Pediatr. 2002;140:93–6.
Pickering LK, Baker CJ, Kimberlin DW, Long SS. Red book: 2009 report of the committee on infectious diseases. 28th ed; 2009.
Spelman D, Buttery J, Daley A, Isaacs D, Jennens I, Kakakios A, et al. Guidelines for the prevention of sepsis in asplenic and hyposplenic patients. Intern Med J. 2008;38:349–56.
Acknowledgments
We thank Dr. D’Arcy Little for help with the imaging and Dr. Toby Rose for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
von Both, I., Pollanen, M.S. Fatal pneumococcal septicemia in a girl with visceral heterotaxy and polysplenia: a case report. Forensic Sci Med Pathol 16, 519–522 (2020). https://doi.org/10.1007/s12024-020-00252-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12024-020-00252-1