Abstract
Purpose
Pituitary neuroendocrine tumors (PitNETs) with invasion of the cavernous sinus (CS) are particularly challenging to treat. Tumor associated fibroblasts (TAFs) are recognized for their pivotal role in reprogramming extracellular matrix (ECM). Herein, we aimed to explore the potential involvement of TAFs in ECM reprogramming and elucidate the underlying mechanism involved.
Methods
We applied dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) to measure tumor vessel permeability and applied atomic force microscopy (AFM) to measure the matrix stiffness of PitNETs located in both CS and sella turcica (ST). Western blotting, immunofluorescence, immunohistochemistry, and quantitative RT-PCR were utilized to analyze the ECM components. Proteomic biochemical analysis was utilized to uncover potential mechanisms governing ECM dynamics.
Results
We found that PitNETs in the CS were stiffer than those in the ST. Increased ECM stiffness within the CS facilitated the acquisition of stem-like properties, enhanced proliferation, and induced epithelial-to-mesenchymal transition (EMT) of GH3 cells. Furthermore, the expression levels of lysyl oxidase (LOX), matrix metallopeptidase 2 (MMP2) and MMP9 in pituitary adenoma cells increased in the stiffer matrix. Proteomic analysis suggested TAFs were activated in the CS area and contributed enhanced matrix stiffness by secreting Col-1 and Col-3. Furthermore, mTOR pathway was activated under higher matrix stiffness and the migration and invasion of GH3 cells be repressed by mTOR inhibitor.
Conclusion
These findings demonstrated that activated TAFs contributed to stiffer matrix and increased ECM stiffness stimulating mTOR pathway in pituitary tumor cells. Our study indicated that mTOR inhibitor was a promising treatment strategy from the standpoint of PitNET biomechanical properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pituitary neuroendocrine tumors (PitNETs), or pituitary adenomas (PAs), are highly prevalent, accounting for 15% of all primary central nervous system tumors [1]. While most are benign, some display aggressive features, and a small subset of these tumors metastasize [2]. Gross total resection is often pursued to alleviate local compressive effects and abnormal secretion of hormones associated with PitNETs. However, the success of gross resection varies due to factors such as size, location, invasiveness, and stiffness of the tumor [3]. Generally, the mean gross total resection rate of noninvasive adenomas in endoscopic series could reach 87% [4]. Conversely, for invasive adenomas, especially those invading the cavernous sinus (CS), the overall gross total resection rate was as low as 40% due to the complex anatomy of the CS, which contains multiple intracranial nerves, internal carotid artery, and the venous sinuses [4,5,6]. Moreover, residual tumors in the CS required multiple therapeutic approaches, including radiotherapy and medications [7]. With the development of endoscopic techniques and a deeper understanding of CS anatomy, full exposure of CS space enables resection and suction of soft tumors within the neurovascular compartment [8,9,10]. However, in clinical practice, tumors located in the CS often exhibit greater stiffness than those located in the sella turcica (ST), leading to challenges in achieving complete resection [8]. Besides, higher matrix stiffness was associated with lower resections rates, higher postoperative complications and worse prognosis [11]. To further reveal intratumor mechanical heterogeneity of invasive pituitary adenomas will facilitate improving the clinical outcome of this troublesome disease.
Considerable efforts have been made to unravel the molecular regulatory mechanism and signaling pathways involved in the progression of invasive pituitary adenomas [12, 13]. However, the impact of variability in mechanical forces within pituitary adenomas has been largely overlooked. The intratumor mechanical heterogeneity of PitNETs is attributed to several factors, including differences in cellular proliferation leading to increased internal solid stress exerted by surrounding normal tissues, as well as extracellular matrix (ECM) alterations that generate external stress within the tumor microenvironment (TME) [2, 12, 13]. Numerous studies have elucidated the effects of these mechanical forces on various cancers such as hepatocellular carcinoma, cholangiocarcinoma, pancreatic ductal adenocarcinoma, colorectal cancer, breast cancer, and glioblastoma [14, 15]. Our previous study also revealed that extracellular matrix-receptor interaction pathway modulated invasion and proliferation function of granulated somatotroph adenomas [16]. While several reports have focused on the intertumoral mechanical heterogeneity among different pituitary tumors [8, 17], few studies have investigated the intratumoral mechanical differences of between CS and ST in the same PitNETs.
In this study, we characterized the mechanical heterogeneity and biological significance of CS tumors and ST tumors within the same PitNET across different perioperative dimensions and to elucidate the potential mechanism responsible for tumor stiffness (Fig. 1). We employed dynamic contrast-enhanced MRI (DCE-MRI), a perfusion imaging technique that reflects the permeability of microvessels and lesion morphology, to analyze the texture of different areas of the adenoma preoperatively. Intraoperatively, we recorded the endoscopic suction strength classification between the CS and ST tumors. Additionally, we utilized atomic force microscopy (AFM), a type of scanning probe microscopy, to measure tumor stiffness at the postoperative fresh tissue level by measuring the Young’s modulus. Subsequent analysis of variations in the molecular and cellular level of the TME between the ST and CS of the adenomas unveil some potential underlying mechanisms contributing to the mechanical heterogeneity of PitNETs. Furthermore, we explored the effects of an adjustable stiffness 3D-culture hydrogel, which mimics the heterogeneous local stiffness of the PA ECM, on the morphology, proliferation, and invasion of GH3 cells. This comprehensive study revealed key processes regulated by heterogeneous mechanical tumor microenvironments in the PitNETs invading the CS, revealing links between mechanical stiffness and adenoma invasion and progression.
Schematic workflow of the experiment. Invasive pituitary adenoma was scanned by dynamic contrast enhanced MRI (DCE-MRI) and resected by endoscopic endonasal surgery. The tumor located in the sella turcica (ST) area and cavernous sinus (CS) was subjected to IHC, qRT-PCR, WB, AFM, and proteomics for intensive analysis. We found tumor in the CS was stiffer compared to the ST due to more activated TAF aggregation
Methods
Tumor tissue collection
Specimens from 18 invasive pituitary adenomas were obtained between April 2022 and May 2023 at Zhongshan Hospital of Fudan University. According to the 2022 WHO classification, 9 patients were diagnosed with PIT1-lineage PitNETs, 3 were diagnosed with TPIT-lineage PitNETs, 3 were diagnosed with SF1-lineage PitNETs, and the remaining 3 were diagnosed with null cell lineage PitNETs (Table S1). This collection of studies concentrated on exploring the variations in intratumoral stiffness heterogeneity across different regions within a single adenoma, thus not engaging in comparisons based on cell lineage classifications. The lineage and pathological endocrine staining of specimens from different regions within the same tumor were consistent. This study was approved by the Ethics Committee of Zhongshan Hospital (B2021-819), and written informed consent was obtained from each patient. The invasive pituitary adenomas included in this study were Knosp grade 4. According to the Mahmoud intraoperative classification method, soft texture is easily removed by suction easily, and tumors with this features were considered grade 1 tumors; fibrous texture is removed by suction with difficulty, and tumors with this features were considered grade 2 tumors; hard texture is not removable by suction and excision en bloc and tumors with this features were considered grade 3 tumors [18]. Fresh pituitary adenoma specimens were quickly frozen in liquid nitrogen, transported to the laboratory within one hour and stored in a −80 °C refrigerator for long-term storage.
DCE-MRI
Of the 18 PitNETs patients, 16 patients received DEC-MRI examination. DCE-MRIs were performed on a UIH uMR 770 3.0 T MR scanner (United Imaging Healthcare, Shanghai, China), except for routine pituitary contrast sequence, the DCE sequences were applied to the previous report [19]. The DCE-MRI data were processed based on DCE analysis (Shanghai United Imaging Healthcare Co., Ltd., China) software.
Label-free 150 min quantification proteomic analysis
The proteomic analysis was performed based on our previous study [20]. Collected samples were washed three times with phosphate buffer saline (PBS) buffer to remove blood and debris. For the proteome profiling samples, peptides were analyzed on a Q Exactive HF-X Hybrid Quadrupole-Orbitrap Mass Spectrometer (Thermo Fisher Scientific) coupled with a high-performance liquid chromatography system (EASY nLC 1200, Thermo Fisher Scientific). The original data of mass spectrometry analysis were RAW files, and iProteome one-stop data analysis cloud platform was used for qualitative and quantitative analysis. These date can be accessed with the iProX accession: Proteome: IPX0007686001, with the following url: https://www.iprox.cn/page/DSV021.html;?url=17017771653606Ilm, and password: dCuF.
Cell culture and sphere formation assay
The rat pituitary adenoma cell line GH3 was obtained from the Chinese Academy of Sciences (Shanghai, China). GH3 cells were cultured in F-12K medium (Meilunbio, China) supplemented with 15% horse serum (Haling, China) and 2.5% fetal bovine serum (Gibco, USA) in a humidified incubator at 37 °C containing 5% CO2. For the sphere formation assay, we first mixed the GH3 culture media with 2X A gel of Biozellen®3D cell organoid culture hydrogel kit at the ratios of 1:1,1:2, and 1:3, representing stiffness of 10 kPa (H), 5 kPa (M), and 1 kPa (L), respectively. Subsequently, approximately 1000 GH3 cells were embedded in a 40 μl drop with different stiffnesses and seeded in 6-well plates (Corning). The jellified droplets were cultured in F-12K based media. The diameter of each cell sphere was measured on the day 3 and day 6.
Wound healing assay and transwell assay
For wound healing, GH3 cells were seeded in a 6-well plate and a 200 ul pipette tip were scratched when the individual wells reached 90% confluence, microscope photoshops were taken at 0 h and 48 h. For transwell migration assay, cells were incubated in a 24-well transewell and incubated with 5% CO2 at 37 °C, then cells were fixed with 4% paraformaldehyde and stained with crystal violet. For transwell invasion assay, cells were incubated in a 24-well Transwell precoated with Matrigel and the invaded cells were fixed, stained and counted.
Isolation and culture of primary tumor associated fibroblasts
TAFs were cultured according to previous studies [21, 22]. In brief, fresh PiNET tumor tissues were obtained during transsphenoidal surgery and stored in F-12K (Meilunbio, China) media supplemented with 100 units/mL of penicillin and 100 μg/mL of streptomycin (Gibco, USA) within 30 min after resection. Fresh tumor blocks were rinsed with magnesium and calcium free phosphate buffered saline (Gibco, USA) and then cut into pieces (approximately 1 mm3) with sterile ophthalmic scissors. Subsequently, the tumor fragments were incubated in 1:10 diluted 0.05% trypsin-EDTA (Gibco, USA) for 3 min and centrifuged at 1000 rpm for 3 min, after which the supernatants were discarded. The separated PiNET tumor cells were mixed with fibroblast media (ScienCell, USA) and transferred to T25 culture flasks (Corning, USA) for incubation at 5% CO2 at 37 °C. The cells in Flasks were observed daily and the fibroblast media were changed every 2–3 days. TAF appeared at the bottom of the culture flasks within one week and TAF within 10 passages were used in this study.
RNA isolation, reverse transcription, and quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
The total RNA was extracted from human pituitary adenoma tissues using the RNaeasy Mini Kit (QIAGEN, Germany) according to the manufacturer’s protocols. The relative quantity of each gene was analyzed using the comparative delta-delta Ct method, adjusted to GAPDH expression level. The PCR primers were shown in Table S2.
Immunohistochemistry (IHC) and Masson staining
Formalin-fixed, paraffin-embedded tissues were cut into 4 μm-thick sections, cleared with xylene, and rehydrated through a series of graded ethanol. After antigenic retrieval and endogenous peroxidase blocking, the sections were blocked with 2% bovine serum albumin (BSA) and incubated overnight with primary antibodies against as listed in Table S3 overnight at 4 °C. Followed by incubation with HRP-conjugated secondary antibodies at room temperature for 2 h. Slides were counterstained with hematoxylin and mounted to detect nuclei. Images of stained sections were recorded and analyzed using a Panoramic MIDI digital slide scanner (3D Histech). Additionally, Masson Trichrome Staining were also performed based on 4 μm-thick sections according to previous published literature [23].
Immunofluorescence staining
For GH3 tumor sphere, cells were firstly pre-embedded with 2% Agarose (BIOWEST, Spanish), and were then fixed with 4% paraformaldehyde (PFA) before being embedded with paraffin. Sections were permeabilized with BSA, followed by overnight incubation with ki67 primary antibodies (Abclonal, 1:100) overnight at 4 °C. After being rinsed with PBS, the slides were incubated with Fluor-conjugated secondary antibody, and nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). The immunofluorescence images were captured using a microscope (Oxympus, Japan).
Western blotting (WB) assay
Tumor tissues were lysed with RIPA (Beyotime, China) buffer containing protease inhibitor (Beyotime, China) cocktail. Equal amount of protein was separated by SDS-PAGF and transferred to PVDF membrane (Millipore, MA, USA). Afterwords, the membranes were gently skimmed mildly at room temperature for 2 h and then incubated with the primary antibodies as shown in Table S3 at 4 °C overnight. Afterwards, the membranes were incubated with appropriate HRP-conjugated secondary antibodies (Beyotime, China) for 1 h at room temperature, The protein bands were detected using ECL chemiluminescence regents (Millipore). The density of protein bands was qualified with public software Image J (National Institutes of Health, USA).
Atomic force microscopy measurements
To assess the stiffness of pituitary adenoma, we utilized AFM System (JPK NanoWizard ULTRA Speed 2; Bruker). AFM and analysis were reported as previously reported [24]. Typically, five 20 μm × 20 μm AFM force maps were obtained on each sample, and each stiffness map was presented as 8 × 8 raster series of indentations using JPK software. Samples were assumed to be incompressible. A Posson’s ratio of 0.5 was used to calculate the Young’s modules of each sample using a fit of Hertz model.
Statistical analysis
All experiments were performed at least three times. GraphPad Prism 8.0 was used to analyze the data. The results were presented as means ± standard deviations (SD). Student’s t test was used for sample comparisons between groups. Nonparametric methods were used in the comparison of Ktrans, Kep and Ve values between CS and ST groups. The correlation analysis of the Ktrans and Ve values between hardness gradings was also performed using nonparametric methods. All differences with a p < 0.05 were considered statistically significant.
Results
Tumor texture analysis based on DCE-MRI and intraoperative view
Pituitary adenoma tissues from the CS and ST were procured via endoscopic transsphenoidal surgery as illustrated in Video S1. The PAs in the ST was characterized by higher values of Ktrans (p < 0.0001) and Ve (p < 0.05) than those in the CS, indicating that CS tumors have lower permeability and exudation. Moreover, Kep was not significantly different between CS tumors and ST tumors, which may be associated with vascular heterogeneity (Fig. 2A–E). Scatter plots of the correlation analysis revealed that the intraoperative PA texture classification of pituitary tumors was negatively correlated with the Ktrans (CS: r = −0.610, P = 0.007; ST: r = −0.503, p = 0.040) and Ve (CS: r = −0.503, P = 0.033; ST: r = −0.472, p = 0.048) values on DCE-MRI (Fig. 2F). This suggested that tumor stiffness was closely related to the MRI features. Additionally, intraoperative observations were consistent with MRI findings; the CS tumor exhibited a fibrous and strip-shaped appearance, making it challenging to suction, whereas the ST tumor appeared soft and could be easily aspirated (Fig. 2G–J).
DCE-MRI of one invasive pituitary adenoma. A ROIs were manually selected in the ST and CS around the ICA at the coronal T1WI. Color images of Ktrans (B), Kep (C), and Ve (D) maps. E Comparison of Ktrans values, Kep values and Ve values between CS and ST groups. F Scatter plots of correlation analysis between radiological parameters and texture classification of CS/ST. ***p < 0.001; *p < 0.05; NS, no significant difference. G Preoperatively MRI showed the adenoma invading the right CS and encased the internal carotid artery (ICA). H During the endoscopic endonasal resection producer, we extensively exposed the bone area including the sella turcica (ST) and right cavernous sinus (CS). I The adenoma in the right CS cannot be suctioned with aspirator, and the tough texture adenoma was sharply dissected from the ICA. The white arrows show the tumor was fibrous and strip shape. J The adenoma in the ST can easily be suctioned with aspirator. The black arrows show the tumor was soft and grainy shape
Increased matrix stiffness of PitNETs invading the cavernous sinus
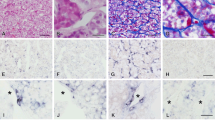
To assess the stiffness of the PitNETs in different regions, AFM was employed. PAs located in the CS exhibited significantly greater stiffness than those in the ST (0.65 Kpa vs. 1.34 Kpa, p < 0.05) (Fig. 3). Further analysis of extracellular matrix components (collagen I[Col-1] and collagen III[Col-3]) and epithelial-to-mesenchymal transition (EMT) markers (E-cadherin [E-cad] and α-smooth muscle actin[α-SMA]) was conducted through western blot assay. The results revealed elevated expression levels of extracellular matrix and mesenchymal cell markers in pituitary adenomas within the cavernous sinus, suggesting a potential correlation with the increased invasiveness in this region (Fig. 4A). These findings were further validated by PCR at the mRNA level (Fig. 4B). Masson’s trichrome stain was used to stain collagen, and the results indicated collagen was more abundant in cavernous sinus tumors. IHC results indicated that the levels of Col-1, Col-3, and α-SMA in CS space were greater than those in the ST space, but E-cad expression showed the opposite trend (Fig. 4C). Collectively, these results suggested the reprogramming of the extracellular matrix in pituitary adenomas invading the cavernous sinus.
Stiffer PA tumor in the CS aera showed more mesenchymal profile and extracellular matrix accumulation. A Western blot analysis of 18 invasive pituitary adenoma for ST tumor and CS tumor. B Quantitative real-time RT-PCR analysis of E-cadherin, α-SMA, Col-1, Col-3 in invasive pituitary adenoma located in ST and CS. C Masson Trichrome Staining and IHC analysis of E-cadherin, α-SMA, Col-1, Col-3 and Sox-2 in invasive pituitary adenoma located in ST and CS
Increased matrix stiffness contributed to EMT and invasion
We constructed matrices with stiffness of 10 kPa (H), 5 kPa (M), and 1 kPa (L) stiffness and embedded GH3 cells in them. Sphere formation assay revealed that GH3 cell spheres grew larger with the increasing stiffness of the matrix in a time-dependent manner (Fig. 5A). Furthermore, Sox-2 expression of PA in the CS was greater than those in the ST (Fig. 4C), suggesting that a stiffer ECM may contribute to the stem-like properties of PAs. Additionally, Ki67 expression also increased when stiffness increased, indicating that the increased stiffness contributed to enhanced cell proliferation (Fig. 5B). Western blot assays revealed elevated expression levels of mesenchymal markers (Vimentin and N-cadherin) and reduced expression of an epithelial marker (E-cadherin) as matrix stiffness increased. LOX, known for inducing collagen crosslinking and facilitating tumor cell growth, invasion, and metastasis, also exhibited increased expression with higher matrix stiffness. Given that MMP2 and MMP9 modulate the dynamic remodeling of the ECM, we focused on their dynamic expression changes and found that a stiffer matrix resulted in greater expression of MMP2 and MMP9 (Fig. 5C). Quantitative RT-PCR assays were subsequently conducted to validate the results of the WB assay (Fig. 5D). Collectively, these results indicated that a stiffer matrix contributed to the proliferation and invasion of PAs.
Stiffer matrix promoted pituitary adenoma tumorigenicity, proliferation abilities, EMT, and ECM reprogramming. A Sphere formation assay demonstrated GH3 cells in stroma with different stiffness on day 3 and day 6. B Immunofluorescence staining of Ki67 at different stiffness on day 6. C The protein expressions of various markers in GH3 cells grown in different stiffness stroma. D mRNA expression various markers in GH3 cells grown in different stiffness stroma; L, low matrix stiffness (1 kPa); M, median matrix stiffness (5kPa); H, high matrix stiffness (10 kPa)
TAFs reprogrammed the ECM of PAs
To obtain comprehensive insight into the proteomic alterations in pituitary tumors between the ST and CS groups, proteomic analysis was conducted, followed by pathway enrichment analysis. The top upregulated genes converged on pathways related to extracellular matrix assembly, extracellular matrix constituent secretion, fibrinolysis, and fibroblast activation (Fig. 6A). Using the xCell method to generate cell type enrichment scores, we found that tumor-associated fibroblasts and fibroblasts had significant differences in protein abundance between ST and CS tumors (Fig. 6B). The protein abundance of brain tumor associated fibroblast and fibroblast was significantly greater in ST tumor than that in CS tumor. Additionally, pathway analysis revealed that regulation of extracellular matrix organization, ECM proteoglycans, regulation of fibroblast proliferation, and blood vessel development related to fibroblast proliferation were significant enriched in CS tumors (Fig. 6C). Furthermore, the abundance of TAF related protein and fibroblast related protein abundance such as LAMB1, LAMB2, PDGFRA, TGFB1, COL1A1, COL3A1, DCN, and ISLR, was significant greater in CS tumor compared to ST tumor, (Fig. S1). Moreover, FAP and α-SMA are important markers of activated fibroblasts [25], We performed immunofluorescence staining of pituitary adenoma in both CS and ST. FAP and α-SMA expression were significantly higher in CS space compared to ST (Fig. 6D). Primary TAF cell lines were cultured from both the ST and CSs, and the morphology of TAF from ST was spindle and regular while those from CS are had long projections (Fig. 6E). FAP and α-SMA expression in the CS were also found to be higher compared to the ST (Fig. 6F). TAFs derived from CS expressed higher levels of extracellular matrix components (such as Col-1 and Col-3) compared to those from ST (Fig. 6F). Therefore, the activation of TAFs played a pivotal role in stiffer extravascular matrix.
Proteomics data analysis of PA located in CS and ST. A Heatmaps of differentially enriched pathways and pathway-related proteins. B Comparison protein enrichment score of TAF and fibroblast tumor tissues in ST and CS. C Functional enrichment analysis of stiffness related proteins. D α-SMA and FAP expression in ST tumor and CS tumor, scale bar 100 μm. E Morphology of primary TAF derived from ST (TAFST) area and CS (TAFcs) area of PA. F Immunofluorescence staining of α-SMA, FAP, Col-1 and Col-3 expression TAFST and TAFCS
The mTOR pathway regulated tumor progression in PitNETs
Previous studies established that the mTOR signaling pathway serves as a central signaling hub of mechanoresponsive-induced signaling cascades to regulate cell growth and invasion [26, 27]. In this study, we explored whether the mTOR pathway regulated tumor progression of invasive pituitary adenomas. Firstly, we found that everolimus, an mTOR inhibitor, significantly inhibited the migration ability of GH3 cell and transwell assay further validated that mTOR kinase inhibitor inhibited the migration and invasion ability of GH3 cells (Fig. 7A, B). Then we found mTOR pathway in the CS tumor was activated under higher matrix stiffness. This was further validated in GH3 cells grown in higher matrix stiffness. Also, we revealed upregulated expression of CCND1, c-Myc, and BCL-2 in the CS of PA tissue, thereby promoting cell cycle, promoting proliferation, and inhibiting apoptosis, respectively. The results were validated in GH3 cells embedded in different matrices with different stiffnesses (Fig. 7C). Furthermore, we administrated an mTOR kinase inhibitor to GH3 cells grown in high matrix stiffness, and found that mTOR pathway activation was repressed in a concentration-dependent manner (Fig. 7D). These results collectively indicated that the mTOR pathway in pituitary tumor cells was activated by stronger biophysical stimulation and mTOR inhibitor repressed the invasion and migration of pituitary tumor cell.
The mTOR pathway in pituitary tumor cell was activated in higher matrix stiffness and mTOR inhibitor could inhibit the invasion and migration of GH3 cell. A Wound healing assay were used to the migration ability of GH3 cells treated by mTOR inhibitors or control. B Transwell assays were used to measure the migration and invasion of GH3 cells treated by mTOR inhibitors or not. C The alteration of mTOR pathway in different region of PA. Also, the mTOR pathway activation altered in GH3 cells grown in different matrix stiffness. D Effect of everolimus in GH3 cells and the effect was determined by WB. Cells was treated with everolimus (0, 2.5, 5, 10, 20, 50 μM) for 72 h. **p < 0.01; ***p < 0.001; NS no significant difference
Discussion
Previous studies rarely focused on the difference in stiffness between invasive pituitary adenoma in the CS and ST. Our current study demonstrated that the tumor texture was stiffer in the CS tumor that of the ST tumor by DCE-MRI, AFM, and histological analysis. We also observed that stiffer matrix contributed to tumor invasion and EMT. Proteomic analysis further revealed that tumor associated fibroblasts could play a critical role in ECM reprogramming and was validated in PA tumor tissues and primary TAFs from CS and ST. Additionally, we found that the mTOR pathway was activated in CS tumors and GH3 cells grown in higher matrix stiffness. Functional assay showed that mTOR inhibitor repressed the migration and invasion of pituitary tumor cells. These findings provided valuable insights into the intratumor heterogeneity of the tumor texture, which could lead to improved treatment efficacy for invasive pituitary adenomas.
In the past, preoperative texture MRI evaluations of PA was all based on the overall evaluation of the adenoma. Our research represented an innovative approach for the analysis of stiffness by introducing regional quantitative measurements via DCE-MRI. Specifically, Ktrans is the permeability rate of the contrast agent from the microvessels to the extravascular ECM space, reflecting the local microvascular blood flow status and surface permeability. Kep represents the speed at which the contrast agent transferred from the extravascular space to microvessels, while Ve mainly reflects the percentage of contrast agent concentration in the extravascular extracellular space in the entire voxel [19]. These parameters have been predominantly utilized for indirect assessment of tumor heterogeneity in texture analysis in breast cancer and glioma studies. In our study, we observed higher Ktrans in the ST compared to the CS, indicating the CS group have a lower contrast agent permeability, potentially associated with a greater stiffness density of the ECM. Furthermore, the higher value of Ve in the CS also suggested a higher contrast agent concentration, potentially indicative of soft ECM in this area. These conclusions were verified by subsequent endoscopic suction strength needed to aspirate tumors.
AFM has been widely utilized to investigate the mechanical profiles of cells, tissues, and macromolecules such as proteins. Particularly, AFM in force mapping mode has been employed to directly measure the stiffness of tumor tissues and normal tissues [28, 29]. There are distinct stiffness peaks between malignant and benign tissues [30, 31]. Therefore, AFM holds significant promise in cancer research for both diagnostic and therapeutic purposes. In our present study, we discovered that pituitary tumor tissue located in the CS was significantly firmer than that located in the ST. AFM provided a quantitative and direct method for determining the stiffness of pituitary tissues in different locations, and these findings were consistent with the results obtained from DCE-MRI.
Tumor stiffness is increasingly recognized as an important contributing factor to disease progression and invasion, and is primarily determined by the amount of ECM, particularly the amount of collagen and hyaluronan within the tumor [32]. The ECM comprises a three-dimensional network of macromolecules, such as proteins, proteoglycans, glycosaminoglycans, and collagens, within which soluble factors such as cytokines, chemokines and growth factors are embedded [33]. Tumor cells and stromal cells can remodel the ECM through the secretion of metalloproteinases (MMPs). Previous studies have shown that high expressions of MMP2 and MMP9 were associated with the invasion and recurrence of pituitary adenoma [34, 35]. In our study, we observed an increase in the expression levels of MMP2 and MMP9 as the ECM became stiffer, potentially contributing to tumor invasion. This highlights the intricate interplay between tumor stiffness, ECM composition, and the activity of MMPs in the context of pituitary adenoma progression.
EMT is a process related to tumorigenesis and tumor progression by which epithelial cells acquired the mesenchymal phenotypes, facilitating the migration, invasion, and metastasis [36]. Gao et al. reported that EMT was a significant hallmark associated with the stiffness of pituitary adenomas [17]. Additionally, stiff pituitary adenomas were found to contain more TAFs and endothelial cells. TAFs have been shown to promote EMT in pituitary adenomas by secreting cytokine factors [17]. This finding suggests a complex interplay between TAFs and EMT in promoting the stiffening of the pituitary adenoma texture. In our study, we found that TAF derived from the CS area expressed higher ECM components, such as Col-1 and Col-3, this implied that activated to higher matrix stiffness. When the ECM stiffness increased, pituitary adenoma cells exhibited increased expression of mesenchymal markers. EMT and mechanotransduction are highly interactive and complex processes, which rely on several mechanisms, such as the Hippo/YAP, Piezo1, TGF-β signaling and so on [37,38,39]. Further exploration of the mechanism of stiffness-related EMT will aid in identifying potential treatment targets for invasive pituitary adenoma.
Our further investigations revealed that the mTOR pathway was activated in CS tumors and GH3 cells with higher matrix stiffness. As the tumor stiffness increased, tumor cells can sense and transmit mechanical cues, which facilitated tumor growth, proliferation, invasion, and EMT [40]. The mTOR is at the central hub of signaling networks that regulate physical phenotype of cancer cells and transmit extracellular mechanical signals [41]. In our study, we observed the activation of the mTOR pathway in pituitary tumors cells in high matrix stiffness, leading to downstream effects. CCND1 is a key regulator of cell cycle and proliferation [42]. c-Myc protein functions as a transcriptional regulator and modulates cell growth, apoptosis, and stem cell formation [43]. Bcl-2 regulates apoptosis, autophagy, and metabolism [44]. As matrix stiffness increased, the expression levels of CCND1, c-Myc and BCL-2 all elevated. We also performed functional assay and demonstrated targeting mTOR pathway could repressed the migration and invasion abilities, which indicated that targeting mTOR pathway was a promising treatment strategy of pituitary adenoma from the standpoint of biomechanical properties. However, crosstalk between TAF and matrix stiffness remained to be elusive and we will carry out further study to discover the molecular mechanism about TAF activation and potential treatment targets.
Conclusions
PitNETs located in the CS exhibited greater matrix stiffness than those in the ST, which was associated with activation of TAFs. Increased matrix stiffness contributed to the enhanced invasion and migration in pituitary tumor cells through mTOR pathway activation and mTOR inhibitors could repressed this biological process. Our study unveiled that mTOR inhibitor was a promising treatment strategy for invasive PitNETs from the standpoint of biomechanical properties.
Abbreviations
- PitNETs:
-
pituitary neuroendocrine tumors
- PAs:
-
pituitary adenomas
- CS:
-
cavernous sinus
- TAFs:
-
tumor associated fibroblasts
- ECM:
-
extracellular matrix
- TME:
-
tumor microenvironment
- DCE-MRI:
-
dynamic contrast-enhanced MRI
- AFM:
-
atomic force microscopy
- ST:
-
sella turcica
- IHC:
-
immunohistochemistry
- EMT:
-
epithelial-to-mesenchymal transition
References
G. Raverot, M.D. Ilie, H. Lasolle, V. Amodru, J. Trouillas, F. Castinetti, T. Brue, Aggressive pituitary tumours and pituitary carcinomas. Nat. Rev. Endocrinol. 17(11), 671–684 (2021)
M.D. Ilie, A. Vasiljevic, P. Bertolino, G. Raverot, Biological and therapeutic implications of the tumor microenvironment in pituitary adenomas. Endocr. Rev. 44(2), 297–311 (2023)
K. Juraschka, O.H. Khan, B.L. Godoy, E. Monsalves, A. Kilian, B. Krischek, A. Ghare, A. Vescan, F. Gentili, G. Zadeh, Endoscopic endonasal transsphenoidal approach to large and giant pituitary adenomas: institutional experience and predictors of extent of resection. J. Neurosurg. 121(1), 75–83 (2014)
S. Dhandapani, H. Singh, H.M. Negm, S. Cohen, V.K. Anand, T.H. Schwartz, Cavernous sinus invasion in pituitary adenomas: systematic review and pooled data meta-analysis of radiologic criteria and comparison of endoscopic and microscopic surgery. World Neurosurg. 96, 36–46 (2016)
S. Melmed, Pituitary-tumor endocrinopathies. N. Engl. J. Med 382(10), 937–950 (2020)
R.D. Almutairi, I.S. Muskens, D.J. Cote, M.D. Dijkman, V.K. Kavouridis, E. Crocker, K. Ghazawi, M.L.D. Broekman, T.R. Smith, R.A. Mekary, H.A. Zaidi, Gross total resection of pituitary adenomas after endoscopic vs. microscopic transsphenoidal surgery: a meta-analysis. Acta Neurochir. (Wien.) 160(5), 1005–1021 (2018)
E. Lefevre, F. Chasseloup, M. Hage, P. Chanson, M. Buchfelder, P. Kamenicky, Clinical and therapeutic implications of cavernous sinus invasion in pituitary adenomas. Endocrine 85(3), 1058–1065 (2024)
M. Cappelletti, A.G. Ruggeri, L. Spizzichino, A. D’Amico, E. D’Avella, R. Delfini, Fibrous pituitary macroadenomas: Predictive role of preoperative radiologic investigations for proper surgical planning in a cohort of 66 patients. World Neurosurg. 121, e449–e457 (2019)
C. Li, H. Zhu, X. Zong, X. Wang, S. Gui, P. Zhao, J. Bai, C. Liu, L. Cao, Z. Li, Y. Zhang, Experience of trans-nasal endoscopic surgery for pituitary tumors in a single center in China: Surgical results in a cohort of 2032 patients, operated between 2006 and 2018. Clin. Neurol. Neurosurg. 197, 106176 (2020)
P. Li, D. Zhang, S. Ma, P. Kang, C. Zhang, B. Mao, W. Zhou, X. Wang, J. Peng, L. Yuan, Y. Wang, J. Diao, W. Jia, Consistency of pituitary adenomas: Amounts of collagen types I and III and the predictive value of T2WI MRI. Exp. Ther. Med. 22(5), 1255 (2021)
A. Acitores Cancela, V. Rodriguez Berrocal, H. Pian Arias, J.J. Diez, P. Iglesias, Effect of pituitary adenoma consistency on surgical outcomes in patients undergoing endonasal endoscopic transsphenoidal surgery. Endocrine 78(3), 559–569 (2022)
M.D. Ilie, D. De Alcubierre, A.L. Carretti, E. Jouanneau, G. Raverot, Therapeutic targeting of the pituitary tumor microenvironment. Pharm. Ther. 250, 108506 (2023)
Z. Yang, X. Tian, K. Yao, Y. Yang, L. Zhang, N. Liu, C. Yan, X. Qi, S. Han, Targeting the tumor immune microenvironment could become a potential therapeutic modality for aggressive pituitary adenoma. Brain Sci. 13(2), 164 (2023)
M. Kalli, M.D. Poskus, T. Stylianopoulos, I.K. Zervantonakis, Beyond matrix stiffness: targeting force-induced cancer drug resistance. Trends Cancer 9(11), 937–954 (2023)
A. Saraswathibhatla, D. Indana, O. Chaudhuri, Cell-extracellular matrix mechanotransduction in 3D. Nat. Rev. Mol. Cell Biol. 24(7), 495–516 (2023)
Y. Tang, T. Xie, S. Wu, Q. Yang, T. Liu, C. Li, S. Liu, Z. Shao, X. Zhang, Quantitative proteomics revealed the molecular characteristics of distinct types of granulated somatotroph adenomas. Endocrine 74(2), 375–386 (2021)
Z. Wang, M. Chang, Y. Zhang, G. Zhou, P. Liu, J. Lou, Y. Wang, Y. Zhang, X. Guo, Y. Wang, X. Bao, W. Lian, Y. Wang, R. Wang, W. Ma, B. Xing, J. Gao, Multi-omics investigations revealed underlying molecular mechanisms associated with tumor stiffness and identified sunitinib as a potential therapy for reducing stiffness in pituitary adenomas. Front Cell Dev. Biol. 10, 820562 (2022)
O.M. Mahmoud, A. Tominaga, V.J. Amatya, M. Ohtaki, K. Sugiyama, T. Sakoguchi, Y. Kinoshita, Y. Takeshima, N. Abe, Y. Akiyama, A.I. El-Ghoriany, A.K. Abd Alla, M.A. El-Sharkawy, K. Arita, K. Kurisu, F. Yamasaki, Role of PROPELLER diffusion-weighted imaging and apparent diffusion coefficient in the evaluation of pituitary adenomas. Eur. J. Radio. 80(2), 412–417 (2011)
Y.Q. Liu, B.B. Gao, B. Dong, S.S. Padikkalakandy Cheriyath, Q.W. Song, B. Xu, Q. Wei, L.Z. Xie, Y. Guo, Y.W. Miao, Preoperative vascular heterogeneity and aggressiveness assessment of pituitary macroadenoma based on dynamic contrast-enhanced MRI texture analysis. Eur. J. Radiol. 129, 109125 (2020)
Y. Wang, R. Luo, X. Zhang, H. Xiang, B. Yang, J. Feng, M. Deng, P. Ran, A. Sujie, F. Zhang, J. Zhu, S. Tan, T. Xie, P. Chen, Z. Yu, Y. Li, D. Jiang, X. Zhang, J.Y. Zhao, Y. Hou, C. Ding, Proteogenomics of diffuse gliomas reveal molecular subtypes associated with specific therapeutic targets and immune-evasion mechanisms. Nat. Commun. 14(1), 505 (2023)
L. Lv, S. Zhang, Y. Hu, P. Zhou, L. Gao, M. Wang, Z. Sun, C. Chen, S. Yin, X. Wang, S. Jiang, Invasive pituitary adenoma-derived tumor-associated fibroblasts promote tumor progression both in vitro and in vivo. Exp. Clin. Endocrinol. Diab 126(4), 213–221 (2018)
P. Marques, S. Barry, E. Carlsen, D. Collier, A. Ronaldson, S. Awad, N. Dorward, J. Grieve, N. Mendoza, S. Muquit, A.B. Grossman, F. Balkwill, M. Korbonits, Pituitary tumour fibroblast-derived cytokines influence tumour aggressiveness. Endocr. Relat. Cancer 26(12), 853–865 (2019)
T. Martinello, F. Pascoli, G. Caporale, A. Perazzi, I. Iacopetti, M. Patruno, Might the Masson trichrome stain be considered a useful method for categorizing experimental tendon lesions? Histol. Histopathol. 30(8), 963–9 (2015)
M. Xia, M. Wu, Y. Li, Y. Liu, G. Jia, Y. Lou, J. Ma, Q. Gao, M. Xie, Y. Chen, Y. He, H. Li, W. Li, Varying mechanical forces drive sensory epithelium formation. Sci. Adv. 9(44), eadf2664 (2023).
M. Nurmik, P. Ullmann, F. Rodriguez, S. Haan, E. Letellier, In search of definitions: Cancer-associated fibroblasts and their markers. Int. J. Cancer 146(4), 895–905 (2020)
A.N. Gargalionis, K.A. Papavassiliou, E.K. Basdra, A.G. Papavassiliou, mTOR signaling components in tumor mechanobiology. Int. J. Mol. Sci. 23(3), 1825 (2022)
Y. You, Q. Zheng, Y. Dong, X. Xie, Y. Wang, S. Wu, L. Zhang, Y. Wang, T. Xue, Z. Wang, R. Chen, Y. Wang, J. Cui, Z. Ren, Matrix stiffness-mediated effects on stemness characteristics occurring in HCC cells. Oncotarget 7(22), 32221–32231 (2016)
T. Nandi, S.R.K. Ainavarapu, Applications of atomic force microscopy in modern biology. Emerg. Top. Life Sci. 5(1), 103–111 (2021)
X. Wei, H. Lou, D. Zhou, Y. Jia, H. Li, Q. Huang, J. Ma, Z. Yang, C. Sun, Y. Meng, S. Xu, X. Yang, X. Li, T. Ji, Z. Guo, Q. Gao, TAGLN mediated stiffness-regulated ovarian cancer progression via RhoA/ROCK. Pathw. J. Exp. Clin. Cancer Res. 40(1), 292 (2021)
J. Najera, M.R. Rosenberger, M. Datta, Atomic force microscopy methods to measure tumor mechanical properties. Cancers (Basel) 15(13), 3285 (2023)
Y. Shen, X. Wang, J. Lu, M. Salfenmoser, N.M. Wirsik, N. Schleussner, A. Imle, A. Freire Valls, P. Radhakrishnan, J. Liang, G. Wang, T. Muley, M. Schneider, C. Ruiz de Almodovar, A. Diz-Munoz, T. Schmidt, Reduction of liver metastasis stiffness improves response to bevacizumab in metastatic colorectal cancer. Cancer Cell 37(6), 800–817 (2020)
V. Gkretsi, T. Stylianopoulos, cell adhesion and matrix stiffness: Coordinating cancer cell invasion and metastasis. Front Oncol. 8, 145 (2018)
P. Marques, M. Korbonits, Tumour microenvironment and pituitary tumour behaviour. J. Endocrinol. Invest 46(6), 1047–1063 (2023)
H.Y. Liu, W.J. Gu, C.Z. Wang, X.J. Ji, Y.M. Mu, Matrix metalloproteinase-9 and -2 and tissue inhibitor of matrix metalloproteinase-2 in invasive pituitary adenomas: A systematic review and meta-analysis of case-control trials. Med. (Baltim.) 95(24), e3904 (2016)
S. Wu, Q. Zheng, X. Xing, Y. Dong, Y. Wang, Y. You, R. Chen, C. Hu, J. Chen, D. Gao, Y. Zhao, Z. Wang, T. Xue, Z. Ren, J. Cui, Matrix stiffness-upregulated LOXL2 promotes fibronectin production, MMP9 and CXCL12 expression and BMDCs recruitment to assist pre-metastatic niche formation. J. Exp. Clin. Cancer Res 37(1), 99 (2018)
P. Marques, A.B. Grossman, M. Korbonits, The tumour microenvironment of pituitary neuroendocrine tumours. Front Neuroendocrinol. 58, 100852 (2020)
M. Lopez-Cavestany, S.B. Hahn, J.M. Hope, N.T. Reckhorn, J.D. Greenlee, S.C. Schwager, J.A. VanderBurgh, C.A. Reinhart-King, M.R. King, Matrix stiffness induces epithelial-to-mesenchymal transition via Piezo1-regulated calcium flux in prostate cancer cells. iScience 26(4), 106275 (2023)
Y. Dong, Q. Zheng, Z. Wang, X. Lin, Y. You, S. Wu, Y. Wang, C. Hu, X. Xie, J. Chen, D. Gao, Y. Zhao, W. Wu, Y. Liu, Z. Ren, R. Chen, J. Cui, Higher matrix stiffness as an independent initiator triggers epithelial-mesenchymal transition and facilitates HCC metastasis. J. Hematol. Oncol. 12(1), 112 (2019)
Y.X. Liu, S. Wan, X.Q. Yang, Y. Wang, W.J. Gan, W.L. Ye, X.S. He, J.J. Chen, Y. Yang, X.M. Yang, X. Guo, X.J. Gao, Y.T. Lu, Z.Y. Deng, G. Hu, H. Wu, TRIM21 is a druggable target for the treatment of metastatic colorectal cancer through ubiquitination and activation of MST2. Cell Chem. Biol. 30(7), 709–725 (2023)
E. Marhuenda, C. Fabre, C. Zhang, M. Martin-Fernandez, T. Iskratsch, A. Saleh, L. Bauchet, J. Cambedouzou, J.P. Hugnot, H. Duffau, J.W. Dennis, D. Cornu, N. Bakalara, Glioma stem cells invasive phenotype at optimal stiffness is driven by MGAT5 dependent mechanosensing. J. Exp. Clin. Cancer Res. 40(1), 139 (2021)
M. Di-Luoffo, Z. Ben-Meriem, P. Lefebvre, M. Delarue, J. Guillermet-Guibert, PI3K functions as a hub in mechanotransduction. Trends Biochem Sci. 46(11), 878–888 (2021)
J. Pawlonka, B. Rak, U. Ambroziak, The regulation of cyclin D promoters - review. Cancer Treat. Res. Commun. 27, 100338 (2021)
M.J. Duffy, S. O’Grady, M. Tang, J. Crown, MYC as a target for cancer treatment. Cancer Treat. Rev. 94, 102154 (2021)
S. Banjara, C.D. Suraweera, M.G. Hinds, M. Kvansakul, The Bcl-2 family: Ancient origins, conserved structures, and divergent mechanisms. Biomolecules 10(1), 128 (2020)
Acknowledgements
We also extend our sincere thanks to Yi Wu for providing the illustrations featured in this manuscript. The professionalism, creativity, and attention to detail have greatly enhanced the visual representation of our research findings. We are grateful for the expertise and contributions to this work.
Author contributions
Conceptualization: T.X., Y.G., X.Z.; Methodology: T.X., Y.G., Q.X., R.L., J.H., Y.G., Q.Y., C.Y.; P.C.; Z.Y., F.H.; Formal Analysis: Y.G., Y.T., Q.X.; Writing—Original Draft: T.X., Y.G.; Writing—Review & Editing: X.Z.; Supervision: T.X., XZ.
Funding
This work is supported by Shanghai Municipal Science and Technology Major Project: (22S31902500, 21ZR1413100); Zhongshan Hospital clinical research special project (ZSLCYJ202344).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
Ethical approval for the study was granted by the ethics committee of Zhongshan Hospital, Fudan University (B2021-819). And all animal studies were approved by the Animal Research Ethics Committee of Zhongshan Hospital, Fudan University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xie, T., Gao, Y., Hu, J. et al. Increased matrix stiffness in pituitary neuroendocrine tumors invading the cavernous sinus is activated by TAFs: focus on the mechanical signatures. Endocrine (2024). https://doi.org/10.1007/s12020-024-04022-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12020-024-04022-9