Abstract
Clinically nonfunctioning pituitary adenomas (NFAs) may be hormonally inactive tumors of differentiated cells, mainly not only gonadotroph adenomas (GAs) but also silent corticotroph adenomas (SCAs) and other differentiated silent adenomas. Recently, the use of transcription factors has been recommended to confirm cytodiffererentiation of these neoplasms. Our objective was to assess the clinical significance of the new classification system using transcription factors. Five hundred sixteen consecutive NFAs were studied retrospectively. They were initially classified based on hormone immunohistochemistry as follows: 119 hormone-negative adenomas (23.1 %), 300 GAs (58.1 %), 51 SCAs (9.9 %), and 46 other silent adenomas. The 119 hormone-negative adenomas were further evaluated for expression of transcription factors including steroidogenic factor-1 (SF-1), estrogen receptor-α (ERα), pituitary-specific transcription factor 1 (Pit-1), and t-box transcription factor (Tpit). One hundred thirteen of 119 (95 %) hormone-negative adenomas showed mutually exclusive lineage-specific differentiation as gonadotrophs (SF-1 positive), corticotrophs (Tpit positive), or somatotrophs/mammosomatotrophs/lactotrophs/thyrotrophs (Pit-1 positive) in 79 cases (66.4 %), 32 cases (26.9 %), and 2 cases, respectively. The 32 ACTH-negative and Tpit-positive adenomas had higher pro-opiomelanocortin mRNA expression levels compared with GAs (P = 0.0001) on quantitative real-time PCR. They showed a female preponderance (P < 0.0001) and were more frequently giant adenomas (P = 0.0028) associated with marked cavernous sinus invasion (P < 0.0001) compared with GAs. These clinical features were identical to those of the 51 ACTH-positive SCAs. Our results justify the complementary role of transcription factors in the precise classification of NFAs that can more accurately characterize biological behavior. Our data suggest that more than one quarter of hormone-negative adenomas are SCAs that share distinct clinicopathological features with ACTH-expressing SCAs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 30–40 % of all surgically treated pituitary adenomas are clinically nonfunctioning adenomas (NFAs) since they lack clinical and biochemical evidence of adenohypophyseal hormone excess [1, 2]. Morphologically, they are heterogeneous and have been traditionally classified into several histological subtypes by various methods including immunohistochemistry using antibodies against adenohypophyseal hormones, electron microscopy, in situ hybridization, or reverse hemolytic plaque assay [1, 3–7]. Some differences in clinical behavior have become evident for each subtype. However, the distinct classification of NFA remains unclear in some adenomas due to negative immunohistochemistry for hormones. Recently, Asa and coworkers [1, 8–10] proposed the application of transcription factors’ immunolocalization to determine adenohypophyseal cell lineages for the accurate subclassification of pituitary adenomas.
Among various types of NFAs, silent corticotroph adenomas (SCAs), which were first reported by Kovacs et al. as a distinct clinicopathological entity [11], represent a clinically important subtype since they often exhibit aggressive clinical features [1, 3, 12–16]. Traditionally, SCAs are distinguished from other NFAs by their ACTH immunoreactivity. However, regardless of the functional status of the tumor, corticotroph adenomas, like nontumorous corticotrophs, express the t-box pituitary transcription factor (Tpit) [1, 8–10, 15, 17].
The aims of the present study were (1) to assess the clinical significance of the new classification system using a complete panel of pituitary transcription factors for NFAs that are traditionally diagnosed in light of hormone immunohistochemistry alone and (2) to detect distinct clinicopathological features of reclassified adenomas.
Materials and Methods
Patients
We retrospectively studied 516 consecutive patients with NFAs who underwent surgery at Toranomon Hospital between 2008 and 2011. These patients lacked clinical and biochemical evidence of adenohypophyseal hormone excess. Basal levels of pituitary and related hormones, apart from prolactin (due to stalk effect), were not elevated. For few patients with high levels of GH or ACTH, glucose tolerant test or low-dose dexamethasone suppression test was performed, respectively, to deny pathological hormone excess. They accounted for 48.2 % (516/1071) of surgically treated pituitary adenomas during this period. The 516 patients included 268 men and 248 women, age 13 to 83 (mean ± standard deviation 53.7 ± 14.2) years. There were 115 cases (22.3 %) which were recurrent adenomas. Informed consent was obtained from all patients, and this study was approved by the ethics committee at Toranomon hospital, Tokyo.
MRI Findings
In addition to patient age and gender, the following three preoperative MRI findings were assessed: giant adenoma (maximum size >40 mm), marked invasion of the cavernous sinus (CSI; Knosp grade 4 [18]), and marked lobulated configuration of the suprasellar tumor (Fig. 1a) [2]. Among the 516 adenomas, giant adenomas, those with marked CSI, and those with lobulated configuration comprised 55 adenomas (10.7 %), 48 adenomas (9.3 %), and 79 adenomas (15.3 %), respectively.
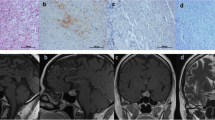
A large nonfunctioning pituitary adenoma with marked cavernous sinus invasion and suprasellar lobulation (a) in a 35-year-old woman. The chromophobic adenoma (b) was ACTH-“negative” except for one positive cell (arrow, c), Tpit-positive (d), SF-1-negative (e), and intensely positive for CAM5.2 (f). A honeycomb Golgi structure (arrow) was observed at ultrastructural examination (g)
Immunohistochemistry
Each pituitary adenoma was subjected to routine histological and immunohistochemical assessment. Immunohistochemistry was performed by using the BenchMark GX automated system (Ventana). The following antibodies were used according to protocols established in our laboratory: growth hormone (GH, 54/9 2A2; BioGenex), prolactin (polyclonal; DAKO), beta-thyroid-stimulating hormone (beta-TSH, 0042; DAKO), adrenocorticotropic hormone (ACTH, 02A3; DAKO), beta-follicle-stimulating hormone (beta-FSH, C10; BioGenex), beta-luteinizing hormone (beta-LH, C93; DAKO), Ki-67 (MIB-1; DAKO), and cytokeratin (CAM5.2; BECTON). Several pathologists reviewed specimens and classified pituitary adenomas as suggested in the 2004 WHO classification [3]. Pituitary adenomas were considered hormone negative if less than 1 % of the cells stained positively for any of the adenohypophyseal hormones. Ultrastructural study was performed in challenging or unusual cases.
Tumors originally classified as hormone-negative NFAs and a few silent adenomas were further subjected to a panel of antibodies against adenohypophyseal cell lineage transcription factors including steroidogenic Factor-1 (SF-1, N1665; Perseus Proteomics), estrogen receptor-α (ERα), pituitary transcription factor 1 (Pit-1, sc-136034; Santa Cruz), and Tpit (antiserum courtesy of Dr. J. Drouin, performed at University Health Network). While SF-1 is the gonadotroph cell lineage-specific transcription factor in the sellar region, ERα can be expressed in both gonadotrophs and lactotrophs. Pit-1 is a GH/prolactin/TSH cell lineage-specific transcription factor. Tpit is specific to pro-opiomelanocortin (POMC)-producing cell lineage. Appropriate positive and negative controls were selected during the evaluation of each antibody. In general, positivity for a transcription factor is diffuse for a specific cell lineage. Since pituitary adenomas can sometimes contain entrapped nontumorous cells of various cell lineage, the transcription factors were considered positive only when nuclear reactivity was identified in more than 80 % of the tumor cells.
Quantitative Real-Time PCR
Expression of POMC mRNA was evaluated in 20 functioning corticotroph adenomas (FCAs), 5 SCAs, 10 hormone-negative and Tpit-positive adenomas, 6 hormone-negative and SF-1-positive adenomas, and 5 gonadotroph adenomas (GAs). Pituitary tumor samples were preserved in RNAlater® (Life Technologies, Carlsbad, CA) solution at −80 °C until RNA extraction. Total RNA was prepared using RNeasy mini kit (Qiagen, Venlo, Netherlands) according to the manufacturer’s protocol. RNA was reverse transcribed using PrimeScript® RT reagent kit (Takara Bio, Otsu, Japan). With the resulting cDNA as a template, quantitative real-time PCR (qRT-PCR) was performed with Power SYBR Green PCR Master Mix (Life Technologies) in an Applied Biosystems® StepOnePlus™ cycler Mix (Life Technologies). The primer sequences were as follows: ACTH precursor polypeptide POMC forward 5′-GCCAGTGTCAGGACCTCAC-3′ and reverse 5′-GGGAACATGGGAGTCTCGG-3′ [19]; and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward 5′-GGCCTCCAAGGAGTAAGACC-3′ and reverse 5′-AGGGGAGATTCAGTGTGGTG-3′. Relative quantification was performed using the standard curve methods. Results of POMC mRNA expression levels were normalized to GAPDH and expressed relative to the mean expression of normal pituitary samples defined as 100 %.
Statistical Analysis
In addition to the chi-square analysis, groups were compared by the Mann-Whitney U test; P < 0.05 was considered significant in all cases. Statistical analysis was carried out with StatView 5.0 software for Macintosh (SAS Institute, Cary, NC).
Results
Based on conventional classification by hormone immunohistochemistry alone, 516 NFAs were initially classified as: 119 hormone-negative adenomas (23.1 %), 300 GAs (58.1 %), 51 SCAs (9.9 %), and 46 silent GH/prolactin/TSH-lineage adenomas (8.9 %) (Fig. 2). Patients with silent SCA and GH/prolactin/TSH-lineage adenomas showed significantly different clinical features from those with GAs. The 51 patients with SCA had a significant female preponderance (P < 0.0001), more frequent CSI (P < 0.0001), and more frequent lobulated configurations (P < 0.0001) compared with 300 GAs. The 46 patients with silent GH/prolactin/TSH-lineage adenomas were younger (P < 0.0001), more frequently associated with CSI (P < 0.0001) and lobulated configurations (P = 0.0281), and showed a higher MIB-1 index (P = 0.0063) compared with 300 GAs. Further immunohistochemical evaluation with pituitary transcription factors revealed that of the 119 hormone-negative adenomas, lineage-specific differentiation was as follows: 79 (66.4 %) gonadotrophs (positive for SF-1 and/or ERα), 32 (26.9 %) corticotrophs (positive for Tpit, Fig. 1), and 2 (1.7 %) somatotroph/lactotroph/mammosomatotroph/thyrotrophs (positive for Pit-1) (Fig. 2). Their expression was mutually exclusive and none of them showed overlapping immunoreactivity beyond each lineage (Fig. 1d, e).
Histological classification of 1071 pituitary adenomas. Functioning adenomas (FA) and nonfunctioning adenomas (NFA) were classified into three distinct groups: gonadotroph adenomas (GA) and the counterpart silent GA (SGA), corticotroph adenomas (CA) and the counterpart silent CA (SCA), and GH/prolactin/TSH-lineage adenomas (somatomammothyrotroph adenomas; SMTA) and the counterpart silent SMTA (SSMTA), respectively. One hundred nineteen hormone-negative adenomas (HNA) were subclassified into four groups: 79 SF-1-positive SGAs, 32 Tpit-positive SCAs, two Pit-1-positive SSMTAs, and six transcription factor-negative null cell adenomas (NCA)
Among the 79 hormone-negative and SF-1 positive GAs, there were very few (less than 1 %) scattered or isolated cell groups mimicking trapped nontumorous adenohypophyseal cells. These cells were faintly positive for beta-FSH and less frequently beta-LH. The age, gender, MRI findings, MIB-1 labeling indices, and clinical features of the 79 GAs resembled those of 300 gonadotropin-positive GAs. They showed slight male preponderance and were less frequently associated with giant adenomas, CSI, and lobulations.
Two “hormone-negative” adenomas were found to be positive for Pit-1. On review, both adenomas were faintly positive for GH. In addition, they were positive for CAM5.2 in the cytoplasmic dot-like pattern characteristic of sparsely granulated somatotroph adenomas. The two patients, a 65-year-old woman and a 57-year-old man, had a macroadenoma without aggressive feature on MRI.
Only six adenomas (5.0 % of 119 hormone-negative adenomas, 1.2 % of 516 NFA, and 0.6 % of 1071 total adenomas) were null cell adenomas with complete negativity for all adenohypophyseal hormones and transcription factors. They were poorly differentiated adenomas on ultrastructural study. Although their MIB-1 labeling indices were not elevated, three of them were recurrent adenomas and two of them were giant adenoma with CSI and lobulated configuration.
There were 32 “hormone-negative” adenomas that were Tpit-positive (Fig. 1d), thus of corticotroph origin. In these 32 Tpit-positive SCAs, there were a very few ACTH-positive cells, which had initially been thought to represent trapped nontumorous corticotrophs at the time of original review (Fig. 1c). Of these 32, 30 adenomas (93.8 %) showed strong positivity for CAM5.2 (Fig. 1f); positive deposits were mainly perinuclear in 27 adenomas, dot-like cytoplasmic in two adenomas, and membranous in one adenoma. On ultrastructural study, a vacuolar change of Golgi complex, the honeycomb Golgi structure (Fig. 1g), was observed in 28 of 30 examined adenomas (93.3 %). On qRT-PCR, expression of POMC mRNA in 10 selected ACTH-negative and Tpit-positive adenomas was significantly higher than that in 5 selected GAs and 6 gonadotropin-negative and SF-1-positive GAs (P = 0.0001), was slightly lower than those in 5 ACTH-positive SCAs (not significant), and was significantly lower than those in 20 FCAs (P = 0.000011) (Fig. 3).
Pro-opiomelanocortin (POMC) mRNA expression levels normalized to GAPDH in functioning corticotroph adenomas (FCA), ACTH-positive silent corticotroph adenomas (SCA), ACTH-negative and Tpit-positive adenomas (Tpit), and gonadotropin-positive gonadotroph adenomas (GA) and gonadotropin-negative, SF-1-positive adenomas (SF-1)
Clinically, 32 cases of ACTH-negative and Tpit-positive adenoma showed a significant female preponderance (P < 0.0001), more frequently were giant adenomas (P = 0.0028) and more frequently were associated with marked CSI (P < 0.0001) compared with 376 cases with GAs and SF-1-positive GAs (Table 1). These clinical features were identical to those of 51 cases of ACTH-positive SCA in this series.
Discussion
The recent advances in molecular biology have clarified the cytodifferentiation pathways involved in the development of adenohypophyseal cells. During development, an organized, complex process of cell differentiation is orchestrated by specific transcription factors. These factors also have a role in determining the cytodifferentiation and hormone production of pituitary adenomas and, thus, can serve as diagnostic markers [1, 8–10, 20, 21]. The three main pathways of cell differentiation and the determined transcription factors are (1) corticotrophs determined by the Tpit transcription factor, (2) somatotrophs/lactotrophs/mammosomatotrophs/thyrotrophs determined by Pit-1, and (3) gonadotrophs determined by SF-1 and/or GATA-2 in the presence of ERα. Recently, Asa and coworkers [1, 8–10] proposed a modern approach to the accurate classification of pituitary adenomas by integrating adenohypophyseal cell lineage transcription factors (also known pituitary transcription factors) into a panel approach that also includes MIB-1, CAM5.2, and monoclonal antibodies against pituitary adenohypophyseal hormones.
Tpit is selectively expressed in both normal and adenomatous corticotrophs and thus represents a reliable marker of POMC-expressing pituitary cells [1, 8–10, 17, 21]. It has been shown that SCAs were commonly positive for ACTH and Tpit, although both POMC and Tpit mRNA levels were lower in SCA than FCA [17]. In a study using fluorescence immunohistochemistry by Cooper et al. [3], SCA was negative for Tpit, whereas corticotroph adenomas and SCAs were positive for SF-1; therefore, they proposed a pathologic and clinically distinct classification of SCAs as silent corticogonadotroph adenomas; these results may be explained by nonspecific fluorescence detection or antisera cross-reactivity. In contrast, the transcription factors were mutually exclusive in the present study. In our series, 51 SCAs were only positive for Tpit, whereas SF-1, ERα, and Pit-1 were all negative. GAs were positive for SF-1 and/or ERα but were negative for Tpit and Pit-1.
In the present classification system, six adenomas were completely negative for all hormones and transcription factors. These unclassified adenomas accounted for 5.0 % of 119 hormone-negative adenomas, 1.2 % of 516 NFA, and 0.6 % of 1071 surgically treated adenomas in the present series. They were poorly differentiated adenomas on ultrastructural study and were frequently associated with aggressive clinical and MRI features. Further studies are necessary for these rare adenomas that likely represent true “null cell” adenomas.
Approximately two thirds of the hormone-negative adenomas were positive for SF-1 and/or ERα and, thus, showed differentiation as gonadotrophs. The clinical and MRI features of these adenomas were identical to those of gonadotropin-positive GA. Since there were very few scattered cells or groups of cells that were faintly positive for gonadotropins, we consider this group to represent GA with low gonadotropin expression. This finding is in agreement with previous suggestions that the many hormone-negative adenomas are indeed SF-1 positive GAs [10]. Consequently, GAs accounted for approximately three quarters of NFAs in the present series. This is also consistent with the view that GAs are the most common NFAs [1–3, 8–10, 22, 23].
Two “hormone-negative” adenomas were positive for Pit-1 and therefore were considered to be in the family of GH/prolactin/TSH-lineage adenomas. While these patients lacked clinical and biochemical evidence of GH excess, both tumors were faintly positive for GH on review. These tumors were positive for CAM5.2 with a prominent cytoplasmic dot-like pattern corresponding to fibrous bodies. Therefore, both tumors were considered to represent sparsely granulated somatotroph adenomas. The very low levels of GH production likely failed to impact circulating GH/IGF1; therefore, these were silent somatotroph adenomas [1]. Thus, it was confirmed that appropriate use of transcription factors could prevent silent adenomas with weak hormone expression from being mistakenly classified as null cell adenomas [10].
On the other hand, 32 hormone-negative adenomas (26.7 %) were positive for Tpit and, thus, showed differentiation along the corticotroph lineage. Although these neoplasms were initially considered to be hormone-negative adenomas, very few ACTH-positive cells, which were initially regarded as trapped nontumorous corticotrophs, were identified in every case. Most adenoma cells were CAM5.2-positive in the perinuclear area and were found to have a honeycomb Golgi structure on ultrastructural study. The former is a common finding in FCAs and SCAs [1, 8–10]. The latter structure is considered to indicate either corticotroph or gonadotroph differentiation [24]. On qRT-PCR, expression of POMC mRNA in ACTH-negative and Tpit-positive adenomas was significantly higher than gonadotropin-positive GAs and SF-1-positive GAs. The latter justifies the role of Tpit over hormones in the determination of corticotroph origin of pituitary adenomas. Since the expression levels were lower than those in FCAs, we propose that nonfunctioning, ACTH-negative and Tpit-positive pituitary adenomas represent SCAs with decreased POMC expression. Furthermore, the clinical features of the 32 patients with ACTH-negative and Tpit-positive adenomas were quite characteristic and identical to those of the ACTH-positive SCAs; they showed significant female preponderance and were more frequently giant adenomas with marked CSI [2, 22].
SCAs are subclassified into two subtypes: SCA type 1 and type 2. The former resemble the densely granulated FCA presenting with Cushing’s disease and show intense ACTH immunoreactivity. On the other hand, type 2 SCAs resemble the uncommon chromophobic corticotroph adenomas (sparsely-granulated corticotroph adenomas) and show weak ACTH immunoreactivity. Both subtypes are positive for Tpit and express the POMC gene and its splicing products [1, 10]. We interpret the results as indicating that the 32 ACTH-negative and Tpit-positive adenomas represent type 2 SCA with low POMC expression. Importantly, our results indicate that the actual frequency of SCAs in NFAs is higher than previously estimated (16.1 % in the present study). Furthermore, immunohistochemistry for ACTH is insufficient to detect SCA, whereas assessment of Tpit expression is required for the accurate diagnosis.
Silent pituitary adenomas of corticotroph origin (SCAs) and those with Pit-1 positivity, indicating differentiation into the somatomammothyrotroph family (silent GH/prolactin/TSH-lineage adenomas), behave more aggressively than GAs. In general, giant adenomas, marked CSI, and lobulated suprasellar configuration represent tumors that tend to be resistance to surgical cure and are often associated with recurrence, indicating aggressiveness [2, 25]. These MRI features were significantly more common in patients with SCAs and silent GH/prolactin/TSH-lineage adenomas compared with those with GAs in the present study and our previous study as well [2, 22]. Approximately one fourth of NFAs belonged to these groups in the present study: SCAs and silent GH/prolactin/TSH-lineage adenomas consisted of 16.7 and 9.3 % of NFAs, respectively.
Our results indicate that in addition to the MIB-1 index, it is very important to achieve a correct histological diagnosis to predict prognosis and to aid in the decision regarding a role for adjuvant radiation therapy after surgery in patients with NFAs [1, 8, 10]. Our data indicate the limitations of achieving accurate classification of NFAs using hormone immunohistochemistry alone. We conclude that immunohistochemistry for pituitary transcription factors has a complementary role in obtaining an accurate diagnosis, particularly in hormone-negative pituitary adenomas.
References
Asa SL (2011) Tumors of the Pituitary Gland. Fascicle 15, 4th series. The Atlas of Tumor Pathology. Armed Forces Institute of Pathology, Washington DC
Nishioka H, Inoshita N, Sano T, Fukuhara N, Yamada S (2012) Correlation between histological subtypes and MRI findings in clinically nonfunctioning pituitary adenomas. Endocr Pathol 23:151-6
Cooper O, Ben-Shlomo A, Bonert V, Bannykh S, Mirocha J, Melmed S (2010) Silent corticotroph adenomas: clinical and cellular characteristics and long-term outcomes. Horm Canc 1: 80-92
Horvath E, Kovacs K (1992) Ultrastructural diagnosis of human pituitary adenomas. Microsc Res Tech 20: 107-135
Jameson JL, Klibanski A, Black PM, Zervas NT, Lindell CM, Hsu DW, Ridgway EC, Habener JF (1987) Glycoprotein hormone genes are expressed in clinically nonfunctioning pituitary adenomas. J Clin Invest 80: 1472-78
Kovacs K, Asa SL, Horvath E, Ryan N, Singer W, Killinger DW, Smyth HS, Scheithauer BW, Ebersold MJ (1990) Null cell adenomas of the pituitary: Attempts to resolve their cytogenesis. In: J Lechago, T Kameya (eds) Endocrine Pathology Update. Field and Wood, Philadelphia, pp 17-31
Yamada S, Asa SL, Kovacs K, Muller P, Smyth HS (1989) Analysis of hormone secretion by clinically nonfunctioning human pituitary adenomas using the reverse hemolytic plaque assay. J Clin Endocrinol Metab 68: 73-80
Al-Brahim NYY, Asa SL (2006) My approach to pathology of the pituitary gland. J Clin Pathol 59: 1245-53
Asa SL (2008) Practical pituitary pathology. What does the pathologists need to know? Arch Pathol Lab Med 132:1231-49
Mete O, Asa SL (2012) Clinicopathological correlations in pituitary adenomas. Brain Pathol 22: 443-453
Kovacs K, Horvath E, Bayley TA, Hassaram ST, Ezrin C (1978) Silent corticotroph cell adenoma with lysosomal accumulation and crinophagy. A distinct clinicopathologic entity. Am J Med 64: 492-9
Baldeweg SE, Pollock JR, Powell M, Ahlquist J (2005) A spectrum of silent corticotroph pituitary adenomas. Br J Neurosurg 19: 38-42
Raverot G, Wierinckx A, Jouanneau E, Auger C, Borson-Chazot F, Lachuer J, Pugeat M, Trouillas J (2010) Clinical, hormonal, and molecular characterization of pituitary adenomas without (silent corticotroph adenomas) and with Cushing’s disease. Eur J Endocrinol 163: 35-43
Sahli R, Christ ER, Seiler R, Kappeler A, Vajtai I (2006) Clinicopathologic correlations of silent corticotroph adenomas of the pituitary: report of four cases and literature review. Pathol Res Pract 202: 457-464
Scheithauer BW, Jaap AJ, Horvath E, Kovacs K, Lloyd RV, Meyer FB, Laws Jr ER, Young Jr WF (2000) Clinically silent corticotroph tumors of the pituitary gland. Neurosurgery 47: 723-730
Webb KM, Laurent JJ, Okonkwo DO, Lopes MB, Vance ML, Laws Jr ER (2003) Clinical characteristics of silent corticotroph adenomas and creation of an internet-accessible database to facilitate their multi-institutional study. Neurosurgery 53: 1076-85
Tateno T, Izumiyama H, Doi M, Yoshimoto T, Shichiri M, Inoshita N, Oyama K, Yamada S, Hirata Y (2007). Differential gene expression in ACTH-secreting and non-functioning pituitary tumors. Eur J Endocrinol 157: 717-24
Knosp E, Steiner E, Kitz K, Matla C (1993) Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33: 610-7
Du L, Bergsneider M, Mirsadraei L, Young SH, Jonker JW, Downes M, Yong WH, Evans RM, Heaney AP (2013) Evidence for orphan nuclear receptor TR4 in the etiology of Cushing disease. Proc Natl Acad Sci USA 110: 8555-60
DeLellis RA, Lloyd RV, Heitz PU, Eng C (2004) Pathology and genetics of tumours of the endocrine organs. IARC press, Lyon
Vallette-Kasic S, Figarella-Branger D, Grino M, Pulichino A-M, Dufour H, Grisoli F, Enjalbert A, Drouin J, Brue T (2003) Differential regulation of proopiomelanocortin and pituitary-restricted transcription factor (TPIT), a new marker of normal and adenomatous human corticotrophs. J Clin Endocrinol Metab 88: 3050-56
Yamada S, Ohyama K, Taguchi M, Takeshita A, Morita K, Takano K, Sano T (2007) A study of the correlation between morphological findings and biological activities in clinically nonfunctioning pituitary adenomas. Neurosurgery 61: 580-5
Young Jr WF, Scheithauer BW, Kovacs KT, Horvath E, Davis DH, Randall RV (1996) Gonadotroph adenoma of the pituitary gland: a clinicopathologic analysis of 100 cases. Mayo Clinic Proc 71: 649-56
Sano T, Mader R, Asa SL, Qian ZR, Hino A, Yamada S (2003) “Honeycomb Golgi” in pituitary adenomas: not a marker of gonadotroph adenomas. Endocr Pathol 14: 363-8
Nishioka H, Hara T, Usui M, Fukuhara N, Yamada S (2011) Simultaneous combined supra-infrasellar approach for giant/large multi-lobulated pituitary adenomas. World Neurosurg 77: 533-39
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Funding
This work was supported by the Okinaka Memorial Institute for Medical Research. This work was also supported in part by JSPS KAKENHI Grant Number 25460468.
Rights and permissions
About this article
Cite this article
Nishioka, H., Inoshita, N., Mete, O. et al. The Complementary Role of Transcription Factors in the Accurate Diagnosis of Clinically Nonfunctioning Pituitary Adenomas. Endocr Pathol 26, 349–355 (2015). https://doi.org/10.1007/s12022-015-9398-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-015-9398-z