Abstract
Ubiquitin-specific protease 10 (USP10), a novel deubiquitinating enzyme, is associated with androgen receptor transcriptional activity and pathological processes of tumor. However, information between USP10 and the adrenal gland is limited. In particular, the role of USP10 in adrenal tumors has not been elucidated yet. This study aims to investigate the expression of USP10 in the human normal adrenal gland and various adrenal tumors. Tissue samples were obtained from 30 adrenocortical adenomas, nine adrenocortical adenocarcinomas, and 20 pheochromocytomas following laparoscopic surgery. Twenty normal adrenal glands were obtained from kidney surgical resection conducted due to renal cell carcinomas. USP10 expression was investigated on protein levels using immunohistochemistry and on mRNA levels using bioinformatics analysis in the Gene Expression Omnibus (GEO) Datasets. In the 20 cases of normal adrenal glands analyzed, USP10 protein was constantly expressed in situ in the cortex of the adrenal glands, but in the medulla of the gland, only the sustentacular cells were detected positive. In adrenal tumors, detectable levels of USP10 protein were found in 100 % (30/30) adrenocortical adenomas, 88.89 % (8/9) adrenocortical carcinomas, and 10 % (2/20) pheochromocytomas. Bioinformatics analysis did not show a significant difference in USP10 messenger RNA (mRNA) expression between adrenal tumors and normal adrenal gland tissues. A positive USP10 immunoreaction can be useful in distinguishing adrenal cortical tumors from pheochromocytoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ubiquitin-specific protease 10 (USP10), also known as UBPO, localized on chromosome 16q24.1, and originally discovered as a deubiquitinating enzyme that interacts with Ras-GAP SH3 domain-binding protein (G3BP) [1], takes part in various biological processes by stabilizing several proteins [2, 3]. Increased USP10 expression has been detected in some breast cancer and glioblastoma samples [4], and USP10 overexpression has been associated with poor prognosis in patients with glioblastoma multiforme [5]. Decreased USP10 expression has been observed in gastric cancer tissues, and its downregulation has been associated with invasion, metastasis, and prognosis of gastric cancer [6]. Moreover, USP10 as a novel regulator of p53 provides an alternative mechanism for p53 inhibition in cancers. USP10 is constitutively downregulated in renal clear cell carcinoma, most of which contain wild-type p53. Meanwhile, elevated USP10 levels have been detected in a subset of renal cell carcinomas that contain p53 mutations [7]. Recent studies have also shown that USP10 suppresses proliferation and growth of pancreatic cancer cells [8]. Therefore, USP10, as a novel deubiquitinating enzyme, has a crucial role in various pathological processes of tumor. However, little information is available concerning USP10 expression in various adrenal tumors and normal adrenal gland.
The adrenal gland is a composite of two endocrine organs: one mesodermally derived (cortex) and the other neuroectodermally derived (medulla). The adrenal cortex is divided into three zones. The outermost layer is the zona glomerulosa, which lies immediately beneath the capsule and is a site of mineralocorticoid production. The zona fasciculata, which is situated between the glomerulosa and reticularis and the widest of the three layers because it composes nearly 80 % of the cortical volume, is responsible for producing glucocorticoid and sex hormones (adrenal androgens). The innermost layer is the zona reticularis, which lies directly next to the medulla, is also involved in the secretion of glucocorticoids and sex hormones (especially for androgens). The biological action of androgens is mediated through androgen receptors (ARs). Relatively high levels of ARs have been detected in the adrenal glands of both male and female rats [9]. Moreover, USP10 is a cofactor that binds to the ARs and stimulates the androgen response of target promoters [10, 11]. Therefore, these findings have promoted us to investigate whether USP10 is constitutively expressed in normal adrenal cortex.
Pheochromocytoma is a catecholamine-producing tumor, with preferential localization in the adrenal medulla. The distinction between adrenal cortical tumors and pheochromocytoma is sometimes difficult to determine because of confounding clinical presentations, overlapping morphologies, and some degrees of immunophenotypic overlap. Thus, this study also aims to confirm whether USP10 immunoreaction can be useful in distinguishing adrenal cortical tumors from pheochromocytomas.
Materials and Methods
Patients and Tissue Samples
All cases were taken consecutively and retrospectively from the Department of Pathology, Renmin Hospital of Wuhan University. Surgical samples were fixed by immersion in 4 % buffered formalin for 24–48 h, sampled, and then paraffin-embedded. After obtaining written consent, demographic and clinical pathological data were collected in the hospitals using a standard interviewer-administered questionnaire and/or medical records. This study was approved by the ethics committee of the hospitals involved.
Tissue was obtained from 30 adrenocortical adenomas, nine adrenocortical carcinomas, and 20 adrenal pheochromocytomas following laparoscopic surgery. A total of 20 normal adrenal glands were obtained from kidney surgical resections, which were conducted because of renal cell carcinomas, free of metastatic disease, and other pathologies. Differential diagnosis between adrenocortical adenomas and carcinomas was performed according to the standard criteria described by Wesis [12]. All carcinoma cases presented vascular and capsular invasion, cellular pleomorphism, atypical mitosis, high mitotic rates, and a variable amount of necrosis. Differential diagnosis between adrenocortical tumors and pheochromocytomas was performed according to morphologic features and immunohistochemical staining results of Melan A and CgA.
Patients for normal adrenal glands were aged from 23 to 75 years old (mean: 55.85 years old), with male predominance (12 males and 8 females). Patients for adrenocortical adenomas ranged from 33 to 76 years old (means: 51.23 years old), with equal sex distribution (15 males and 15 females). Patients for adrenocortical carcinomas ranged from 34to 62 years old (mean: 50.22 years old), with female predominance (two males and seven females). Patients for pheochromocytomas ranged from 15 to 71 years old (mean: 44.50 years old), with female predominance (six males and 14 females). A wide range in tumor sizes was seen in adrenocortical adenomas (0.8 to 6 cm, mean 2.27 cm), adrenocortical carcinomas (1.7 to 9 cm, mean 5.27 cm), and pheochromocytomas (0.8 to 6 cm, mean 4.96 cm). All pheochromocytomas lacked metastatic disease at the time of resection.
Immunohistochemistry
Immunostaining was performed as described previously [6]. Briefly, deparaffinized sections were treated with 3 % H2O2, and then subjected to antigen retrieval by citric acid (pH 6.0). After overnight incubation with primary antibody (anti-USP10, 1:250, ab109219, Abcam, Cambridge, MA; anti-CgA (MAB-0142, DO-7) and anti-Melan A (MAB-0589, A42C7), ready-to-use, Maixin Bio, Fujian, China) at 4 °C, the sections were incubated for 15 min at room temperature with horseradish peroxidase-labeled polymer conjugated with secondary antibody ((MaxVision™ Kits) and 1 min with diaminobenzidine. Afterward, the sections were lightly counterstained with hematoxylin. The sections without primary antibody served as negative controls. The immunohistochemical staining results of USP10 in all sections were evaluated by two independent observers (Zeng and Zhou) unaware of the disease outcome.
Gene Expression Data Analysis
Human normal adrenal gland and adrenal tumor data sets, as well as their corresponding clinical data, were downloaded from the public available Gene Expression Omnibus (GEO) datasets (http://www.ncbi.nlm.nih.gov/gds/). Two independent data sets from GDS1096 and GDS3113 were utilized to analyze the relative expression level of USP10 messenger RNA (mRNA) in various types of human normal tissues [13, 14]. Two independent data sets from GSE10927 and GSE39716 were utilized to analyze the ratio of USP10/GAPDH mRNA in human normal adrenal cortex (n = 10) and medulla (n = 8) [15, 16]. One independent data set from GSE68889 was utilized to analyze the ratio of USP10/GAPDH mRNA in different layers of the human normal adrenal gland cortex (n = 4). One independent data set from GSE10927 was utilized to analyze the ratio of USP10/GAPDH mRNA in the human normal adrenal gland cortex (n = 10), adrenocortical adenoma (n = 22), and adrenocortical carcinoma (n = 33). One independent data set from GSE39716 was utilized to analyze the ratio of USP10/GAPDH mRNA in the human normal adrenal gland medulla (n = 8) and pheochromocytoma (n = 21). For GDS1096, GSE10927, and GSE68889, the intensity of probe 209137_s_at was used to represent the USP10 expression level, whereas the intensity of probe 212581_x_at was used to represent the GAPDH expression level. For GDS3113, the intensity of probe 222023 was used to represent the USP10 expression level. For GSE39716, the intensity of probe 7997633 was used to represent the USP10 expression level, whereas the intensity of probe 7953385 was used to represent the GAPDH expression level. The housekeeping gene GAPDH expression was used as internal control.
Statistical Analysis
All the experimental data were analyzed by SPSS 15.0 statistical software (SPSS, Inc., Chicago, IL, USA). Comparisons of proportions were evaluated using a chi-square test. Student’s two-tailed t test was used to compare data between two groups. ANOVA was used to compare data between three groups. A p value of less than 0.05 was regarded as statistically significant.
Results
USP10 Protein Expression in Human Normal Adrenal Glands
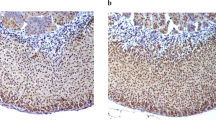
In all normal adrenal glands examined, USP10 protein was constantly expressed in situ in the cortex of adrenal glands, but in the medulla of the adrenal gland, only the sustentacular cells were detected positive (Fig. 1a, b). In addition, USP10 expression was differently distributed in different layers of the adrenal cortex. In glomerular cell layer, positive reaction was most frequently observed in the cytoplasm in a diffuse but weak form (Fig. 1e). In the zona fasciculata, cells showed intense and continuous immunoreactions in the cytomembrane (Fig. 1d). In the zona reticularis, cells showed intense and diffused immunoreactions in the cytoplasm (Fig. 1c). All adrenal medulla cells were always completely negative, except for sustentacular cells (Fig. 1c).
Immunohistochemical detection of USP10 protein in normal adrenal gland. (a) HE staining of normal adrenal gland (original magnification, ×40). (b) Sections are stained with a monoclonal antibody against USP10 and counterstained with hematoxylin in normal adrenal gland (original magnification, ×40). (c, d, e) Slides represent different layers of the adrenal gland in Fig. 2b (original magnification, ×200). Zg: zona glomerulosa; Zf: zona fasciculata; Zr: zona reticularis; M: adrenal medulla
USP10 mRNA Expression in Human Normal Adrenal Glands
To obtain USP10 mRNA expression level, two independent microarray data sets containing various types of human normal tissues were analyzed, including GDS1096 and GDS3113. The data sets showed that USP10 mRNA can be detected in human normal adrenal gland tissues, as well as other multiple tissues. The data set from GDS1096 showed that the relative expression levels of USP10 mRNA in the adrenal gland, brain, kidney, breast, and stomach were 1, 0.45, 0.94, 0.84, and 0.76, respectively (Fig. 2a). The data set from GDS3113 showed that the relative expression levels of USP10 mRNA in the adrenal gland, brain, kidney, and breast were 1, 1.12, 0.71, and 0.57, respectively (Fig. 2b). Afterward, we further analyzed the relative mRNA expression level of USP10 in the human normal adrenal cortex and medulla from GSE10927, GSE39716, and GSE68889. The data sets showed that USP10 mRNA can be detected in the zona fasciculata, zona reticularis, zona glomerular, and medulla. However, no significant difference among these regions was found (Fig. 2c and d).
USP10 mRNA expression in the human normal adrenal gland and other tissues is detected by bioinformatics analysis in the GEO datasets. (a) The data set from GDS1096 shows the relative expression level of USP10 mRNA in the adrenal gland, brain, kidney, breast, and stomach. (b) The data set from GDS3113 shows the relative expression level of USP10 mRNA in the adrenal gland, brain, kidney, and breast. (c) The data sets from GSE10927 and GSE39716 shows the ratio of USP10/GAPDH mRNA in the human normal adrenal cortex and medulla (Student’s two-tailed t test). (d) The data set from GSE68889 shows the ratio of USP10/GAPDH mRNA in the zona glomerulosa, zona fasciculata, and zona reticularis (ANOVA)
USP10 Protein Expression in Adrenal Tumors
In adrenal tumors, USP10 protein expression was detected in 100 % (30/30) adrenocortical adenomas, and 88.9 % (8/9) adrenocortical carcinomas (Fig. 3). In adrenocortical adenomas, two different patterns of USP10 staining can be observed in the each case: (1) diffused and strong cytoplasmic reactivity with USP10 (Fig. 4a) and (2) continuous and strong immunoreactions in the cytomembrane (Fig. 4b). In adrenocortical carcinomas, diffused and moderate intensity cytoplasmic USP10 reactivity was identified in eight of nine cases (Fig. 4c). Pheochromocytoma did not have a constant appearance. Two different cases presented an immunoreaction comparable with adrenocortical adenomas, where positivity was restricted to focal areas; whereas, 18 cases were completely negative (Fig. 3). In addition, sustentacular cells immunostained for USP10 protein at the periphery of the tumor nests can be observed in all pheochromocytomas (Fig. 4d).
Immunohistochemical detection of USP10 protein in adrenal tumors. (a) Adrenocortical adenoma: neoplastic cells show intense and diffused immunoreactions in the cytoplasm. (b) Adrenocortical adenoma: neoplastic cells show intense and continuous immunoreactions in the cytomembrane. (c) Adrenocortical carcinoma: tumor showing USP10-positivity. (d) Pheochromocytoma: tumor showing USP10-negative. DAB staining (brown); nuclear counterstaining (hematoxylin); original magnification, ×200
USP10 mRNA Expression in Adrenal Tumors
To obtain USP10 mRNA expression level in adrenal tumors, two independent microarray data sets containing various adrenal tumors were analyzed, including GSE10927 and GSE39716. The data set from GSE10927 showed that USP10 mRNA can be detected in benign and malignant adrenocortical tumors and in normal adrenal gland cortex. However, the ratio of USP10/GAPDH mRNA in the human normal adrenal gland cortex (n = 10), adrenocortical adenoma (n = 22), and adrenocortical carcinoma (n = 33) were 0.74 ± 0.01, 0.73 ± 0.01, and 0.75 ± 0.03, respectively. No significant difference among these groups was observed (Fig. 5a). The data set from GSE39716 showed that USP10 mRNA can also be detected in the human normal adrenal gland medulla and pheochromocytoma. However, the ratio of USP10/GAPDH mRNA in the human normal adrenal gland medulla (n = 8) and pheochromocytoma (n = 21) were 0.71 ± 0.03 and 0.71 ± 0.02, respectively. No significant difference was observed between these two groups (Fig. 5b).
USP10 mRNA expression in adrenal tumors. (a) The data set from GSE10927 shows the ratio of USP10/GAPDH mRNA in the human normal adrenal cortex, adrenocortical adenoma, and adrenocortical carcinoma (ANOVA). (b) The data set from GSE39716 shows the ratio of USP10/GAPDH mRNA in the human normal adrenal medulla and pheochromocytoma (Student’s two-tailed t test)
Discussion
USP10 is a protein consisting of 798 amino acids and belongs to the USPs family of cysteine proteases. USP10 has a critical role in modulating ARs function. Overexpression of USP10 enhances ARs transcriptional activity, whereas its knockdown has the opposite effect. Moreover, USP10 was originally detected in DNA-bound ARs complexes, two of which may interact with each other [10, 11]. The adrenal gland is an endocrine organ. The zona fasciculata and reticularis of the adrenal cortex are sites for sex hormone production (adrenal androgens). In addition, the biological action of androgens is mediated through the ARs. These findings promoted us to investigate the expression of USP10 in human adrenal glands.
In this study, we demonstrated that USP10 protein is constantly expressed in situ in the adrenal gland cortex, mostly in the zona fasciculata and reticularis, but not in the medulla. In the zona fasciculata, cells showed intense and continuous immunoreactions in the cytomembrane. In the zona reticularis, cells show intense and diffused immunoreactions in the cytoplasm. In the glomerular cell layer, positive reaction is most frequently observed in the cytoplasm in a diffused but weak form. The distribution of USP10 protein and androgen production showed an overlap in the adrenal gland, indicating that the function of USP10 in the adrenal gland cortex may be associated with androgen through the regulation of AR protein stabilization. In addition, we further analyzed the expression of USP10 mRNA in the human normal adrenal gland using bioinformatics analysis in the GEO datasets. USP10 expression can be detected in multiple human normal tissues, including the brain, kidney, breast, and stomach. Therefore, these tissues were used as an internal control. In this study, the data sets showed that the expression level of USP10 mRNA in the human normal adrenal gland was similar to that in the brain, kidney, breast and stomach. Further bioinformatics analysis in the GEO datasets demonstrated that the expression level of USP10 mRNA also can be observed in the adrenal gland medulla, which indicated that the absence of USP10 protein in the medulla occurred at post-transcriptional level.
Recent studies demonstrated that USP10 is involved in various biological processes through regulation of protein stabilization [17]. For example, USP10 plays a role in facilitating the deubiquitination of CFTR in early endosomes, thereby enhancing the endocytic recycling of CFTR [18, 19]. Th2-dominated inflammation can be inhibited by USP10 through deubiquitination and stabilization of T-bet [2]. USP10 antagonizes c-myc transcriptional activation through SIRT6 stabilization to suppress tumor formation [20]. USP10 deubiquitylates the histone variant H2A.Z and functions in AR-mediated gene activation [11]. USP10 is a host factor that inhibits stress-induced reactive oxygen species production and apoptosis in HTLV-1-infected T cells [21]. USP10 inhibits genotoxic NF-κB activation by MCPIP1-facilitated deubiquitination of NEMO [22]. USP10 immunoreactivity has been reported in a limited number of tumors. Increased USP10 expression has been detected in some breast cancer and glioblastoma samples [4, 5]. Decreased USP10 expression has been found in gastric cancer and renal cancer tissues [6]. However, little information is available concerning USP10 protein expression in adrenal tumors. In the present study, we investigated the expression of USP10 protein in various adrenal tumors and found that the frequency of USP10 protein-positive expression in adrenal cortical tumors (100 % adrenocortical adenomas and 89 % adrenocortical carcinomas) was higher than that in pheochromocytomas (10 %). However, the result of bioinformatics analysis in the GEO datasets showed that no significant differences among adrenocortical adenomas, adrenocortical carcinomas, and pheochromocytomas. These findings suggest that only a positive USP10 protein-immunoreaction can be useful in distinguishing adrenal cortical tumors from pheochromocytoma.
Differential diagnosis between benign and malignant adrenal cortical tumors can be a difficult task. Therefore, we also wanted to know whether USP10 is a useful marker in distinguishing adrenocortical adenoma and carcinoma. However, in this study, the result of USP10 on protein levels using immunohistochemistry and on mRNA levels using bioinformatics analysis in GEO datasets failed to distinguish adrenocortical adenoma from adrenocortical carcinoma.
References
Soncini C, Berdo I, Draetta G (2001) Ras-GAP SH3 domain binding protein (G3BP) is a modulator of USP10, a novel human ubiquitin specific protease. Oncogene 20 (29):3869-3879. doi:10.1038/sj.onc.1204553
Pan L, Chen Z, Wang L, Chen C, Li D, Wan H, Li B, Shi G (2014) Deubiquitination and stabilization of T-bet by USP10. Biochem Biophys Res Commun 449 (3):289-294. doi:10.1016/j.bbrc.2014.05.037
Liu J, Xia H, Kim M, Xu L, Li Y, Zhang L, Cai Y, Norberg HV, Zhang T, Furuya T, Jin M, Zhu Z, Wang H, Yu J, Hao Y, Choi A, Ke H, Ma D, Yuan J (2011) Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell 147 (1):223-234. doi:10.1016/j.cell.2011.08.037
Deng S, Zhou H, Xiong R, Lu Y, Yan D, Xing T, Dong L, Tang E, Yang H (2007) Over-expression of genes and proteins of ubiquitin specific peptidases (USPs) and proteasome subunits (PSs) in breast cancer tissue observed by the methods of RFDD-PCR and proteomics. Breast Cancer Res Treat 104 (1):21-30. doi:10.1007/s10549-006-9393-7
Grunda JM, Nabors LB, Palmer CA, Chhieng DC, Steg A, Mikkelsen T, Diasio RB, Zhang K, Allison D, Grizzle WE, Wang W, Gillespie GY, Johnson MR (2006) Increased expression of thymidylate synthetase (TS), ubiquitin specific protease 10 (USP10) and survivin is associated with poor survival in glioblastoma multiforme (GBM). J Neurooncol 80 (3):261-274. doi:10.1007/s11060-006-9191-4
Zeng Z, Wu HX, Zhan N, Huang YB, Wang ZS, Yang GF, Wang P, Fu GH (2014) Prognostic significance of USP10 as a tumor-associated marker in gastric carcinoma. Tumour Biol 35 (4):3845-3853. doi:10.1007/s13277-013-1509-1
Yuan J, Luo K, Zhang L, Cheville JC, Lou Z (2010) USP10 regulates p53 localization and stability by deubiquitinating p53. Cell 140 (3):384-396. doi:10.1016/j.cell.2009.12.032
Liu H, Xu XF, Zhao Y, Tang MC, Zhou YQ, Lu J, Gao FH (2014) MicroRNA-191 promotes pancreatic cancer progression by targeting USP10. Tumour Biol 35 (12):12157-12163. doi:10.1007/s13277-014-2521-9
Bentvelsen FM, McPhaul MJ, Wilson CM, Wilson JD, George FW (1996) Regulation of immunoreactive androgen receptor in the adrenal gland of the adult rat. Endocrinology 137 (7):2659-2663. doi:10.1210/endo.137.7.8770883
Faus H, Meyer HA, Huber M, Bahr I, Haendler B (2005) The ubiquitin-specific protease USP10 modulates androgen receptor function. Mol Cell Endocrinol 245 (1-2):138-146. doi:S0303-7207(05)00405-3
Draker R, Sarcinella E, Cheung P (2011) USP10 deubiquitylates the histone variant H2A.Z and both are required for androgen receptor-mediated gene activation. Nucleic Acids Res 39 (9):3529-3542. doi:10.1093/nar/gkq1352
Aubert S, Wacrenier A, Leroy X, Devos P, Carnaille B, Proye C, Wemeau JL, Lecomte-Houcke M, Leteurtre E (2002) Weiss system revisited: a clinicopathologic and immunohistochemical study of 49 adrenocortical tumors. Am J Surg Pathol 26 (12):1612-1619
Ge X, Yamamoto S, Tsutsumi S, Midorikawa Y, Ihara S, Wang SM, Aburatani H (2005) Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 86 (2):127-141. doi:S0888-7543(05)00111-4
Dezso Z, Nikolsky Y, Sviridov E, Shi W, Serebriyskaya T, Dosymbekov D, Bugrim A, Rakhmatulin E, Brennan RJ, Guryanov A, Li K, Blake J, Samaha RR, Nikolskaya T (2008) A comprehensive functional analysis of tissue specificity of human gene expression. BMC Biol 6:49. doi:10.1186/1741-7007-6-49
Giordano TJ, Kuick R, Else T, Gauger PG, Vinco M, Bauersfeld J, Sanders D, Thomas DG, Doherty G, Hammer G (2009) Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res 15 (2):668-676. doi:10.1158/1078-0432.CCR-08-1067
Giubellino A, Shankavaram U, Bullova P, Schovanek J, Zhang Y, Shen M, Patel N, Elkahloun A, Lee MJ, Trepel J, Ferrer M, Pacak K (2014) High-throughput screening for the identification of new therapeutic options for metastatic pheochromocytoma and paraganglioma. PLoS One 9 (4):e90458. doi:10.1371/journal.pone.0090458
Cheng LL, Itahana Y, Lei ZD, Chia NY, Wu Y, Yu Y, Zhang SL, Thike AA, Pandey A, Rozen S, Voorhoeve PM, Yu Q, Tan PH, Bay BH, Itahana K, Tan P (2012) TP53 genomic status regulates sensitivity of gastric cancer cells to the histone methylation inhibitor 3-deazaneplanocin A (DZNep). Clin Cancer Res 18 (15):4201-4212. doi:10.1158/1078-0432.CCR-12-0036
Bomberger JM, Barnaby RL, Stanton BA (2010) The deubiquitinating enzyme USP10 regulates the endocytic recycling of CFTR in airway epithelial cells. Channels (Austin) 4 (3):150-154. doi:11223
Bomberger JM, Barnaby RL, Stanton BA (2009) The deubiquitinating enzyme USP10 regulates the post-endocytic sorting of cystic fibrosis transmembrane conductance regulator in airway epithelial cells. J Biol Chem 284 (28):18778-18789. doi:10.1074/jbc.M109.001685
Lin Z, Yang H, Tan C, Li J, Liu Z, Quan Q, Kong S, Ye J, Gao B, Fang D (2013) USP10 antagonizes c-Myc transcriptional activation through SIRT6 stabilization to suppress tumor formation. Cell Rep 5 (6):1639-1649. doi:10.1016/j.celrep.2013.11.029
Takahashi M, Higuchi M, Makokha GN, Matsuki H, Yoshita M, Tanaka Y, Fujii M (2013) HTLV-1 Tax oncoprotein stimulates ROS production and apoptosis in T cells by interacting with USP10. Blood 122 (5):715-725. doi:10.1182/blood-2013-03-493718
Niu J, Shi Y, Xue J, Miao R, Huang S, Wang T, Wu J, Fu M, Wu ZH (2013) USP10 inhibits genotoxic NF-kappaB activation by MCPIP1-facilitated deubiquitination of NEMO. EMBO J 32 (24):3206-3219. doi:10.1038/emboj.2013.247
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81571147 and 81301019) and Independent Scientific Research Subject for Young Teachers of Wu Han University (Grant No. 2042015kf0076).
Authors’ Contribution
Zeng Z: Project development and manuscript writing
Zhou ZY, Zhan N, Yuan JP, and Ye BX: Data collection or management
Gu LJ, Wang J, and Jian ZH: Data analysis
Xiong XX: Protocol development and manuscript editing
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the ethics committee of Renmin Hospital of the Wuhan University and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Zeng, Z., Zhou, Z., Zhan, N. et al. USP10 Expression in Normal Adrenal Gland and Various Adrenal Tumors. Endocr Pathol 26, 302–308 (2015). https://doi.org/10.1007/s12022-015-9406-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-015-9406-3