Abstract
Some thyroid nodules such as follicular adenomas (FAs), follicular variant of papillary thyroid carcinomas (FVPTCs), and follicular thyroid carcinomas (FTCs) exhibit similar clinical presentations and gross morphologic appearances. The differential diagnosis of these lesions is sometimes difficult based on morphologic, cytologic, or clinical features alone. miR-146b-5p and miR-21 deregulation has been associated with progression and metastasis of thyroid cancers. However, the utility of in situ hybridization (ISH) to determine the cellular localization, diagnostic, and prognostic significance of miR-146b-5p and miR-21 expression in thyroid tumors has not been extensively analyzed. In order to examine the expression of miR-146b-5p and miR-21 in benign and malignant thyroid tissues and to determine if these microRNAs could be assigned to distinct histomorphological types of thyroid nodules, we analyzed miR-146b-5p and miR-21 expression in thyroid nodules on tissue microarrays (TMAs) with 193 thyroid specimens by ISH. miR-146b-5p and miR-21 expression in thyroid tissues was also analyzed by quantitative reverse transcription–polymerase chain reaction (qRT-PCR). miR-146b-5p was highly expressed (89 %) in papillary thyroid carcinomas (PTCs) and 41 % of FVPTC. The expression of miR-146b-5p was not expressed in most FTCs, anaplastic thyroid carcinomas (ATCs), poorly differentiated thyroid carcinomas (PDTCs), or FAs (7, 8, 0, and 0 %, respectively). MiR-21 was overexpressed in 83 % of ATCs, 79 % of PTCs, 34 % of FVPTCs, and 19 % of PDTCs. The expression of miR-21 was not expressed in most FAs (9 %) or FTCs (4 %). Normal thyroid tissues and most benign goiters were negative for miR-146b-5p and miR-21. qRT-PCR analysis supported the ISH findings. PTC cases with positive expression of miR-146b-5p and miR-21 had significantly poorer disease-free survival rates. Immunohistochemical staining for HBME-1 showed positive staining in PTCs (100 %) and FVPTCs (92 %) with a subset of FTC (40 %) staining positive, while all FAs were negative. Since miR-146b-5p was mainly expressed in PTC including FVPTC and was not expressed in most FTC, PDTC, or ATC, it may serve as a useful diagnostic marker for PTC. ISH is a useful method to analyze microRNA expression in formalin-fixed paraffin-embedded thyroid tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid cancer is one of the most rapidly increasing malignancies in the USA [1]. Follicular variant of papillary thyroid carcinomas (FVPTCs), follicular carcinomas (FTCs), and follicular adenomas (FAs) have similar clinical presentations and gross morphologic features. The differential diagnosis is sometimes difficult based on morphologic, cytologic, or clinical features alone. Recently, microRNAs have been shown to function as tumor suppressor genes or oncogenes and have been shown to be useful for cancer classification and prognostication [2]. MicroRNAs have been reported to be dysregulated in many human cancers, including all variants of thyroid cancer [3–5]. miR-146b-5p [6, 7] and miR-21 [8] deregulation has been associated with progression and metastasis of thyroid cancers. miR-146b-5p and miR-21 have been shown to be upregulated at least tenfold in several studies when comparing classic variant of papillary thyroid carcinoma (CPTC) to normal thyroid tissue by quantitative reverse transcription–polymerase chain reaction (qRT-PCR) analysis [6–8]. miR-21 has also been shown to be upregulated in ATCs and PTCs by qRT-PCR analyses [9–11].
qRT-PCR is a highly sensitive and specific technique. However, the use of qRT-PCR as a screening method for detecting microRNA may not be completely reliable for the suboptimal quality of RNAs obtained from formalin-fixed paraffin-embedded (FFPE) tissues available in pathology laboratories. ISH can localize specific miRNA within tissue in different compartments of the cytoplasm and in the nucleus. Wienholds et al. [12] introduced locked nucleic acid (LNA) into ISH probes and increased the affinity to target microRNA. The use of LNA-modified oligonucleotide probes has been shown to significantly improve the sensitivity and specificity of microRNA detection in endocrine tissues [13, 14]. In this study, we aimed to study the expression patterns of miR-146b-5p and miR-21 in thyroid lesions and to determine their possible utility in differentiating malignant from benign thyroid lesions.
Material and Methods
Tissue Microarray
Formalin-fixed paraffin-embedded tissue blocks of 193 thyroid specimens were selected according to tissue availability for construction of three tissue microarrays (TMAs) as previously reported [15]. Briefly, the TMA consisted of 10 normal thyroids (NTs), 10 nodular goiters (NGs), 32 follicular adenomas (FAs), 28 follicular carcinomas, 57 papillary thyroid carcinomas (PTCs), 21 poorly differentiated carcinomas (PDCs), and 35 anaplastic thyroid carcinomas (ATCs). Fifty-seven PTCs consist of 29 FVPTCs and 26 classical PTCs and two tall cell variants of PTCs. The first TMA from the University of Wisconsin had 147 cases while two TMAs constructed in Turin, Italy, consisted of 21 PDTCs and 25 ATCs, respectively. The study was approved by the IRB at the University of Wisconsin Medical Center and University of Turin, Turin, Italy. The TMA consisted of triplicate 0.6 mm of each case made by using a manual tissue microarray (Beecher Instruments, Sun Prairie, WI). Normal thyroid tissue was obtained from the opposite (histologically normal) lobe in patients with follicular or papillary carcinomas. Tissues from three independent patients of FVPTC were used to validate the findings from the TMA.
In Situ Hybridization
Formalin-fixed paraffin-embedded (FFPE) TMA sections were used for ISH with double digoxigenin (DIG)-labeled LNA probes labeled at both 3′ and 5′ ends. LNA in situ hybridization (ISH) on paraffin tissue sections with probes specific for human miR-146b-5p and miR-21 was performed according to the manufacturer’s instructions (Exiqon, USA) as previously reported [13, 14]. In brief, 4-μm sections of archived paraffin-embedded specimens were deparaffinized in xylenes and then rehydrated through an ethanol dilution series (from 100 to 70 %). Sections were treated with proteinase K at 37 °C for 10 min and then dehydrated through an ethanol dilution series (from 70 to 100 %). DIG-labeled probes were denatured at 94 °C for 5 min. Slides were incubated in a DIG-labeled probe diluted to 250 nMol in hybridization buffer at 55 °C for 2 h. Stringent washes were performed with 5× SSC, 1× SSC, and 0.2× SSC buffers at 50 °C for 33 min, DIG blocking reagent (Roche, Germany) in maleic acid buffer containing 2 % sheep serum at 30 °C for 15 min, alkaline phosphatase-conjugated anti-digoxigenin (diluted 1:800 in blocking reagent, Roche) at room temperature for 60 min, enzymatic development using 4-nitro-blue tetrazolium (NBT) and 5-bromo-4-chloro-30-Indolyl-phosphate (BCIP) substrate (Vector laboratories, USA) developing at 30 °C for 120 min, followed by nuclear fast red counterstain for 1 min. The slides were then placed in water, dehydrated in alcohol and xylene, and then mounted with mounting medium. Scrambled probe was used as a negative control. U6 served as positive control. ISH scoring was performed by two independent observers (Z.G. and R.V.L.) using conventional bright field microscopy, and differences in interpretation were reviewed for consensus. Cytoplasmic staining was interpreted based on the intensity as negative (0), weak (1+), moderate (2+), and strong (3+). Cases showing 1+, 2+, and 3+ intensity of staining were considered as positive. The expression was considered focal when 1–25 % of cells were positive and diffuse when >25 % of cells were positive. Staining of <1 % of all tumor cells was considered to be negative.
RNA Isolation and Quantitative RT-PCR
Total RNA was isolated with TRIzol Reagent (Invitrogen, Grand Island, NY, USA) according to manufacturer’s instructions. RNA quality and concentration were assessed on the NanoDrop 1000 (Thermo Scientific, Pittsburgh PA, USA). Total RNA of 1 μg was reverse transcribed using the All-In-One First-Strand cDNA Synthesis Kit (GeneCopoeia, Rockville, MD, USA). Validated PCR primers for miR-146b-5p, miR-21, and RN5 were purchased from GeneCopoeia. The qRT-PCR was performed on the CFX96 PCR detection system (Bio-Rad, USA). Relative quantities were determined by the delta–delta CT method.
Immunohistochemical Staining
Immunohistochemical staining of the TMA from the University of Wisconsin with an antibody to HBME-1 (Dako, Carpinteria, CA) using a 1/150 dilution was done as previously reported [15]. Negative control slides consisted of substituting normal serum for the primary antibody. A whole section of a PTC was used as a positive control. The sections were evaluated using 0 for negative staining, 1+ for weak staining, 2+ for moderate staining, and 3+ for strong staining.
Statistical Analysis
Data were expressed as mean ± SEM. Continuous variables were assessed by Student’s t test. Categorical variables were compared with the chi-squared test. The correlations of microRNA expression with various clinicopathological parameters were evaluated with χ 2 test. The Kaplan–Meier method and log-rank test were used to estimate survival; hazard ratios (HRs) were calculated using unadjusted univariate Cox regression analysis. Multivariate Cox regression analysis was used to test for independent prognostic factors. All statistical analyses were performed using the SPSS statistical software program (version 21.0; SPSS Inc.) or GraphPad Prism (version 6.0; GraphPad Inc.). A P value (two-tail) of less than 0.05 was considered to be statistically significant.
Results
miR-146b-5p Was Highly Expressed in the Cytoplasm of Papillary Thyroid Carcinoma
Double DIG LNA probe stained slides were used to detect microRNA expression in thyroid specimens. Cytoplasmic expression was considered positive. Overexpression of miR-146b-5p was present in 12 of 29 (41.4 %) FVPTCs and 25 of 28 (89 %) of PTCs (Table 1; Fig. 1a, b), suggesting perhaps an association of miR-146b-5p expression with PTC subtypes. Two of 28 (7 %) FTCs and 3 of 35 ATCs (8 %) showed 1+ positivity. Normal thyroid tissues, FAs, and PDCs were all negative for this miRNA (Table 1). The reaction with the negative and U6-positive control probes were consistent with the expected results (results not shown).
In situ hybridization analysis of miR-146b-5p and miR-21 expression in follicular variant of papillary thyroid carcinoma (FVPTC). a In situ hybridization of whole tissue sections in FVPTC 2+ miR-146b-5p staining; the adjacent normal thyroid (upper left corner) was negative. b Higher magnification of a FVPTC with 3+ miR-146b-5p staining. c Follicular adenoma with negative staining. d FVPTC with 1+ miR-21 staining. Cytoplasm staining (blue) was considered as positive staining ×200. Scale bar = 75 μm. Inset: low magnification of the corresponding tissue core
miR-21 Was Highly Expressed in Papillary Thyroid Carcinoma and Anaplastic Thyroid Carcinoma
miR-21 showed cytoplasmic expression in PTCs, ATCs, and in some of the PDCs but was less commonly expressed in FTCs and FAs. miR-21 was highly expressed in 29 of 35 ATCs, 22 of 28 (79 %) PTCs, 10 of 29 (34 %) of FVPTCs, and 4 of 21 (19 %) PDCs (Fig. 1d and Table 2).
Expression of miR-146b-5p and miR-21 in PTC Were Associated with Disease-Free Survival
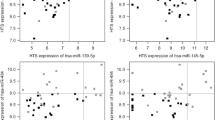
Expression of miR-146b-5p and miR-21 in thyroid cancers was not associated with tumor size, LN metastasis, extrathyroidal extension, or vascular invasion. Kaplan–Meier analysis showed that PTC cases with expression of both miR-146b-5p and miR-21 had significantly poorer disease-free survival rates (Fig. 2a, b). However, Cox regression showed that miR-146b-5p and miR-21 were not independent prognostic factors (P = 0.769, P = 0.975, respectively).
ISH for miR-146b-5p and miR-21 Expression Differences in FVPTC and FA
Comparison of miR-146b-5p expression in FVPTCs and FAs showed significant differences. The expression of miR-146b-5p was seen in 12 cases (41 %) of FVPTC, but was not observed in FAs. The expression of miR-21 was seen in 10 cases (34 %) of FVPTC and in 3 cases of FAs (9 %). miR-146b-5p was very specific for the detection of FVPTC, but was not very sensitive.
miR-146b-5p and miR-21 Expression by Real-Time PCR
Three cases of thyroid tumors and normal thyroid tissues were analyzed using real-time PCR. The average miR-146b-5p expression in PTCs was significantly higher than in the other lesions (mean PTC vs NTs; PTC vs FTC; PTC vs ATC, P < 0.001). The average miR-21 expressions in ATCs were significantly higher than in the other specimens (mean ATC vs NTs; ATC vs FTC; ATC vs PTC, P < 0.05) (Fig. 3).
miR-146b-5p and miR-21 expression in thyroid FFPE tissue was analyzed by qRT-PCR. a Significant increase in miR-146b-5p expression was present in papillary thyroid carcinomas (PTCs). ***P < 0.001 for PTC compared to normal thyroid (NT), follicular adenoma (FA), follicular thyroid carcinoma (FTC), and anaplastic thyroid carcinoma (ATC). b Significant increase in miR-21 expression was present in ATCs. *P < 0.05 for ATC compared to NT, FA, FTC, and PTC
Immunohistochemical Staining
Immunohistochemical staining for HBME-1 on the largest TMA which contained representation of all of the lesions studied showed positive staining in 100 % of the PTCs and 92 % of the FVPTC. Positive staining was also present in 40 % of the FTC although the immunostaining in FTC was usually weaker than in the PTCs. All of the other groups including the normal thyroid, nodular goiters, and follicular adenomas were negative for HBME-1. A combination of ISH for miR-146b-5p and immunostaining for HBME-1 increased the sensitivity of diagnosing FVPTC on the TMA (Table 3).
Discussion
In this study, we detected the expression and analyzed the possible diagnostic and clinical value of miR-146b-5p and miR-21 in patients with PTC based on ISH analyses. There is not much information available about the utility of ISH to determine the diagnostic and prognostic significance of miR-146b-5p and miR-21 in human PTC tissue samples. This method has recently demonstrated its reliability in predicting prognosis in some malignancies [16–18]. Our study showed the expression of miR-146b-5p was present in 89 % of PTC cases and 41 % of FVPTC but is not expressed in most FA, FTC, PDTC, or ATC. The expression of miR-21 was present in 83 % of ATC, 79 % of PTC, and 34 % of FVPTC cases, but not expressed in most cases of FA or FTC. In addition, our study determined that PTC cases with higher expression of miR-146b-5p and miR-21 had significantly poorer disease-free survival rates. Our findings with miR-146b-5p and miR-21 are in agreement with previous studies [19, 20] which reported high expression of miR-146b-5p and miR-21 by RT-PCR in PTCs. To the best of our knowledge, however, there is no study detecting expression of these specific miRNAs in thyroid cancer by ISH. Therefore, our data provides further insight into the specific localization of miR-146b-5p and miR-21 in thyroid lesions and is a useful method to analyze expression of specific microRNAs in thyroid tissues.
High expression of miR-146b-5p has been reported in PTCs [21]. miR-146b-5p is a negative regulator of the TGF beta signaling pathway in normal and neoplastic thyroid cells. Other studies have found that miR-146b-5p is frequently downregulated in prostate cancer, pancreatic cancer, glioblastoma [22], and large B-cell lymphoma [23] and it is upregulated in lung [24] and breast cancer [25]. These opposing findings support the hypothesis that miR-146b-5p may play different roles as an oncogene or as a tumor suppressor gene in different cancer types. Specific inhibition of miR-146b-5p with a LNA-modified anti-miR-146b oligonucleotide probe significantly increased SMAD4 levels in human PTC cell lines [26]. Suppression of miR-146b-5p increased the cellular response to the TGF beta anti-proliferative signal, significantly decreasing the proliferation rate [26]. miR-146b-5p is highly expressed in PTCs and may be a potential diagnostic marker since the levels of this microRNA in normal thyroid and other thyroid cancer subtypes are very low.
miR-21 is overexpressed in ATCs and PTCs. miR-21 was significantly upregulated in PTC tissues compared with non-tumor tissues in studies with microRNA microarray chips [27]. Another study showed that miR-21 was upregulated in human ATC cell lines [28]. This microRNA has been shown to have an important role in oncogenic Ras-induced cell proliferation [8]. miR-21 may play an oncogenic role by directly targeting PDCD4 in the cellular processes of PTC [27]. One study recently showed that miR-21 targets the thyroid hormone receptor [29]. Our findings with miR-146b-5p and miR-21 are in agreement with previous studies [19, 20] which reported high expression of miR-146b-5p and miR-21 by RT-PCR in PTCs. To the best of our knowledge, however, there is no study detecting expression of these specific miRNAs in thyroid cancer by ISH. Therefore, our data provided further insight into the specific localization of miR-146b-5p and miR-21 in thyroid lesions.
In a recent report from the Cancer Genome Atlas Research Network [30] analyzing 496 PTCs, miR-21 and miR-146b were found to be epigenetically regulated and were differentially expressed between PTCs and normal thyroids. miR-21 expression correlated with highly variable DNA methylation and suggested that this microRNA expression may contribute to the clinically aggressive nature of some PTCs such as the Tall Cell variant by genetic dysregulation. miR-146b expression exhibited similar patterns of differential expression and also correlated with DNA methylation [30]. Our recent study of miR-146b-5p regulation in an epithelial mesenchymal transition model of tumor progression of PTC also supports a role of this microRNA in PTC progression [31].
Although miR146b-5p is relatively specific in separating PTC and FVPTC from FA or FTC in this study, because of its low sensitivity, it cannot be used as a solitary biomarker for this purpose. However, a combination of ISH for miR146b-5p with immunohistochemical staining for HBME-1 [32] could be very useful in distinguishing between FVPTC and FA in difficult cases (Table 3). In the current study, 100 % of the PTCs and 92 % of the FVPTCs were positive for HBME-1, while all of the FAs were negative.
In summary, ISH demonstrated high levels of expression of cytoplasmic miR-146b-5p in PTCs, including the FVPTC, but not in normal, benign, or other malignant thyroid tissues. The highest levels of cytoplasmic miR-21 were detected in PTCs and ATCs. PTC cases with higher expression of miR-146b-5p and miR-21 had significantly poorer disease-free survival rates. qRT-PCR findings in a subset of cases supported the ISH findings. ISH is another method of analyzing microRNA expression in thyroid tumors and may be useful in the differential diagnosis of difficult thyroid tumors especially in small biopsy specimens.
References
Siegel R, Naishadham D & Jemal A. Cancer statistics, CA Cancer J Clin 63:11–30, 2013.
Wiemer EA. The role of microRNAs in cancer: no small matter. Eur J Cancer 43:1529–44, 2007.
Gao Y, Wang C, Shan Z, Guan H, Mao J, Fan C, et al. miRNA expression in a human papillary thyroid carcinoma cell line varies with invasiveness. Endocr J 57:81–6, 2010.
Menon MP, Khan A. Micro-RNAs in thyroid neoplasms: molecular, diagnostic and therapeutic implications. J Clin Pathol 62:978–85, 2009.
Nikiforova MN, Chiosea SI, Nikiforov YE. MicroRNA expression profiles in thyroid tumors. Endocr Pathol 20:85–91, 2009.
Chou CK, Yang KD, Chou FF, et al. Prognostic implications of miR-146b expression and its functional role in papillary thyroid carcinoma. J Clin Endocrinol Metab 98:E196-205, 2013.
Dettmer M, Perren A, Moch H, Komminoth P, Nikiforov YE, Nikiforova MN. Comprehensive microRNA expression profiling identifies novel markers in follicular variant of papillary thyroid carcinoma. Thyroid. 23:1383–1389, 2013.
Frezzetti D, De Menna M, Zoppoli P, Guerra C, Ferraro A et al. Upregulation of miR-21 by Ras in vivo and its role in tumor growth. Oncogene 30:275–286, 2011.
He H, Jazdzewski K, Li W, Liyanarachchi S, Nagy R, Volinia S, et al. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci U SA 102:19075–19080, 2005.
Nikiforova MN, Tseng GC, Steward D, Diorio D & Nikiforov YE. MicroRNA expression profiling of thyroid tumors: biological significance and diagnostic utility. J Clin Endocrinol Metab 93: 1600–1608, 2008.
Keutgen XM, Filicori F, Crowley MJ, Wang Y, Scognamiglio T, Hoda R, et al. A panel of four miRNAs accurately differentiates malignant from benign indeterminate thyroid lesions on fine needle aspiration. Clin Cancer Res 18: 2032–2038, 2012.
Wienholds E, Kloosterman WP, Miska E, Alvarez-Saavedra E, Berezikov E, de Bruijn et al. Science 309:310–311, 2005.
Ruebel K, Leontovich AA, Stilling GA, Zhang S, Righi A, Jin L, et al. MicroRNA expression in ileal carinoid tumors: downregulation of microRNA-133a with tumor progression. Mod Pathol 23:367–375, 2010.
Stilling G, Sun Z, Zhang S, Jin L, Righi A, Kovacs G, et al. MicroRNA expression in ACTH-producing pituitary tumors: up-regulation of microRNA-122 and -493 in pituitary carcinomas. Endocrine 38:67–75, 2010.
Buehler D, Hardin H, Shan W, Montemayor-Garcia C, Rush PS, Asioli S, Chen H, Lloyd RV. Expression of epithelial-mesenchymal transition regulators SNAI2 and TWIST1 in thyroid carcinomas. Mod Pathol 26:54–61, 2013.
Nielsen BS, Jorgensen S, Fog JU, Sokilde R, Christensen IJ, et al. High levels of microRNA-21 in the stroma of colorectal cancers predict short disease-free survival in stage II colon cancer patients. Clin Exp Metastasis 28: 27–38, 2011.
Liu Y, Ding Y, Huang J, Wang S, Ni W, Guan J, et al. MiR-141 suppresses the migration and invasion of HCC cells by targeting Tiam1. PLoS One 9:e88393. 2014.
Sempere LF, Christensen M, Silahtaroglu A, Bak M, Heath CV, et al. Altered MicroRNA expression confined to specific epithelial cell subpopulations in breast cancer. Cancer Res 67: 11612–11620, 2007.
Swierniak M, Wojcicka A, Czetwertynska M, Stachlewska E, Maciag M, Wiechno W, et al In-depth characterization of the microRNA transcriptome in normal thyroid and papillary thyroid carcinoma. J Clin Endocrinol Metab 98:E1401–1409, 2013
Zhang J, Liu Y, Liu Z, Wang XM, Yin DT, Zheng LL, et al. Differential expression profiling and functional analysis of microRNAs through stage I-III papillary thyroid carcinoma. Int J Med Sci. 10:585–592, 2013.
Pallante P, Visone R, Croce CM, Fusco A. Deregulation of microRNA expression in follicular-cell-derived human thyroid carcinomas. Endocr Relat Cancer 17: F91–104, 2010.
Li Y, Wang Y, Yu L, Sun C, Cheng D, Yu S, et al. miR-146b-5p inhibits glioma migration and invasion by targeting MMP16. Cancer Lett 339:260–9, 2013.
Wu PY, Zhang XD, Zhu J, Guo XY, Wang JF. Low expression of microRNA-146b-5p and microRNA-320d predicts poor outcome of large B-cell lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone. Hum Pathol 45:1664–1673, 2014.
Patnaik SK, Kannisto E, Mallick R, Yendamuri S. Overexpression of the lung cancer-prognostic miR-146b microRNAs has a minimal and negative effect on the malignant phenotype of A549 lung cancer cells. PLoS One 6(7):e22379, 2011.
Buisson M, Bertrand P, Rimokh R, Rouleau E, Lopez BS, et al Down-regulation of BRCA1 expression by miR-146a and miR-146b-5p in triple negative sporadic breast cancers. Garcia AI1. EMBO Mol Med. 5:279–284, 2011.
Geraldo MV, Yamashita AS, Kimura ET. MicroRNA miR-146b-5p regulates signal transduction of TGF-β by repressing SMAD4 in thyroid cancer. Oncogene 31:1910–1922, 2011.
Zhang J, Yang Y, Liu Y, Fan Y, Liu Z, Wang X, et al MicroRNA-21 regulates biological behaviors in papillary thyroid carcinoma by targeting programmed cell death 4. J Surg Res 189:68–74, 2014.
Mitomo S, Maesawa C, Ogasawara S, Iwaya T, Shibazaki M, Yashima-Abo A, Izutsu N, et al. Downregulation of miR-138 is associated with overexpression of human telomerase reverse transcriptase protein in human anaplastic thyroid carcinoma cell lines. Cancer Sci 99: 280–286, 2008.
Jazdzewski K, Boguslawska J, Jendrzejewski J, Liyanarachchi S, Pachucki J, Wardyn KA, et al. Thyroid hormone receptor {beta}(THRB) is a major target gene for microRNAs deregulated in papillary thyroid carcinoma (PTC). J Clin Endocrinol Metab 96:E546–53, 2010.
Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 159: 676–690, 2014.
Hardin H, Guo Z, Shan W, Montemayor-Garcia C, Asioli S, Yu X-M, et al. The roles of the epithelial-mesenchymal transition marker PRRX1 and miR-146b-5p in papillary thyroid carcinoma progression. Am J Pathol 184:2342–2354, 2014
Nakamura N, Erickson LA, Jin L, Kajita S, Zhang H, Qian X, et al. Immunohistochemical separation of follicular variant of papillary thyroid carcinoma and follicular adenoma. Endocrine Pathol 17: 213–224, 2006.
Acknowledgments
We thank the Translational Research in Pathology (TRIP) Laboratory at the University of Wisconsin for the technical support.
Compliance with Ethical Standards
This work was supported in part by NIH Cancer Support Grant P30-CA 014520-39 from the University of Wisconsin Carbone Cancer Center (RVL).
Conflict of Interest
All other authors declare that they have no conflict of interest.
Informed Consent
This is a retrospective study and informed consent is not required.
Animal Rights Statement
This study does not contain studies with animals by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, Z., Hardin, H., Montemayor-Garcia, C. et al. In Situ Hybridization Analysis of miR-146b-5p and miR-21 in Thyroid Nodules: Diagnostic Implications. Endocr Pathol 26, 157–163 (2015). https://doi.org/10.1007/s12022-015-9363-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-015-9363-x