Abstract
To compare the utility of PAX6 and PAX8 as immunohistochemical markers for neuroendocrine tumors (NETs) of pancreatic origin, we performed PAX6 and PAX8 immunostains on 178 NETs, including 110 primary NETs (26 pancreatic, 10 gastric, 12 duodenal, 22 jejuno-ileal, 10 rectal, 30 pulmonary) and 68 NETs metastatic to the liver (24 pancreatic, 1 duodenal, 37 jejuno-ileal, 1 rectal, 5 pulmonary). Among primary NETs, PAX6 and PAX8 were positive in 65 % (17/26) and 73 % (19/26) of pancreatic, 0 % (0/10) and 10 % (1/10) of gastric, 92 % (11/12) and 92 % (11/12) of duodenal, 0 % (0/22) and 0 % (0/22) of jejuno-ileal, 90 % (9/10) and 80 % (8/10) of rectal, and 0 % (0/30) and 23 % (7/30) of pulmonary NETs, respectively. PAX6 and PAX8 positivity was seen in 46 % (11/24) and 50 % (12/24) of metastatic pancreatic NETs to the liver, respectively. None of the nonpancreatic NETs metastatic to the liver were immunoreactive for either PAX6 or PAX8. PAX6 showed a slightly but statistically significant higher specificity for pancreatic NETs than did PAX8 (P = 0.039), while the sensitivities were similar (P = 0.51). PAX6 had the additional advantages over PAX8 of not exhibiting nonspecific cytoplasmic staining of tumor cells and only infrequently staining background lymphocytes. Since rectal NETs rarely present with metastatic disease, positive staining of a metastatic NET of unknown primary origin for PAX6 and/or PAX8 favors a pancreatic or duodenal origin. This information may be helpful in directing further diagnostic studies to identify the primary site of the metastatic tumor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroendocrine tumors (NETs) mostly arise within the bronchopulmonary system, gastrointestinal tract, and pancreas and may present as distant metastases (often to the liver) before discovery of the primary tumor. Identification of the primary site in a patient with metastatic NET has become increasingly important in the consideration of surgical, pharmacological, or other targeted therapies. CDX2 and TTF1 are well-known markers for gastrointestinal (particularly jejuno-ileal) and pulmonary NETs, respectively. Recently, PAX8 (a paired box transcription factor) has been added as a pancreatic marker in the immunohistochemical panel for metastatic NETs of unknown origin [1–3]. In our previous study [4], we demonstrated that both PAX8 and Islet1 are helpful in distinguishing metastatic NETs of pancreatic origin from those of ileal origin.
Recently, Lorenzo et al. demonstrated that monoclonal PAX8 antibody does not stain pancreatic NETs and that the immunoreactivity of pancreatic NETs for PAX8 reported in prior studies is due to use of a polyclonal PAX8 antibody which cross-reacts with PAX6, another paired box transcription factor and differentiation marker of pancreatic neuroendocrine cells [5]. However, immunohistochemical staining for PAX6 in NETs arising in various anatomic sites and the comparison of PAX6 and PAX8 as immunohistochemical markers for NETs of pancreatic origin have previously not been well studied. The current study was therefore designed to compare the sensitivity and specificity of PAX6 and PAX8 as markers for NETs of pancreatic origin by investigating their immunoreactivity in a group of primary NETs from various sites (including pancreas, stomach, duodenum, jejuno-ileum, rectum, and lung) and in a group of NETs metastatic to the liver from a similar range of primary sites. The relationship between clinicopathologic features of pancreatic NETs and immunohistochemical positivity for PAX6 and PAX8 was also evaluated.
Material and Methods
Case Selection
A total of 178 well-differentiated NETs, including 110 primary NETs (26 pancreatic, 10 gastric, 12 duodenal, 22 jejuno-ileal, 10 rectal, and 30 pulmonary) and 68 NETs metastatic to the liver from various primary sites (24 pancreatic, 1 duodenal, 37 jejuno-ileal, 1 rectal, and 5 pulmonary), were retrieved from the surgical pathology archives of Cedars-Sinai Medical Center from 2005 to 2011. Institutional review board approval was obtained for the study. Documented prior history or concurrent resection of the primary and metastatic tumors was used to determine the primary site of origin of the metastatic NETs. The clinical records and relevant pathology reports were reviewed to determine the functional status of the pancreatic NETs, the presence or absence of metastasis to lymph nodes and/or liver, and the WHO tumor grade [6], as determined by the reported Ki-67 index (low grade, Ki-67 index ≤2 %; intermediate grade, Ki-67 index = 3–20 %), quantified by visual estimation and/or computer-assisted image analysis.
Immunohistochemistry
Immunostains for PAX6 and PAX8 were performed on all 178 tumors. The majority of the tumors, including 94 primary NETs (26 pancreatic, 6 gastric, 22 jejuno-ileal, 10 rectal, and 30 pulmonary) and 68 NETs metastatic to the liver, had been previously stained for PAX8 as part of our recently reported study [4]. Tissue sections (4 μm) were cut from paraffin-embedded formalin-fixed tissue blocks and stained with antibodies against PAX6 (clone PAX6, mouse monoclonal, dilution 1:150, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and PAX8 (polyclonal, dilution 1:50, Proteintech, Chicago, IL, USA). Immunohistochemical staining for PAX6 was performed on the Leica Bond III (Chicago, IL) and PAX8 on the Ventana Benchmark Ultra (Tucson, AZ). Pretreatment was performed with on-board antigen retrieval method. External positive control tissues used included normal pancreatic tissue for PAX6 and non-neoplastic kidney tissue for PAX8.
Using a scale similar to that used in our prior study [4], the extent of positive staining was semiquantitatively assessed as 0 = <5 % staining, 1+ = 5–25 % staining, 2+ = 26–50 % staining, 3+ = 51–75 % staining, and 4+ = >75 % staining. The intensity of nuclear staining was evaluated as weak, moderate, or strong, based on comparison with staining of external positive controls or internal positive controls if present. Tumors showing moderate to strong nuclear staining of at least 5 % of cells were considered positive. In those tumors exhibiting only weak staining, the threshold for positivity was increased to at least 10 % of tumor cells in an effort to avoid interpreting nonspecific staining as positive. Cytoplasmic staining in the absence of nuclear staining was scored as negative. Nuclear immunoreactivity for PAX6 and PAX8 was evaluated by two investigators (JL and DD). A consensus opinion was achieved for any discordance between the pathologists (<5 % of cases).
Statistical Analysis
Sensitivity and specificity were calculated according to the standard definitions. The sensitivity and specificity of PAX6 and PAX8 antibodies for pancreatic NETs were compared using McNemar’s test for related proportions. For the 95 % confidence interval, the exact binomial proportion confidence limit was used. Binary variables were compared across independent groups by the Fisher’s exact test. Tumor size was compared across independent groups by the Wilcoxon rank sum test. SAS version 9.2 (SAS Institute, Cary, NC) was used for all statistical calculations. The 5 % significance level was used throughout.
Results
PAX6 and PAX8 Expression in Normal Tissues
Both PAX6 and PAX8 were strongly expressed in pancreatic islets and in neuroendocrine cells in the gastrointestinal epithelium, which served as positive internal controls. There was no difference in the expression of either PAX6 or PAX8 in the normal neuroendocrine cells relative to their location in the gastrointestinal tract (stomach, duodenum, ileum, or rectum). While PAX8 expression was universally present in lymphoid follicles wherever present, PAX6 was rarely seen in the lymphoid follicles. With the PAX8 antibody, nonspecific cytoplasmic staining was sometimes identified in non-neoplastic epithelium, particularly in gastric oxyntic mucosa. No cytoplasmic staining was observed with the PAX6 antibody.
PAX6 and PAX8 Expression in Primary Pancreatic NETs (Table 1)
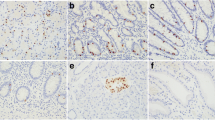
PAX6 was positive in 65 % (17/26) of primary pancreatic NETs, including 14 cases with moderate to strong, 1+ to 4+ staining (Fig. 1a) and 3 cases with weak, 2+ to 4+ staining. PAX8 was positive in 73 % (19/26) of primary pancreatic NETs, including 14 cases with moderate to strong, 1+ to 4+ staining (Fig. 1b) and 5 cases with weak, 1+ to 4+ staining. There was concordance between PAX6 and PAX8 staining in 20 of 26 cases (77 %). The discordances included four cases that were PAX8 positive (two with weak positivity) but PAX6 negative and two cases that were PAX6 positive but PAX8 negative. Cytoplasmic staining for PAX8 was seen in nine cases, including seven that lacked nuclear staining; two of these seven cases were positive for PAX6. No cytoplasmic staining was observed using the PAX6 antibody.
PAX6 and PAX8 expression in pancreatic and gastric NETs. a Strong nuclear positivity for PAX6 in a low grade pancreatic NET; b strong nuclear positivity for PAX8 in the same tumor as in a, with some associated weak cytoplasmic staining; c low grade gastric NET negative for PAX6; d same tumor as in c showing weak positivity for PAX8 (a–d, ×400)
In comparison with the strong staining intensity observed in surrounding non-neoplastic islets, there was decreased to absent staining for both PAX6 and PAX8 in six (35 %) of the primary pancreatic NETs.
PAX6 and PAX8 Expression in Metastatic Pancreatic NETs (Table 1)
PAX6 and PAX8 were positive in 11 (46 %) and 12 (50 %) of the 24 pancreatic NETs metastatic to the liver. Concordant PAX6 and PAX8 staining was seen in 21 of 24 cases (88 %). There were two cases in which PAX8 was positive but PAX6 was negative and one case in which PAX6 was positive but PAX8 was negative. Six cases showed only cytoplasmic staining for PAX8; all six of these cases were negative for PAX6. None of the tumors showed cytoplasmic staining with PAX6.
There were only six matched primary and corresponding metastatic pancreatic NETs. In one case, PAX8 was positive in the primary tumor but negative in the metastasis, whereas PAX6 was negative in both the primary and metastatic tumors. In another case, PAX6 was positive in the metastasis but negative in the primary tumor; PAX8 was positive in both the primary and metastatic tumors in this case. In the remaining four cases, there was concordant staining in the primary and metastatic tumors with both PAX6 and PAX8.
Association of Immunoreactivity for PAX6 and PAX8 with Clinicopathologic Features of Pancreatic NETs
Primary Pancreatic NETs
The cohort of primary pancreatic NETs (n = 26) included 10 males and 16 females, with a median age of 51 years (range 16–79). Fourteen tumors (54 %) were low grade, and 12 (46 %) were intermediate grade. There were 12 (46 %) functioning tumors and 14 (54 %) nonfunctioning tumors. Fifteen patients presented with metastatic disease, four of which had metastasis to lymph nodes alone and 11 of which had metastasis to the liver, with or without involvement of lymph nodes.
The distribution of PAX6 and PAX8 positive and negative cases according to the clinicopathologic characteristics of the primary pancreatic NETs is displayed in Table 2. The tumors tended to be larger in both the PAX6 and PAX8 negative groups, though the difference was statistically significant only when comparing PAX6 positive and negative tumors (P = 0.032). Positive staining for PAX6 and PAX8 was noted in 12 (86 %) of 14 low grade primary pancreatic NETs, whereas only 5 (42 %) and 7 (58 %) of 12 intermediate grade primary pancreatic NETs showed positive staining for PAX6 and PAX8, respectively. Low grade pancreatic NETs had a significantly higher PAX6 positive rate than intermediate grade tumors (P = 0.038), but a statistically significant difference was not observed with PAX8 (P = 0.19). Positive staining for PAX6 was noted in 10 (91 %) of 11 patients without metastasis and in 7 (47 %) of 15 patients who had metastasis. There was a significant association of PAX6 negativity with the presence of metastatic disease (liver and/or lymph nodes) (P = 0.036). However, the difference was not statistically significant using PAX8; positive staining for PAX8 was noted in 10 (91 %) of 11 patients without metastasis and in 9 (60 %) of 15 patients who had metastasis (P = 0.18). There was no association of PAX6 or PAX8 staining with the functional status of the tumor (P = 0.43 and P = 0.67)
Metastatic Pancreatic NETs
The cohort of metastatic pancreatic NETs (n = 24) included 9 males and 15 females, with a median age of 49 years (range 34–79). Nine tumors (37.5 %) were low grade and 15 (62.5 %) were intermediate grade. There were 9 (37.5 %) functioning tumors and 15 (62.5 %) nonfunctioning tumors. The distribution of PAX6 and PAX8 positive and negative cases according to the clinicopathologic characteristics of the metastatic pancreatic NETs is displayed in Table 3. There was no association of PAX6 or PAX8 staining with the WHO grade or functional status of the metastatic tumor (P > 0.05).
PAX6 and PAX8 Expression in Nonpancreatic NETs (Table 1)
Gastric NETs
PAX6 was completely negative in all ten primary gastric NETs (Fig. 1c), whereas positive PAX8 staining (1+, weak) was observed in one of the cases (Fig. 1d). Nonspecific cytoplasmic staining was noted with the PAX8 antibody in two of the ten primary gastric NETs, whereas cytoplasmic staining was not observed using the PAX6 antibody.
Duodenal NETs
Both PAX6 and PAX8 were positive (moderate to strong, 3+ or 4+) in 11 of 12 (92 %) of the primary duodenal NETs (Fig. 2a, b). All of the positively stained tumors were low grade NETs, whereas the one intermediate grade NET was negative for both PAX6 and PAX8. Nonspecific cytoplasmic staining for PAX8 was apparent in two duodenal NETs, but no cytoplasmic staining was seen using the PAX6 antibody. One metastatic low grade duodenal NET to the liver was negative for both PAX6 and PAX8.
Jejuno-ileal NETs
None of the 22 primary jejuno-ileal NETs were scored as positive for either PAX6 or PAX8 (Fig. 2c, d), though focal (5–10 %) weak nuclear staining for PAX8 was observed in one case, and nonspecific cytoplasmic staining for PAX8 was observed in another case. No cytoplasmic staining was observed in any of the cases using the PAX6 antibody. The 37 jejuno-ileal NETs metastatic to the liver were all negative for both PAX8 and PAX6.
Rectal NETs
PAX6 was positive (2+ to 4+, weak to strong) in nine of ten (90 %) of the primary rectal NETs, and PAX8 was positive (3+ to 4+, moderate to strong) in eight of ten (80 %) of the tumors (Fig. 3a, b). One rectal NET metastatic to the liver was negative for both PAX6 and PAX8.
Pulmonary NETs
All 30 primary pulmonary NETs were negative for PAX6 (Fig. 3c), whereas positive staining for PAX8 (1+ to 4+, weak to strong) was observed in 7 of the 30 cases (23 %) (Fig. 3d). Among the seven PAX8-positive cases, five were typical carcinoids and two were atypical carcinoids. With PAX8 antibody, nonspecific cytoplasmic staining was observed in seven cases, six of which lacked nuclear staining. No cytoplasmic staining was noted using the PAX6 antibody. Five pulmonary NETs metastatic to liver were negative for both PAX8 and PAX6.
Comparison of the Sensitivity and Specificity of PAX6 and PAX8 for Pancreatic NETs (Table 4)
Grouping primary and metastatic tumors together, PAX6 showed a slightly but statistically significant higher specificity for pancreatic NETs than did PAX8 (84 vs 79 %; P = 0.039). The sensitivity of PAX6 for pancreatic NETs appeared to be slightly lower than that of PAX8 (56 vs 62 %), but this difference was not statistically significant (P = 0.51). When primary tumors were analyzed separately, PAX6 continued to show a statistically significant higher specificity than PAX8 for pancreatic NETs (76 vs 68 %, P = 0.039), but the difference in sensitivity (65 vs 73 %) was not statistically significant (P = 0.69). In the group of metastatic NETs, no difference in specificity was noted between PAX6 and PAX8, since both PAX6 and PAX8 were 100 % specific for pancreatic tumors in this setting; in this group, the difference in sensitivity between PAX6 and PAX8 (46 vs 50 %) was not statistically significant (P = 0.99).
In the combined group of primary and metastatic NETs, when positivity of tumors for PAX6 was compared to positivity for either PAX6 or PAX8, a significant improvement in sensitivity for pancreatic NETs was attained by using both antibodies (68 vs 56 %, P = 0.03), but this increase in sensitivity was achieved at the expense of a significant decrease in specificity (78 vs 84 %, P = 0.008).
Discussion
Our previous study [4] and several other studies [1–3] have demonstrated that PAX8 is a reliable immunohistochemical marker for pancreatic NETs and is useful in conjunction with other markers (such as TTF1 and CDX2) in the workup of a metastatic NET of unknown primary site of origin. Recently, it has been demonstrated that immunoreactivity of pancreatic NETs for PAX8 is due to cross-reactivity of the polyclonal PAX8 antibody with PAX6 [5, 7]. PAX6, a member of the paired box gene family, is a transcription factor known to be crucial for islet cell differentiation and function through transcriptional control of key genes involved in glucagon and insulin biosynthesis and secretion [7–11]. PAX6 has also been demonstrated to play an essential role in the development and function of endocrine cells in the gastrointestinal tract, particularly in the stomach and duodenum [12, 13]. Although PAX6 has been demonstrated to be expressed by the majority of pancreatic NETs [14], the comparison of PAX6 with PAX8 as immunohistochemical markers for pancreatic NETs has not been previously reported nor has the immunohistochemical expression of PAX6 in nonpancreatic NETs. In the study reported herein, we therefore compared the sensitivity and specificity of PAX6 and PAX8 as markers for pancreatic NETs by evaluating their immunohistochemical expression in 178 NETs, including 110 primary NETs and 68 metastatic NETs to the liver from various primary sites.
In our study, the PAX6 and PAX8 antibodies showed similar sensitivities as markers for pancreatic NETs; the slightly decreased sensitivity of PAX6 relative to PAX8 was not statistically significant. The monoclonal PAX6 antibody showed a slightly but statistically significant higher specificity for pancreatic NETs than did the polyclonal PAX8 antibody. The increased specificity of the monoclonal PAX6 antibody relative to the polyclonal PAX8 antibody was due to the finding of reactivity for PAX8 in 1 of 10 gastric NETs and in 7 of 30 pulmonary NETs, whereas all of the gastric and pulmonary NETs were negative for PAX6. PAX8 immunoreactivity in gastric NETs has been reported in previous studies [1, 3]. The improved specificity of PAX6 for pancreatic NETs may be attributable to the monoclonal nature of the PAX6 antibody used in our study, whereas the PAX8 antibody used in this and most other studies was polyclonal in nature. PAX8 polyclonal antibody has previously been shown to cross-react with PAX5 [15], a transcription factor involved in B cell differentiation which has also been demonstrated to be expressed in a subset of NETs of the lung (most commonly in intermediate and high grade tumors) [16]. Hence, cross-reactivity with PAX5 may potentially explain the immunoreactivity for PAX8 observed in several of the pulmonary NETs in our study, thereby reducing the specificity of PAX8 for pancreatic NETs.
The specificity of both the PAX6 and PAX8 antibodies for pancreatic NETs was reduced by the finding of positive staining with both of these antibodies in the majority of duodenal and rectal NETs. Previous studies have also demonstrated PAX8 staining in duodenal and rectal NETs [1, 3, 4]. Like PAX8, PAX6 was negative in all of the jejuno-ileal NETs examined, indicating that positive staining of a metastatic NET for either PAX6 or PAX8 argues strongly against a jejuno-ileal primary site of origin.
If only one marker for NETs of pancreatic origin is to be included in a panel of immunostains for metastatic NETs of unknown primary origin, the slightly increased specificity of the monoclonal PAX6 antibody favors the use of this antibody over the polyclonal PAX8 antibody. In addition, PAX6 has the additional advantages over PAX8 of not staining lymphoid cells in most cases and not exhibiting nonspecific cytoplasmic staining, observations which were seen using the polyclonal PAX8 antibody in a number of cases. The staining of lymphocytes with the PAX8 antibody may also be attributable to cross-reactivity of the PAX8 antibody with PAX5, a marker of B lymphocytes [15].
Since staining for PAX6 and PAX8 was not concordant in all pancreatic NETs, the potential value of including PAX8 in addition to PAX6 in the immunohistochemical panel for metastatic tumors of unknown site of origin was evaluated, with a positive result being defined as positive staining of the tumor with either of these antibodies. We found that the sensitivity for pancreatic NETs is increased by using both PAX6 and PAX8 antibodies (compared to using PAX6 alone), but that this increase in sensitivity is achieved at the expense of a significant decrease in specificity. For this reason, and keeping cost-effectiveness in mind, we believe that it is preferable to use PAX6 alone (rather than both PAX6 and PAX8) in an immunohistochemical panel for metastatic NETs of unknown primary origin.
An additional interesting observation was the reduction of staining for PAX6 and PAX8 in intermediate grade primary pancreatic NETs, in comparison with low grade primary pancreatic NETs; this difference was statistically significant in the case of PAX6 (P = 0.04) but did not quite achieve statistical significance in the case of PAX8 (P = 0.19). Reduced staining for PAX8 in higher grade pancreatic NETs was previously reported in the study by Long et al. [1]. In contrast, Sangoi et al. did not find any association of PAX8 staining with the tumor grade [3]. In addition, we found that PAX6 negativity in pancreatic NETs was significantly associated with the presence of metastatic disease. In contrast, PAX8 staining did not show any relationship with the presence of metastatic disease, a finding similar to that of Sangoi et al. [3]. Because of the inconsistent results between different studies, it is still unclear whether there is in fact an inverse relationship between PAX6 expression and tumor aggressiveness. We did not find any association of PAX6 or PAX8 staining with the functional status of the tumor. Previous studies have also shown similar findings with PAX8 [3].
In summary, this is the first study to compare the utility of PAX6 and PAX8 as markers for pancreatic NETs. Our results indicate that (1) the monoclonal PAX6 antibody is a slightly more specific marker than the polyclonal PAX8 antibody for pancreatic NETs; (2) the monoclonal PAX6 antibody has the additional advantages over the polyclonal PAX8 antibody of not exhibiting nonspecific cytoplasmic staining and less frequently staining background lymphocytes; (3) in an immunohistochemical panel for metastatic NETs of unknown primary origin, use of the PAX6 monoclonal antibody is preferable to use of the polyclonal PAX8 antibody as a marker for NETs of pancreatic origin; (4) use of both PAX6 and PAX8 in an immunohistochemical panel for metastatic does achieve some increase in sensitivity for NETs of pancreatic origin, but this is achieved at the expense of decreased specificity, and therefore is not favored; (5) neither PAX6 nor PAX8 is specific for pancreatic NETs, with staining for both being seen in a high percentage of duodenal and rectal NETs; and (6) in the setting of a metastatic NET of unknown primary, positive staining for PAX6 or PAX8 favors a pancreatic or duodenal primary site, since rectal NETs rarely present with metastatic disease. This information may be helpful in directing further diagnostic studies to identify the primary site.
References
Long KB, Srivastava A, Hirsch MS, Hornick JL. PAX8 expression in well-differentiated pancreatic endocrine tumors: correlation with clinicopathologic features and comparison with gastrointestinal and pulmonary carcinoid tumors. Am J Surg Pathol 2010; 34:723–729.
Haynes CM, Sangoi AR, Pai RK. PAX8 is expressed in pancreatic well-differentiated neuroendocrine tumors and in extrapancreatic poorly differentiated neuroendocrine carcinomas in fine-needle aspiration biopsy specimens. Cancer Cytopathol 2011; 119:193–201.
Sangoi AR, Ohgami RS, Pai RK, Beck AH, McKenney JK, Pai RK. PAX8 expression reliably distinguishes pancreatic well-differentiated neuroendocrine tumors from ileal and pulmonary well-differentiated neuroendocrine tumors and pancreatic acinar cell carcinoma. Mod Pathol 2011; 24:412–424.
Koo J, Mertens RB, Mirocha JM, Wang HL, Dhall D. Value of Islet 1 and PAX8 in identifying metastatic neuroendocrine tumors of pancreatic origin. Mod Pathol 2012; 25:893–901.
Lorenzo PI, Jimenez Moreno CM, Delgado I, Cobo-Vuilleumier N, Meier R, Gomez-Izquierdo L, Berney T, Garcia-Carbonero R, Rojas A, Gauthier BR. Immunohistochemical assessment of Pax8 expression during pancreatic islet development and in human neuroendocrine tumors. Histochem Cell Biol. 2011; 36:595–607.
Rindi G, Arnold R, Bosman FT, et al. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND (eds). WHO Classification of Tumors of the Digestive System. IARC Press, Lyon, 2010, pp 13–14.
Moreno CM, Lorenzo PI, Delgado I, Cobo-Vuilleumier N, Gomez-Izquierdo L, Garcia-Carbonero R, Rojas A, Gauthier BR. Pax8 detection in well-differentiated pancreatic endocrine tumors: how reliable is it? Am J Surg Pathol. 2011; 35:1906–8.
St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature. 1997; 387:406–9.
Sander M, Neubüser A, Kalamaras J, Ee HC, Martin GR, German MS. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev. 1997; 11:1662–73.
Gosmain Y, Katz LS, Masson MH, Cheyssac C, Poisson C, Philippe J. Pax6 is crucial for β-cell function, insulin biosynthesis, and glucose-induced insulin secretion. Mol Endocrinol. 2012; 26:696–709.
Gosmain Y, Cheyssac C, Heddad Masson M, Dibner C, Philippe J. Glucagon gene expression in the endocrine pancreas: the role of the transcription factor Pax6 in α-cell differentiation, glucagon biosynthesis and secretion. Diabetes Obes Metab. 2011; 13 Suppl 1:31–8.
Larsson LI, St-Onge L, Hougaard DM, Sosa-Pineda B and Gruss P. Pax4 and 6 regulate gastrointestinal endocrine cell development. Mech Dev 1998; 79: 153–9.
Hill ME, Asa SL, Drucker DJ. Essential requirement for Pax6 in control of enteroendocrine proglucagon gene transcription. Mol Endocrinol. 1999; 13:1474–86.
Zhang L, Smyrk TC, Oliveira AM, Lohse CM, Zhang S, Johnson MR, Lloyd RV. KIT is an independent prognostic marker for pancreatic endocrine tumors: a finding derived from analysis of islet cell differentiation markers. Am J Surg Pathol. 2009; 33:1562–9.
Moretti L, Medeiro S, Kunkalla K, Williams MD, Singh RR, Vega F. N-terminal PAX8 polyclonal antibody shows cross-reactivity with N-terminal region of PAX5 and is responsible for reports of PAX8 positivity in malignant lymphomas. Mod Pathol. 2012; 25:231–6.
Song J, Li M, Tretiakova M, Salgia R, Cagle PT, Husain AN. Expression patterns of PAX5, c-Met, and paxillin in neuroendocrine tumors of the lung. Arch Pathol Lab Med. 2010; 134:1702–5.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lai, JP., Mertens, R.B., Mirocha, J. et al. Comparison of PAX6 and PAX8 as Immunohistochemical Markers for Pancreatic Neuroendocrine Tumors. Endocr Pathol 26, 54–62 (2015). https://doi.org/10.1007/s12022-014-9346-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-014-9346-3