Abstract
WHO classifications should be used for comparing the results from different groups of pathologist and clinicians by standardized histopathological methods. Our present report describes the important parameters of pituitary adenoma pathology as demand of the WHO classification for correlation to endocrine data and prognosis. The combination of HE stain based structures with immunostainings for pituitary hormones allows subclassification of adenomas as the best method not only for correlations to clinical hyperfunctions but also for statements to the sensitivity of drug therapies (somatostatin analogs, dopamine agonists). GH-, PRL- and ACTH-secreting pituitary adenomas are further classified based on the size and number of their secretory granules by electron microscopy, or as is mostly the case nowadays by cytokeratin staining pattern, into densely and sparsely granulated. Granulation pattern may be considered for the prediction of treatment response in patients with GH-secreting adenomas, since the sparsely granulated subtype was shown to be less responsive to somatostatin analog treatment. For prognosis, it is important to identify aggressive adenomas by measurements of the Ki-67 index, of the number of mitoses, and of nuclear expression of p53. Among the criteria for atypical adenomas, high Ki-67 labeling index and invasive character are the most important adverse prognostic factors. Promising molecular markers have been identified that might supplement the currently used proliferation parameters. For defining atypical adenomas in a future histopathological classification system, we propose to provide the proliferative potential and the invasive character separately.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The WHO classification of 2004 [1] for pituitary tumors should be used by all pathologists reporting pituitary specimens. Only a standardized consistent classification basing on standardized histopathological methods can ensure that pathologist and clinicians around the world can compare their results. As a matter of principle, a report of pituitary adenoma should include details of immunohistolologic hormone content, of proliferation, and of regressive changes.
In this multi-disciplinary article, we studied on the base of the WHO classification the value of pathological parameters for clinical diagnoses, postoperative treatment, and prognosis which was done in only few papers [2–4].
General Pathology of Pituitary Adenomas
A typical sinusoidal basic structure is typical for gonadotroph adenomas and null cell adenomas. A diffuse architecture is found in most GH and Prolactin secreting tumors, but also in TSH and ACTH adenomas. A strong eosinophilic cytoplasm is present in densely granulated GH adenomas, and in weaker fashion in densely granulated Prolactin cell adenomas. The staining intensity correlates to the amount of secretory granules which is also characteristic for the PAS positivity of densely and sparsely granulated ACTH adenomas. Typically chromophobic tumors are the gonadotroph and null cell adenomas. These criteria may be used for correlations to immunostainings for hormones: the stronger the staining the stronger are mostly the immunoreactions for GH and ACTH.
Amyloid [5, 6] in stroma or psammoma bodies [7] can be seen in some Prolactin adenomas. Global PAS positive inclusions [8] in the cytoplasm are typical for TSH adenomas, but all criteria do not replace immunostainings.
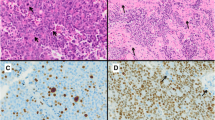
Signs of proliferation are delivered by the number of mitoses [9], which are rarely found in adenomas, by Ki-67 index [10] which should not exceed 3 % in non-aggressive adenomas, and by the p53 index [9] which is negative in most adenomas or limited to very sparse nuclei in non-aggressive adenomas.
Regressive changes are represented by necroses of cells, shrinkage of nuclei, and fibroses of stroma. Necroses are more frequent in silent ACTH cell adenomas. Shrinkage and fibroses are frequently found in Prolactin adenomas treated with dopamine agonists [11, 12]. Some more fibroses are demonstrable in some GH adenomas treated with somatostatin analogs [13].
Use of Immunohistochemical Stainings in Evaluating the Hormone Expression Status
Classification
The use of reliable antibodies to detect hormone expression in pituitary adenoma specimens is mandatory and represents the basis of the currently valid version of the WHO classification system [1]. But, similar to other neuroendocrine tumors, the functional status of pituitary adenomas is defined by the presence of clinical symptoms, not by immunohistochemistry [14]. Therefore, a close cooperation between pathologists and clinicians (i.e., interdisciplinary tumor boards) is recommended. Inadequate clinical evaluation should not be a reason to make the diagnosis of a “silent” lesion and the term “silent adenoma” should be restricted to tumors that have no evidence of clinical or biochemical abnormalities [15–17]. Immunohistochemically detectable hormone expression in clinically silent tumors can be very helpful in the sometimes difficult differentiation of sellar lesions (e.g., metastases vs. adenoma). Drug administration can result in reversible morphological changes in the adenoma tissue. The interpretation of these findings, e.g., marked shrinkage in cell size, can be challenging as the morphological features resemble lymphoma or lymphocytic hypophysitis. In such cases, immunohistochemical stainings are required to address this differential diagnosis. Furthermore, it can be helpful to uncover lymphocytic infiltrations within the tumor and to identify the cell population responsible for proliferation [3]. For the group of gonadotroph cell adenomas, no obvious correlation is apparent between histologic pattern, type, and extent of immunoreactivity and the hormonal activity of tumors as shown by currently used hormone assays [1]. Null cell adenomas represent immunonegative and hormonally inactive tumors. Therefore, such a diagnosis can only be made by using immunohistochemistry. The fact that the currently valid WHO classification system allows single tumor cells with FSH and or LH expression in this subtype is problematic because of tumor heterogeneity and missing reliable standards. Growth hormone secreting adenomas are predominantly clinically associated with acromegaly. They show a homogenous and intense (densely granulated) or more variable (sparsely granulated) staining pattern using antibodies against human growth hormone. Clinically silent patients and tumor specimens without immunohistochemically detectable hormone expression are exceptional cases. The immunoprofile of mixed somatotroph and lactotroph adenomas resembles that of mammosomatotroph adenomas and the distinction is usually an academic challenge rather than prognostic [3]. Sparsely granulated lactotroph tumors show generally a distinct and strong paranuclear located („Golgi pattern“) expression pattern whereas the rarely densely granulated cases display a diffuse cytoplasmatic staining and a marked acidophilic phenotype. Immunohistochemistry is just recommended to confirm the clinical diagnosis, because these tumors are nearly always clinically active and elevated levels of Prolactin are detected by currently used hormone assays. TSH producing adenomas show a variable extent of immunohistochemically detectable hormone expression. Sometimes interspersed Prolactin and Growth hormone positive cells can be detected within the tumor. Usually, the diagnosis is already supposed prior surgery and immunohistochemistry is just used for confirmation purposes. In cases of ACTH producing adenomas, both clinically silent and Cushing disease associated tumors show a varying extent of immunoreactivity. Immunohistochemistry is mandatory to detect the subtype of silent corticotroph adenomas, which are reportedly more aggressive characterized by high recurrence rates, invasive growth, and the propensity to undergo hemorrhagic infarction [14, 18]. Furthermore, the diagnosis and subtyping of plurihormonal adenomas can only be performed using immunohistochemistry.
Prognostic Purpose
Pituitary adenomas that are associated with aggressive behavior include sparsely granulated somatotroph adenomas, densely granulated lactotroph adenomas, acidophil stem cell adenomas, thyrotroph adenomas, sparsely granulated corticotroph adenomas, Crooke’s cell adenomas, silent subtype 3 adenomas, and null cell adenomas [3, 14, 19]. The use of immunohistochemistry is obligate to diagnose and discriminate these adenoma subgroups. No single immunohistochemically detectable biomarker has been found to independently predict aggressive behavior in pituitary neoplasms [14, 20–22]. The best prognosticator still remains accurate subtyping of the adenoma based on hormone content and subtype classification [14–16, 19, 23]. As indicated above, the most important clinical and prognostic features of pituitary adenomas remain the hormonal profile and subtype classification. The lack of accurate codes to reflect this classification will result in a continuing inability to collect statistics and represents a failure on the part of the pathology community to play an important role in clinical epidemiology [23].
Predictive Value
In the past, a diagnosis of “adenoma” was considered sufficient for many patients, but the advances in pituitary medicine demand a more thorough clinicopathological diagnosis that will guide patient management [15]. Today, no correlation between histomorphology of pituitary adenomas alone and clinical outcome exists and has, therefore, no impact on treatment decisions. Additional immunohistochemistry can be helpful and may have an impact on planning the therapeutic strategy.
Problems
There is wide variability depending on tissue fixation, antibodies, and method of immunolocalization [1].
Electron Microscopy
The most detailed electron microscopic classification of pituitary adenomas was published by Kovacs and Horvath [24] in 1986. In that time, the ultrastructure appeared to be the best method for classification, since some adenoma types, especially the acidophil stem cell adenoma showing giant mitochondria and fibrous bodies [25], the sparsely granulated GH adenoma with fibrous bodies and sparse small secretory granules [26], and later the silent subtype 3 adenoma [25] with strong development of smooth and rough endoplasmic reticulum, the large Golgi fields and the focal accumulation of secretory granules need the electron microscope for identification.
The development of immunostaining since the eighties and nineties of the former century replaced the requirement of the electron microscope in most cases. So, the fibrous bodies of sparsely granulated GH cell adenomas and acidophil stem cell adenomas could be identified by Keratin-positive globules in the light microscope. The giant mitochondria in the acidophil stem cell adenomas could be seen as large vacuoles in very good paraffin sections.
The silent subtype 3 [25] adenomas cannot be identified with certainty in the light microscope. If the distinct diagnosis of this special adenoma type appears to be important, the use of electron microscopy is not avoidable.
Granule Size and Distribution in Pituitary Tumors
The observation of secretory granules in varying numbers and size in pituitary tumors by electron microscopy prompted their use in the pathological classification of GH-, PRL- and ACTH-secreting pituitary adenomas into ‘densely’ and ‘sparsely’ granulated subtypes.
GH-Secreting Pituitary Adenomas
Ultrastructural classification by electron microscopy in densely and sparsely granulated was traditionally seen as a pathological characteristic of the GH-secreting pituitary adenomas. The finding that sparsely granulated tumors contain a cytokeratin-rich aggregation of intermediate filaments, called fibrous body, in the cytoplasm in close proximity to the nucleus enabled the classification by light microscopy using anti-cytokeratin antibodies. Immunohistochemistry with the Cam 5.2 antibody in GH-secreting tumors shows two staining patterns: a dot pattern that stains the fibrous bodies that are juxtanuclear globular aggregations of cytokeratin intermediate filaments characteristic of sparsely granulated tumors and a perinuclear pattern seen in somatotroph cells of the normal pituitary and in densely granulated tumors [27]. An intermediate staining pattern is also observed with cells showing clearly dot pattern and cells having a perinuclear staining within the same tumor, as well as ‘transitional’ cells with the dot pattern forming into a ring around the nucleus [28]; however, immunohistochemical analysis in a big number of cases (n = 104) revealed that the intermediate subtype shares clinical characteristics with the densely granulated and could therefore be included in this category.
The formation of the fibrous body responsible for the dot pattern in the sparsely granulated GH-secreting tumors may be attributed to low E-cadherin expression [29, 30]. E-cadherin anchors cytokeratin filaments to the cell membrane promoting effective cell to cell adhesion. When absent, these intermediate filaments collapse into cytoplasmic aggregates that may form the fibrous bodies [29] Interestingly, under the electron microscope, densely granulated cells retain the normal somatotroph cell morphology, while sparsely granulated tumor cells look less differentiated.
Mutations in the alpha subunit of the stimulatory regulatory G protein (Gs protein; gsp), are observed in around 40 % of acromegalic tumors [31]. However, no correlation was found between gsp mutational status and granulation pattern [32, 33].
Sparsely granulated GH-secreting pituitary adenomas are less common and are more associated with tumor invasiveness and a more aggressive phenotype; they tend to be macroadenomas and occur in younger, female patients [28, 33–35].
Sparsely granulated tumors produce only GH, while the densely granulated type may produce in addition to GH, PRL, beta subunit of TSH and/or alpha subunit [34]. No significant associations were found between granulation pattern and symptoms of acromegaly, incidence of diabetes II mellitus and hypertension [36]. In contrast, there is a strong correlation between granulation pattern and treatment outcome. Incomplete surgical resection and re-operations are more frequent in acromegalic patients with sparsely granulated tumors with a shorter time interval between operations [35]. Furthermore, patients with sparsely granulated tumors showed poor response to somatostatin analog treatment [28, 30, 32, 33, 37–39]. Densely granulated GH-secreting pituitary tumors have higher SSTR2 immunoreactivity and this may explain the good response of these tumors to the currently used SSTR2-binding somatostatin analog treatment [32, 39, 40]. In contrast, they have lower SSTR5 expression with SSTR5 immunoreactivity being observed exclusively in sparsely granulated tumors [40]. Therefore, for the management of the treatment resistant acromegalic patients with sparsely granulated tumors, somatostatin analogs that strongly bind SSTR5 (pasireotide) could be considered instead of the standard SSTR2 somatostatin analogs (octreotide, lanreotide) [16].
Prolactinomas
Sparsely granulated lactotrophs are hypothetically actively synthesizing prolactin, while the bigger densely granulated cells are storing it [41]. The majority of prolactinomas are sparsely granulated, respond to dopamine agonist treatment, and generally have a good prognosis [3]. Densely granulated prolactinomas are rare (0.3 % in the German Pituitary Tumor Registry [42]), present with diffuse PRL immunoreactivity, and are mainly found in male patients with aggressive tumors. They are frequently resistant to dopamine agonist treatment [43, 44].
Corticotrophinomas
Contrary to GH- and PRL-secreting tumors, the 2004 WHO classification does not classify corticotrophinomas according to granulation. Nevertheless, electron microscopy and low molecular weight keratin (LMWK)-CAM5.2 staining (of keratins 7 and 8) shows perinuclear bundles of cytokeratin filaments and distinguishes corticotrophinomas into densely granulated and sparsely granulated [45]. Densely granulated corticotroph tumors are morphologically similar to the normal corticotrophs, present with strong and diffuse ACTH immunoreactivity, and are the most common cause of Cushing’s disease. Sparsely granulated corticotrophinomas are usually low secreting macroadenomas that are associated with the development of Nelson’s syndrome after bilateral adrenalectomy [3].
Crooke’s cell adenoma is a very rare and atypical form of corticotrophinoma that shows accumulation of hyaline material in the cytoplasm. This corticotrophinoma subtype may be endocrinologically active, is characterized by an aggressive and invasive behavior, and is very difficult to manage by surgery and radiotherapy [46]. It also stains strongly with the CAM5.2 antibody and shows a characteristic intense ring-like pattern, while electron microscopy shows dense presence of perinuclear keratin [47].
Silent corticotrophinomas are ACTH immunopositive tumors that are not accompanied by physical and biochemical signs of cortisol excess [48, 49]. They are also divided into densely granulated (subtype 1) and sparsely granulated (subtype 2) that is characterized by large cells and is invasive to the cavernous and sphenoid sinus [50].
Granulation Patterns in Other Pituitary Tumor Types
TSH-secreting pituitary adenomas or thyrotrophinomas are sparsely granulated with the secretory granules scattered into the cell periphery [51]. Null cell adenomas that do not stain for any of the pituitary hormones also present with few secretory granules [52]. Gonadotrophinomas are characterized by smaller number and size of secretory granules compared to the non-tumorous gonadotroph cells, which may explain the lack of gonadotrophin hormone excess and endocrine phenotype (clinically inactive) in patients bearing these tumors [52].
Conclusion and Critical Outlook of Granulation as Diagnostic Tool
The clinical importance of granulation pattern as diagnostic tool is more evident in acromegaly in regard to treatment response [53]. Sparsely granulated GH- and PRL-secreting pituitary tumors are usually resistant to somatostatin analog and dopamine agonist treatment, respectively, aggressive and more difficult to manage. Accordingly, the granulation pattern may be taken into consideration when deciding treatment options.
Special Adenoma Subtypes
By definition, null cell adenomas are endocrine inactive adenomas without expression of pituitary hormones. Adenoma cells are small or medium-sized and chromophobic. Ultrastructurally, the cytoplasma is poor in organelles showing sparse small secretory granules [24]. Few null cell adenomas strongly express alpha-subunit (alpha-subunit only adenomas) [54] but this is not a special adenoma type in the WHO classification. The same is true for the oncocytic adenoma. This is an inactive adenoma composed of oncocytic tumor cells characterized by huge amounts of slightly pleomorphic mitochondria in the cytoplasm as seen in the electron microscope [55] whereas in the light microscope, an enlarged slightly eosinophilic cytoplasm with cloudy granular structures is demonstrable. Nowadays, the oncocytic character can be identified by immunostaining with antibodies against mitochondrial proteins [56]. Not only null cell adenomas but also FSH-LH adenomas, GH adenomas, and TSH adenomas can contain oncocytic parts [56] but are never totally composed of oncocytes. The acidophil stem cell adenoma [25] is a kind of oncocytic adenoma [57], too, due to its high amounts of mitochondria.
Crooke’s cell adenomas is a special type of ACTH adenoma composed nearly completely of Crooke’s which are defined as ACTH cells suppressed by high levels of cortisol and found in the tumor-free pituitary in Cushing’s syndrome [58]. Crooke’s cell adenomas show large cells with a typical hyaline ring, paranuclear vacuoles, and a peripheral PAS positive granulation. Ultrastructurally, the hyaline ring is composed of densely arranged cytofilaments, whereas the vacuoles are large lysosomes. Thirty-five percent of these adenomas are clinically inactive, whereas 65 % are hyperactive in Morbus Cushing [59]. Eighty-one percent of Crooke cell adenomas are macroadenomas and 72 % invasive. Recurrences develop in 60 % [59].
Acidophil stem cell adenomas (see above) induce a hyperprolactinemia and in some cases a mostly slight acromegaly [60]. All are macroadenomas and invasive [4, 57]. It is, therefore, a member of the group of aggressive adenomas [2, 57]. They are ultrastructurally characterized by giant mitochondria and fibrous bodies [25].
Silent subtype 3 adenomas are also aggressive invasive macroadenomas. They are definitively endocrine inactive but may show a slight hyperprolactinemia. The light microscopic structure [25] shows large chromophobic cells in diffuse arrangement. Some blotchy acidophilia and PAS positive granulation can be seen. Immunostainings [25] reveal mostly hormone-negative parts but also areas with GH-, Prolactin- or TSH-positive cells. ACTH-, FSH- or LH-positive cells are rarely found. The amount of Prolactin-positive cells does not correlate to the level of hyperprolactinemia. For an unequivocal evidence of this adenoma type, electron microscopy is necessary (see above).
Atypical and Aggressive Adenomas
Introduction
The 2004 WHO classification of pituitary adenomas [1] differentiates three histopathological grades, namely typical adenomas, atypical adenomas, and pituitary carcinomas [1]. The classification reflects the different biological behavior of pituitary adenomas. The typical adenoma is considered a benign and slow-growing tumor. Pituitary carcinomas show signs of malignant behavior and are defined by metastatic deposits through systemic spread or dissemination along the cerebro-spinal fluid pathways. Pituitary carcinomas account for less than 1 % of pituitary adenomas [42].
Atypical adenomas represent an intermediate biological type. It is intended to summarize in this category those adenomas with uncertain behavior that might show a more aggressive behavior, an increased growth potential, and a higher likelihood of recurrence. In the German Pituitary Tumor Registry, 2.7 % of the adenomas were classified as atypical [42]. Others [61] reported on 15 % atypical adenomas. In their study, invasive character was not included as a criterion for the atypical adenomas.
The WHO classification uses the cell cycle markers Ki-67 and p53 for assessing proliferative potential of pituitary adenomas. Atypical adenomas are defined by a Ki-67 labeling index >3 %, an excessive p53 immunoreactivity and an increased rate of mitoses [1]. In addition, an invasive adenoma growth is a prerequisite for the classification as atypical adenoma.
Proliferation Markers: Ki-67 Labeling Index
Ki-67 is a cell cycle specific antigen, which is expressed in the proliferative phases of the cell cycle but not in the quiescent G0 phase. The monoclonal antibody MiB-1 is used for immunohistochemical staining for Ki-67. Ki-67 is a measure of the cellular growth fraction. Ki-67 labeling index and MiB-1 labeling index are used in a synonymous manner.
Sufficient evidence is provided in the literature to allow the conclusion that a high Ki-67 labeling index is significantly correlated to a more aggressive behavior of pituitary adenomas and to a higher propensity to recur [62–64]. Recent publications with large case numbers are particularly confirmatory [65–67]. As this correlation was not uniformly found in all studies, it has been suggested that Ki-67 alone has limited prognostic ability to predict recurrence accurately and the combination with other biological markers may be more promising [68].
A few studies have assessed the growth velocity of pituitary adenomas and found a positive correlation with Ki-67 labeling index [69–71]. However, if fast- and slow-growing adenomas are compared, a significant overlap of Ki-67 labeling index is observed. Hence, the predictive value of Ki-67 labeling index for the growth rate in an individual case is low. Hsu et al. [72] examined postoperative adenoma progression and found a correlation of tumor volume doubling time (TVDT) and Ki-67 labeling index among progressive adenomas. Interestingly, mean Ki-67 labeling indices were only slightly higher in progressive adenomas as compared to non-progressive adenomas.
A correlation of pituitary tumor subtypes with their hormone production and Ki-67 labeling index is not clearly established and the data from the literature are controversial [68, 73].
A cut-off level of 3 % has been adopted in the WHO classification [1]. However, cut-off values for Ki-67 labeling index in pituitary adenomas are a matter of debate [73]. By far, lower cut-off value has been proposed and a rapid decrease of sensitivity for predicting recurrence with increasing cut-off values has been reported [10, 62, 65, 70, 72]. Righi et al. [10] have shown that a Ki-67 labeling index >3 % is associated with a low sensitivity for predicting recurrence or progression of adenomas. Gejman et al. [62] have stressed that the assessment of the Ki-67 labeling index is subject to a number of variables and discrepancies are explained by methodological differences. The mean values of Ki-67 labeling index reported in different studies on pituitary adenomas varied from 0.84 to 2.72 [72]. Salehi et al. [73] have reviewed the role of Ki-67 in pituitary adenomas and concluded that uniform definitions and methods, as well as new markers, are key to improved treatment of pituitary tumors.
Expression of p53
p53 is a tumor suppressor protein encoded by the TP53 gene [74]. P53 has a low level of expression in normal cells. P53 immunostaining detects both mutated p53 protein and wild-type p53 protein. Whether p53 overexpression in pituitary adenomas is caused by TP53 gene mutation resulting in mutated p53 protein or is caused by dysregulation of wild-type p53 protein is still unsettled [75]. The significance of p53 for predicting progression and recurrence is not as clear as for Ki-67. While some studies have shown that excessive p53 immunoreactivity is a significant predictor for increased proliferation and recurrence [66, 73] in pituitary adenomas, other studies have not confirmed this finding [62, 65]. Therefore, the relevance of p53 expression as a marker of recurrence has been questioned [73]. It has been shown that the use of Ki-67 labeling index alone is equal in discriminating recurring and non-recurring adenomas compared to the application of all three parameters for proliferation that are used in the current WHO classification for discriminating typical from atypical adenomas [67]. Due to methodological differences among centers, a well-defined cut-off is not provided in the WHO classification.
Mitotic Activity
The prognostic value of a higher mitotic activity for the biological behavior of pituitary adenomas has not been sufficiently investigated. In the large study by Lee et al. [66], the mitotic index failed to reach significance for predicting recurrence.
Additional Biological Markers
The role of cell cycle regulators for growth of pituitary adenomas has also been examined. In the study of Lee et al. [66], normostaining for p16 and overstaining for pRB protein and cyclin D1 predicted recurrence in functioning pituitary adenomas. Salehi et al. [73] reviewed the biomarkers in pituitary adenomas for various cellular processes, including cell-cycle progression, cell proliferation, apoptosis, cell adhesion, and tumor vascularity. Some markers, such as fibroblast growth factor, fibroblast growth factor receptor and matrix metalloproteinases, were found to be promising for identification of pituitary tumors with aggressive behavior.
Invasion
In the 2004 WHO classification, the invasive behavior in pituitary adenomas is ill-defined. Invasive behavior can be assessed by histological [76] or radiological criteria [66] by the surgeon’s intraoperative impression [77]. Due to application of different criteria for invasion, the clinical significance of invasive character as reported in the literature has to be considered in a differentiated manner.
It has been clearly shown that the radiological criteria of invasiveness strongly correlate with surgical outcome in terms of persistence in functioning adenomas and in terms of recurrence [65–67, 78]. A high Knosp grade [79] for cavernous sinus invasion has been found a highly significant and independent factor for recurrence of pituitary adenomas [65, 66]. Major cavernous sinus invasion prohibits gross total tumor removal and effects residual adenoma on postoperative MRI. It is obvious that recurrence is likely under these circumstances as compared to enclosed adenomas that can be radically resected.
The surgeon’s intra-operative impression is subjective and should not be used alone for defining invasive adenoma growth.
The dura invasion is difficult to assess. Usually, only a small central specimen of the basal dura can be collected for histopathological examination at the stage of opening the pituitary fossa during transsphenoidal surgery. It is even not representative for the whole basal dura. The major site of dura invasion is the medial wall of the cavernous sinus which is usually not accessible for biopsy or biopsy is associated with a risk of surgical morbidity. Only few centers systematically provide specimens of the dura for histopathological examination. Meij et al. [76] assessed the long-term significance of microscopic dural invasion in 354 patients treated for pituitary adenomas by transsphenoidal surgery. In their study, recurrence rate was not related to dural invasion in a consistent or significant fashion.
Most publications are divided into the two categories—invasive or non-invasive. This approach oversimplifies the clinical implications. Circumscribed invasion at one site (f.e. one-sided cavernous sinus invasion or sole sphenoid sinus invasion) might still be accessible to complete surgical resection or residual adenoma at this site might be sufficiently treated by single-shot radio-surgery. Invasion at two sites (f.e. bilateral cavernous sinus invasion) is by far a greater clinical challenge and disease control is impeded. Some adenomas even show general invasive character with involvement of all contiguous structures (cavernous sinus, sphenoid sinus, clivus).
Correlation of Invasion and Tumor Size with Proliferative Activity
It is still a controversial issue, whether an increased proliferation as indicated by high Ki-67 LI and high p53 expression is correlated to invasive growth behavior of pituitary adenomas [61, 68]. Several publications show a correlation of proliferation and invasion [61, 75, 80], while many other publications do not find an association and claim that proliferation and invasive behavior are independent properties of pituitary adenomas [67, 70, 81]. Furthermore, no correlation between growth velocity as a direct measure of proliferation and cavernous sinus invasion has been found [70, 71].
Proliferative activity is not clearly correlated to tumor size [73, 81, 82]. Even in giant adenomas, mitotic rates, p53 expression, and Ki-67 labeling indices were only found minimally increased [83]. On the other hand, incidentalomas in the microadenoma stage are less likely to grow during observation than macroadenomas.
Aggressive Adenomas
Aggressive pituitary adenomas are considered a clinical subset of adenomas with frequent recurrence and resistance to conventional therapy [84]. A significant number of aggressive adenomas have been shown to be responsive to temozolomide chemotherapy [84]. The WHO classification does not provide an accurate correlate for clinically aggressive adenomas [85] as many atypical adenomas do not behave aggressively. Mete et al. [74] claim that the histological subtypes of pituitary adenomas remain the best independent predictor of aggressive behavior with sparsely granulated somatotroph adenomas, densely granulated lactotroph adenomas, acidophil stem cell adenomas, thyrotroph adenomas, sparsely granulated corticotroph adenomas, Crooke cell adenomas, silent subtype 3 adenomas, and null cell adenomas being associated with aggressive behavior.
Proposals to Improve the Classification
The major shortcoming of the currently valid WHO classification is the linkage of proliferation parameters and evidence of invasion for defining “atypical adenomas.”
There are two scenarios where an aggressive adenoma can be missed by the current criteria for atypical adenomas:
-
1.
If an adenoma exhibits major invasive behavior, a surgical cure is impossible and the adenoma is prone to recur even if the Ki-67 labeling index is low.
-
2.
If an adenoma exhibits a high proliferation index and is fast growing and aggressive, the absence of invasive character defines the adenoma as a typical (i.e., totally benign) adenoma.
Based on the French collaborative study on prognostic factors of pituitary tumors, Trouillas et al. [67] have proposed a new 5-grade classification for pituitary adenomas:
-
Grade 1a: non-invasive tumor
-
Grade 1b: non-invasive and proliferative tumor
-
Grade 2a: invasive tumor
-
Grade 2b: invasive and proliferative tumor
-
Grade 3: metastatic tumor
They provide the following modifications of the current WHO classification:
-
1.
Invasiveness and proliferation are uncoupled.
-
2.
They have more clearly defined invasion:
-
Histological and/or radiological (MRI) signs of cavernous sinus or sphenoid sinus invasion.
-
-
3.
A more precise and dichotomous evaluation of proliferation parameters is provided:
Proliferation is considered on the presence of at least two of the three criteria:
-
Ki-67 labeling index: >1 % (Bouin-Hollande fixative) or >3 % (formalin fixative)
-
Mitoses: n >2/10 HPF
-
P53: positive (>10 strongly positive nuclei/10 HPF)
-
Based on the above mentioned data, we suggest the following modifications for grading of pituitary adenomas:
-
1.
Invasiveness is only based on radiological criteria.
-
2.
Invasive character is regarded more differentially and divided into three grades:
-
Non-invasive
-
Invasion at one site (i.e., cavernous sinus, sphenoid sinus, or clivus)
-
Invasion at two or more sites (bilateral cavernous sinus invasion included)
-
-
3.
With the current WHO classification [1] and with the classification of Trouillas et al. [67], a definite histopathological diagnosis is only available, if the clinician and neuroradiologist provide the information of invasive character [86]. We recommend that the proliferation grade is provided independent from clinical data. It allows a pure histopathological classification differentiating non-proliferative (grade 1) and proliferative (grade 2) adenomas. The radiological information should be provided separately (f.e. with an alphabetic character). With this modification, histopathological classification without information on invasiveness can be provided.
Recommended Classification
Grade 1: Non-proliferative adenoma
-
1a: noninvasive
-
1b: invasion at one site
-
1c: invasion at two or more sites
Grade 2: Proliferative adenoma
-
2a: invasion at one site
-
2b: invasion at one site
-
2c: invasion at two or more sites
-
2d: pituitary carcinoma
The 2004 WHO classification defines atypical adenomas in the presence of increased proliferation markers (Ki-67 LI, p53 expression, mitosis rate) and invasive growth. The proliferation markers are considered predictive for progression and recurrence of pituitary adenomas reflecting uncertain or more aggressive adenoma behavior. Sufficient evidence exists for Ki-67 while the usefulness of p53 as a marker for progression and recurrence has been questioned. These markers are best established and widely used. However, the sensitivity and specificity of each marker for predicting enhanced growth of pituitary adenomas is limited. It is paramount to standardize the methods and the criteria for interpretation of proliferation markers. This is a prerequisite for reliable and comparable classification of pituitary adenomas.
Further promising molecular markers have been identified that might supplement or replace the currently used proliferation parameters. In particular, markers that reliably predict clinically aggressive pituitary adenomas are urgently needed.
Invasive character is an important predictor both for immediate surgical outcome and for risk of recurrence. The prognostic value of radiological criteria is superior to microscopic criteria. The invasive behavior has far-reaching implications for the clinical management and for follow-up schedules in pituitary adenomas.
Increased proliferation potential and invasive growth pattern have to be considered as independent qualities of adenomas. Therefore, both qualities should not be intermingled in a histopathological classification system. For improvement of the WHO classification, we propose to provide the proliferative potential and the invasive character separately.
References
Lloyd RV, Kovacs K, Young WF, Jr., Farrell WE, Asa SL, Trouillas J, et al. Tumours of the pituitary. In: DeLellis RA, Lloyd RV, Heitz PU, editors. Pathology and Genetics. Tumours of Endocrine Tumours. 1 ed. Lyon: International Agency for Research and Cancer (IARC); 2004. p. 9–48.
Mete O, Asa SL. Therapeutic implications of accurate classification of pituitary adenomas. Semin Diagn Pathol 2013;30(3):158–64.
Mete O, Asa SL. Clinicopathological Correlations in Pituitary Adenomas. Brain Pathology 2012;22(4):443–53.
Scheithauer BW, Gaffey TA, Lloyd RV, Kovacs KT, Horvath E, Yapicier O, et al. Pathobiology of pituitary adenomas and carcinomas. Neurosurgery 2006;59(2):341–53.
Saitoh Y, Mori H, Matsumoto K, Ushio Y, Hayakawa T, Mori S, et al. Accumulation of amyloid in pituitary adenomas. Acta Neuropath (Berlin) 1985;68(2):87–92.
Röcken C, Uhlig H, Saeger W, Linke RP, Fehr S. Amyloid deposits in pituitaries and pituitary adenomas: Immunohistochemistry and in situ hybridization. Endocr Pathol 1995;6:135–43.
Lipper S, Isenberg HD, Kahn LB. Calcospherites in pituitary prolactinomas. A hypothesis for their formation. Arch Pathol Lab Med 1984 Jan;108(1):31–4.
Scheithauer BW, Horvath E, Lloyd RV, Kovacs K. Pathology of pituitary adenomas and pituitary hyperplasia. In: Thapar K, Kovacs K, Scheithauer BW, Lloyd RV, editors. Diagnosis and management pituitary tumors. 1 ed. Totowa,NJ: Humana Press; 2001. p. 91–154.
Kontogeorgos G. Predictive markers of pituitary adenoma behavior. Neuroendocrinology 2006;83(3–4):179–88.
Righi A, Agati P, Sisto A, Frank G, Faustini-Fustini M, Agati R, et al. A classification tree approach for pituitary adenomas. Hum Pathol 2012;43(10):1627–37.
Kovacs K, Stefaneanu L, Horvath E, Lloyd RV, Lancranjan I, Buchfelder M, et al. Effect of dopamine agonist medication on prolactin producing pituitary adenomas. A morphological study including immunocytochemistry, electron microscopy and in situ hybridization. Virchows Arch A Pathol Anat Histopath 1991;418(5):439–46.
Saeger W. Effect of drugs on pituitary ultrastructure. Microsc Res Techn 1992 Jan 15;20(2):162–76.
Sautner D, Saeger W, Tallen G, Lüdecke DK. Effects of octreotide on morphology of pituitary adenomas in acromegaly. Pathol Res Pract 1993;189:1044–51.
Asa SL. Pituitary adenomas. In: Silverberg SG, Gardner WA, Sobin LH, editors. Tumors of the pituitary gland. 1 ed. Washington: Armed Forces Institute of Pathology; 2011. p. 55–172.
Al Brahim NYY, Asa SL. My approach to pathology of the pituitary gland. Journal of Clinical Pathology 2006;59(12):1245–53.
Asa SL. Practical pituitary pathology - What does the pathologist need to know? Arch Pathol Lab Med 2008;132(8):1231–40.
Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, et al. The prevalence of pituitary adenomas - A systematic review. Cancer 2004;101(3):613–9.
Jahangiri A. A comprehensive long-term retrospective analysis of silent corticotrophic adenomas vs hormone-negative adenomas. Neurosurgery 73, 8–17 Discussion 17–18. 2013.
Asa SL, Ezzat S. The pathogenesis of pituitary tumors. Annu Rev Pathol 4, 97–126. 2015.
Sivapragasam M, Rotondo F, Lloyd RV, Scheithauer BW, Cusimano M, Syro LV, et al. MicroRNAs in the Human Pituitary. Endocr Pathol 2011;22(3):134–43.
Wierinckx A. Integrated genomic profiling identifies loss of chromosome 11p impacting transcriptomic activity in aggressive pituitary PRL tumors. Brain Pathol 21, 533–543. 2015.
Cornelius A, Cortet-Rudelli C, Assaker R, Kerdraon O, Gevaert MH, Prevot V, et al. Endothelial Expression of Endocan Is Strongly Associated with Tumor Progression in Pituitary Adenoma. Brain Pathology 2012;22(6):757–64.
Al Shraim M, Asa SL. The 2004 World Health Organization classification of pituitary tumors: What is new? Acta Neuropathologica 2006;111(1):1–7.
Kovacs K, Horvath E. Tumors of the pituitary gland. Washington,D.C.: Armed Forces Institute of Pathology; 1986.
Horvath E, Kovacs K, Smyth HS, Cusimano M, Singer W. Silent adenoma subtype 3 of the pituitary - Immunohistochemical and ultrastructural classification: A review of 29 cases. Ultrastruct Pathol 2005;29(6):511–24.
Horvath E, Kovacs K. Ultrastructural classification of pituitary adenomas. J Canad Sci Neurol 1976;3:9–21.
Sano T, Ohshima T, Yamada S. Expression of glycoprotein hormones and intracytoplasmic distribution of cytokeratin in growth hormone-producing pituitary adenomas. Pathol Res Pract 1991 Jun;187(5):530–3.
Obari A, Sano T, Ohyama K, Kudo E, Qian ZR, Yoneda A, et al. Clinicopathological features of growth hormone-producing pituitary adenomas: Difference among various types defined by cytokeratin distribution pattern including a transitional form. Endocr Pathol 2008;19(2):82–91.
Nishioka H, Haraoka J, Akada K. Fibrous bodies are associated with lower GH production and decreased expression of E-cadherin in GH-producing pituitary adenomas. Clin Endorin 2003;59(6):768–72.
Bakhtiar Y, Hirano H, Arita K, Yunoue S, Fujio S, Tominaga A, et al. Relationship between cytokeratin staining patterns and clinico-pathological features in somatotropinomae. Eur J Endocrin 2010;163(4):531–9.
Spada A, Vallar L, Faglia G. G-Protein oncogenes in pituitary tumors. Trends Endocrinol Metab 1992;3:355–60.
Fougner SL, Casar-Borota O, Heck A, Berg JP, Bollerslev J. Adenoma granulation pattern correlates with clinical variables and effect of somatostatin analogue treatment in a large series of patients with acromegaly. Clin Endorin 2012;76(1):96–102.
Larkin S, Reddy R, Karavitaki N, Cudlip S, Wass J, Ansorge O. Granulation pattern but nit GSP or GHR mutation is associated with clinical characteristics in somatostatin-naive patients with somatotroph adenomas. Eur J Endocrinol 2013;168(4):491–9.
Yamada S, Aiba T, Sano T, Kovacs K, Shishiba Y, Sawano S, et al. Growth hormone-producing pituitary adenomas: correlations between clinical characteristics and morphology. Neurosurgery 1993 Jul;33(1):20–7.
Mazal PR, Czech T, Sedivy R, Aichholzer M, Wanschitz J, Klupp N, et al. Prognostic relevance of intracytoplasmic cytokeratin pattern, hormone expression profile, and cell proliferation in pituitary adenomas of akromegalic patients. Clin Neuropathol 2001;20(4):163–71.
Ito M, Yoshida K, Kyo E, Ayhan A, Nakayama H, Yasui W, et al. Expression of several growth factors and their receptor genes in human colon carcinomas. Virchows Arch B Cell Pathol 1990;59(3):173–8.
Ezzat S, Kontogeorgos G, Redelmeier DA, Horvath E, Harris AG, Kovacs K. In vivo responsiveness of morphological variants of growth hormone-producing pituitary adenomas to octreotide. Eur J Endocrin 1995;133(6):686–90.
Bhayana S, Booth GL, Asa SL, Kovacs K, Ezzat S. The implication of somatotroph adenoma phenotype to somatostatin analog responsiveness in acromegaly. J Clin Endocrinol Metab 2005;90(11):6290–5.
Brzana J, Yedinak CG, Gultekin SH, Delashaw JB, Fleseriu M. Growth hormone granulation pattern and somatostatin receptor subtype 2A correlate with postoperative somatostatin receptor ligand response in acromegaly: a large single center experience. Pituitary 2013;16(4):490–8.
Mayr B, Buslei R, Theodoropoulou M, Stalla GK, Buchfelder M, Schofl C. Molecular and functional properties of densely and sparsely granulated GH-producing pituitary adenomas. Eur J Endocrin 2013;169(4):391–400.
Velkeniers B, Hooghe-Peters EL. From prolactin cell to prolactinoma: implications of ontogenic mechanisms in diagnosis and management. Endocr Relat Cancer 1998;5:27–36.
Saeger W, Lüdecke DK, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol 2007;156:205–17.
Asa SL, Ezzat S. Medical management of pituitary adenomas: structural and ultrastructural changes. Pituitary 2002;5(2):133–9.
Asa SL, Ezzat S. The pathogenesis of pituitary tumors. Annu Rev Pathol 2009;4:97–126.
Reed CT, Adams K, Shenoy V. Xanthogranulomatous Adrenalitis: A Case Report of a Diabetic, 55-Year-Old Male. Endocr Pathol 2015;26(3):229–31.
Di Ieva A, Davidson JM, Syro LV, Rotondo F, Montoya JF, Horvath E, et al. Crooke’s Cell Tumors of the Pituitary. Neurosurgery 2015;76(5):616–22.
Syro LV, Rotondo F, Cusimano MD, Di Ieva A, Horvath E, Restrepo LM, et al. Current status on histological classification in Cushing’s disease. Pituitary 2015;18(2):217–24.
Horvath E, Kovacs K, Killinger DW, Smyth HS, Platts ME, Singer W. Silent corticotropic adenomas of the human pituitary gland. A histologic, immunocytologic, and ultrastructural study. Amer J Pathol 1980;98:617–38.
Reincke M, Allolio B, Saeger W, Kaulen D, Winkelmann W. A pituitary adenoma secreting high molecular weight adrenocorticotropin without evidence of Cushing’s disease. J Clin Endocrinol Metab 1987;65:1296–300.
Osamura RY, Kajiya H, Takei M, Egashira N, Tobita M, Takekoshi S, et al. Pathology of the human pituitary adenomas. Histochemistry and Cell Biology 2008;130(3):495–507.
Saeger W, Lüdecke DK. Pituitary adenomas with hyperfunction of TSH. Frequency, histological classification, immunocytochemistry and ultrastructure. Virchows Archiv 1982;394:255–67.
Horvath E, Kovacs K. Gonadotroph adenomas of the human pituitary: sex-related fine-structural dichotomy. A histologic, immunocytochemical, and electron-microscopic study of 30 tumors. Am J Pathol 1984 Dec;117(3):429–40.
Grossman AB. The 2004 World health organization classification of pituitary tumors: is it clinically helpful? Acta Neuropathologica 2006;111(1):76–7.
Landolt AM, Heitz PU. Alpha-subunit-producing pituitary adenomas. Immunocytochemical and ultrastructural studies. Virchows Arch A Pathol Anat Histopath 1986;409(4):417–31.
Saeger W. Elektronenoptische Untersuchungen zur Problematik der onkocytären Hypophysenadenome. Verhandlungen der Deutschen Gesellschaft für Pathologie 58, 544. 1974.
Niveiro M, Aranda FI, Paya A, Boix E, Peiro G, Pico A. Oncocytic transformation in pituitary adenomas - Immunohistochemical analyses of 65 cases. Arch Pathol Lab Med 2004;128(7):776–80.
Asa SL. Tumors of the pituitary gland. Washington,D.C.: Armed Forces Institute of Pathology; 1998.
Crooke AC. A change in the basophil cells of the pituitary gland common to conditions which wxhibit the syndrome attributed to basophil adenoma. J Pathol Bacteriol 1935;41:339–49.
George DH, Scheithauer BW, Kovacs K, Horvath E, Young WF, Lloyd RV, et al. Crooke’s cell adenoma of the pituitary - An aggressive variant of corticotroph adenoma. Amer J Surg Pathol 2003;27(10):1330–6.
Lim JS, Ku CR, Lee MK, Kim TS, Kim SH, Lee EJ. A case of fugitive acromegaly, initially presented as invasive prolactinoma. Endocrine 2010;38(1):1–5.
Zada G, Woodmansee WW, Ramkissoon S, Amadio J, Nose V, Laws ER. Atypical pituitary adenomas: incidence, clinical characteristics, and implications Clinical article. J Neurosurg 2011;114(2):336–44.
Gejman R, Swearingen B, Hedley-Whyte ET. Role of Ki-67 proliferation index and p53 expression in predicting progression of pituitary adenomas. Hum Pathol 2008;39(5):758–66.
Mizoue T, Kawamoto H, Arita K, Kurisu K, Tominaga A, Uozumi T. MIB-1 immunopositivity is associated with rapid regrowth of pituitary adenomas. acta neurochirurgica (Wien,Oester.) 139, 426–432. 1997.
Widhalm G, Wolfsberger S, Preusser M, Fischer I, Woehrer A, Wunderer J, et al. Residual nonfunctioning pituitary adenomas: prognostic value of MIB-1 labeling index for tumor progression Clinical article. J Neurosurg 2009;111(3):563–71.
Chiloiro S, Bianchi A, Doglietto F, de Waure C, Giampietro A, Fusco A, et al. Radically resected pituitary adenomas: prognostic role of Ki 67 labeling index in a monocentric retrospective series and literature review. Pituitary 2014;17(3):267–76.
Lee EH, Kim KH, Kwon JH, Kim HD, Kim YZ. Results of Immunohistochemical Staining of Cell-Cycle Regulators: The Prediction of Recurrence of Functioning Pituitary Adenoma. World Neurosurgery 2014;81(3–4):563–75.
Trouillas J, Roy P, Sturm N, Dantony E, Cortet-Rudelli C, Viennet G, et al. A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case–control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathologica 2013;126(1):123–35.
Aguilar PH, Aires R, Laws ER, Isolan GR, Logullo A, Patil C, et al. Labeling index in pituitary adenomas evaluated by means of MIB-1: is there a prognostic role? A critical review. Neurological Research 32, 1060–1071. 2010.
Ekramullah SM, Saitoh Y, Arita N, Ohnishi T, Hayakawa T. The correlation of Ki-67 staining indices with tumor doubling times in regrowing non-functioning pituitary adenomas. acta neurochir (Wien) 1996;138:1449–55.
Honegger J, Prettin C, Feuerhake F, Petrick M, Schulte-Monting J, Reincke M. Expression of Ki-67 antigen in nonfunctioning pituitary adenomas: correlation with growth velocity and invasiveness. J Neurosurg 2003;99(4):674–9.
Tanaka Y, Hongo K, Tada T, Sakai K, Kakizawa Y, Kobayashi S. Growth pattern and rate in residual nonfunctioning pituitary adenomas: correlations among tumor volume doubling time, patient age, and MIB-1 index. J Neurosurg 2003;98(2):359–65.
Hsu CY, Guo TY, Chien CP, Ho DM. MIB-1 labeling index correlated with magnetic resonance imaging detected tumor volume doubling time in pituitary adenoma. Europ J Endocrinol 162, 1027–1033. 2010.
Salehi F, Agur A, Scheithauer BW, Kovacs K, Lloyd RV, Cusimano M. Ki-67 in Pituitary Neoplasms: A Review-Part I. Neurosurgery 2009;65(3):429–37.
Mete O, Ezzat S, Asa SL. Biomarkers of aggressive pituitary adenomas. Journal of Molecular Endocrinology 2012;49(2):R69-R78.
Thapar K, Scheithauer BW, Kovacs K, Pernicone PJ, Laws ER, Jr. p53 expression in pituitary adenomas and carcinomas: Correlation with invasiveness and tumor growth fractions. Neurosurgery 1996;38(4):765–70.
Meij BP, Lopes MBS, Ellegala DB, Alden TD, Laws ER. The long-term significance of microscopic dural invasion in 354 patients with pituitary adenomas treated with transsphenoidal surgery. J Neurosurg 2002 Feb;96(2):195–208.
Mastronardi L, Guiducci A, Spera C, Puzzilli F, Liberati F, Ruggeri A, et al. Adrenocorticotropic hormone secreting pituitary adenomas: Analysis of growth fraction using the MIB-1 antibody. Tumori 2000 May;86(3):229–32.
Chacko G, Chacko AG, Kovacs K, Scheithauer BW, Mani S, Muliyil JP, et al. The clinical significance of MIB-1 labeling index in pituitary adenomas. Pituitary 2010;13(4):337–44.
Knosp E, Steiner E, Kitz K, Matula C. Pituitary Adenomas with Invasion of the Cavernous Sinus Space - A Magnetic Resonance Imaging Classification Compared with Surgical Findings. Neurosurgery 1993;33:610–8.
Thapar K, Kovacs K, Scheithauer BW, Stefaneanu L, Horvath E, Pernicone PJ, et al. Proliferative activity and invasiveness among pituitary adenomas and carcinomas: An analysis using the MIB-1 antibody. Neurosurgery 1996;38(1):99–106.
Sarkar S, Chacko AG, Chacko G. An analysis of granulation patterns, MIB-1 proliferation indices and p53 expression in 101 patients with acromegaly. Acta Neurochirurgica 2014;156(12):2221–30.
Mastronardi L, Guiducci A, Puzzilli F. Lack of correlation between Ki-67 labellin index and tumor size of anterior pituitary adenomas. Bmc Cancer 1, 12–16. 2001.
Madsen C, Schroder HD. Ki-67 immunoreactivity in meningiomas - determination of the proliferation potential of meningiomas using the monclonal antibody Ki-67. Clinical Neuropathology 16, 137–142. 1997.
Raverot G, Castinetti F, Jouanneau E, Morange I, Figarella-Branger D, Dufour H, et al. Pituitary carcinomas and aggressive pituitary tumours: merits and pitfalls of temozolomide treatment. Clin Endorin 2012;76(6):769–75.
Di Ieva A, Rotondo F, Syro LV, Cusimano MD, Kovacs K. Aggressive pituitary adenomas-diagnosis and emerging treatments. Nature Reviews Endocrinology 2014;10(7):423–35.
Miermeister CP, Petersenn S, Buchfelder M, Fahlbusch R, Lüdecke DK, Hölsken A, et al. Histological criteria for atypical adenomas: data from German Pituitary Tumor Registry suggest modifications. acta neuropath communications . 2015.
Acknowledgments
The funding for the German Pituitary Tumor Registry to WS from Novartis Pharma GmbH (Nuremberg, Germany), Novo Nordisk Pharma GmbH (Mainz, Germany), Pfizer Pharma GmbH (Karlsruhe, Germany), and Ipsen Pharma GmbH (Ettlingen, Germany) is gratefully acknowledged. We thank all colleagues for sending tumor material to the German Pituitary Tumor Registry.
Authors Contributions
W. Saeger: General pathology, electron microscopy, special adenoma subtypes.
J. Honegger: Atypical adenomas.
M. Theodoropoulou: Granulation.
R. Buslei: Immunocytochemistry.
U.J. Knappe: Invasion.
C. Schöfl: Correlation of clinic and morphology.
St. Petersen: Correlation of clinic and morphology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Due to the character and kind (review) of the theme of the manuscript, an approval on ethics appears to be not necessary.
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Saeger, W., Honegger, J., Theodoropoulou, M. et al. Clinical Impact of the Current WHO Classification of Pituitary Adenomas. Endocr Pathol 27, 104–114 (2016). https://doi.org/10.1007/s12022-016-9418-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-016-9418-7