Abstract
Background
Levothyroxine (LT4) monotherapy is the standard treatment for hypothyroidism; however, 10–15% of patients have persistent hypothyroid symptoms despite normalizing thyroid hormone levels with LT4. This study aims to summarize the best available evidence on interventions to improve symptomatology in patients with hypothyroidism and persistent symptoms.
Methods
A systematic search was conducted in March 2022 for randomized controlled trials and observational studies on interventions for adult patients with persistent hypothyroid symptoms despite biochemical euthyroidism on thyroid hormone replacement.
Results
A total of 277 articles were reviewed and seven fulfilled the inclusion criteria. 455 participants were included. Most intervention participants were female (78.6%) with a mean age of 47.5 (±2.8) years. Five clinical trials evaluating ginger (vs. starch), L-carnitine (vs. placebo), combination LT4 and liothyronine (LT3) (vs. LT4 or placebo), and surgery for patients with serum antithyroid peroxidase (TPO Ab) titers greater than 1000 IU/ml (vs. LT4) found inconsistent improvement in hypothyroidism related symptoms and general health. The two clinical trials with the largest improvement in fatigue scores were the use of ginger and surgery. One observational study comparing thyroidectomy vs observation found no significant difference on general health. Lastly, another observational study evaluating combination LT4/LT3 (vs. LT4 monotherapy) found improvement in fatigue and quality of life. There were 31 (12%) adverse events in the intervention group and 18 (10.8%) in the comparator group.
Conclusions
There is no high-quality evidence supporting any intervention for persistent symptoms in hypothyroidism. Available evidence, limited by the risk of bias, inconsistency, and heterogeneity, suggests that some persistent symptoms, particularly fatigue, could improve with ginger and thyroidectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypothyroidism is a common endocrine disorder resulting from low thyroid hormone levels. Untreated hypothyroidism leads to low quality of life (QoL) due to symptoms and can be fatal in severe cases [1]. The most common symptoms of hypothyroidism include cold intolerance, dry skin, hair loss, constipation, fatigue, sleep disturbance, weight gain, irregular menses, and cognitive impairment such as memory loss. This clinical presentation can vary with age, sex, and time between onset and diagnosis [2].
The standard treatment for patients with hypothyroidism is thyroid hormone replacement therapy with levothyroxine (LT4) [3]. LT4 monotherapy is effective at restoring thyroid levels and improving hypothyroid symptoms in most patients. However, 10–15% of patients with hypothyroidism treated with LT4 complain of persistent hypothyroid symptoms even when their thyroid hormone levels are normalized [4]. While the etiology of these symptoms is under investigation, their presence affects patients’ QoL, satisfaction with care, and patient-clinician relationship [5]. Patients affected by persistent symptoms are often labeled as having “unexplained” medical symptoms and struggle to find care responsive to their needs. In addition, clinicians caring for patients with persistent symptoms face the challenge of not having evidence-based recommendations to guide their next steps [6].
There is a need to systematically examine and appraise the evidence on interventions for patients with hypothyroidism and persistent symptoms to guide decision-making and future research efforts. The purpose of this systematic review was to summarize the available evidence on pharmacologic and non-pharmacologic interventions to improve symptomatology in patients with hypothyroidism who experience persistent symptoms despite achieving biochemical euthyroidism on thyroid hormone replacement.
Materials and methods
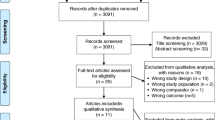
A protocol was developed to perform this study. This manuscript is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [7] (Figure 1).
Eligibility criteria
We included randomized controlled trials (RCTs) and comparative observational studies that assessed pharmacological and non-pharmacological interventions for hypothyroid adults with persistent symptoms despite biochemical euthyroidism. To be considered for inclusion, studies must have reported the effect of the intervention on QoL or hypothyroid symptoms. There were no restrictions on language. We excluded studies that detailed data on pregnancy or planned pregnancy. Animal studies, case reports, case series, reviews, and letters were also excluded.
Persistent symptoms despite biochemical euthyroidism were defined as patients experiencing fatigue, memory loss, concentration disturbance, slow thinking, dizziness, depression, weight gain, cold intolerance, skin dryness, and constipation after failing to respond to the first treatment line. The thyroid function test had to be within normal limits, with a TSH range of 0.2–4.5 mIU/L.
Data sources and searches
A comprehensive search of several databases from January 1st, 2000, to March 11th, 2022 was conducted. The databases included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy was conducted by an experienced librarian with input from the study’s principal investigator. Controlled vocabulary supplemented with keywords was used to search for the treatment of hypothyroidism with persistent hypothyroid symptoms in adult patients. References of selected studies were searched to identify additional publications. Furthermore, articles suggested by our expert investigators were reviewed to consider for inclusion. The full search strategy is described in Supplementary Material.
Study selection
The references from the search strategy process were uploaded into the systematic review software program Rayyan. Reviewers working independently in duplicate screened titles, abstracts, and full text articles for eligibility using standardized criteria. Before the title and abstract screening phase, a pilot test was conducted with 20 articles to ensure adequate inter-rater agreement; a Kappa statistic of >0.70 was set as an appropriate inter-rater agreement. Studies included by at least one reviewer at the title and abstract phase were considered for full-text screening. Before starting the full-text screening, a second pilot test was conducted with 10 articles between the reviewers. Any disagreements between reviewers were resolved by consensus.
Data collection and management
For all included articles, one reviewer extracted the study data into a spreadsheet in excel and a second reviewer checked the data for accuracy and completeness. The variables extracted were: (i) study characteristics (author name, publication year, country, period when the study was conducted, study design); (ii) patient characteristics (sample size, age, sex, body mass index [BMI], TSH, free T4, free T3, cause of hypothyroidism, follow-up duration, high-density lipoprotein, triglycerides, waist circumference, systolic blood pressure and fasting blood glucose; (iii) pharmacological interventions (LT4, combination therapy with LT4 and liothyronine [LT3]); (iv) non-pharmacological interventions (surgery, exercise, diet, natural products); (v) primary outcomes (impact on hypothyroid symptoms, QoL); (vi) secondary outcomes (adverse events); and (vii) risk of bias of included studies.
Statistical analysis
A narrative synthesis of the included studies was conducted due to inconsistencies in intervention, comparators, and outcome assessment. The statistical program R Studio for R software was employed to perform the analysis (Supplemental Table S1).
Risk of bias in individual studies
Study quality was assessed by two independent reviewers using the Cochrane risk of bias tools for parallel and cross-over RCTs [8], and the nine-star Newcastle Ottawa Scale (NOS) tool for cohort studies, according to the study design [9]. A third reviewer resolved any disagreements.
Results
Study characteristics and risk of bias
A total of 277 records were identified through our electronic database search and 7 studies fulfilled the inclusion criteria (Figure 1) [10,11,12,13,14,15,16]. Five were RCTs [10,11,12, 15, 16] and two were comparative observational studies [13, 14]. One study was only available as a conference abstract [16]. Two studies assessed the natural health products ginger and L-carnitine [10, 11], two studies evaluated total thyroidectomies [12, 13], and three studies assessed combination therapy with LT3 and LT4 [14,15,16]. Supplemental Table S2 summarizes additional details of these interventions. The studies originated predominantly from the United States (28.5%) (Table 1), and the overall risk of bias was judged to be high in all studies due to selection bias, lack of blinding assessment, and ascertainment of outcomes (Supplemental Table S3).
Overall characteristics of participants according to treatment group
A total of 455 participants were included across all studies. In the intervention group, there were 166 (78.6%) females with an overall mean age of 47.5 (±2.8) years. The overall mean baseline BMI of participants in the intervention group was 26.2 kg/m2 (±2.4), waist circumference 96.3 cm (±10/51) and the overall mean initial TSH, FT4, and FT3 were 1.6 mIU/L (±0.9), 1.5 mIU/L (±0.6), and 4.3 mIU/L (±1.4), respectively. Follow-up duration was between 1 month and 18 months. The most common causes of hypothyroidism were autoimmune disease (n = 141; 67.1%) and post-thyroidectomy (n = 37; 17.6%). The characteristics of the comparator group were similar and are shown in Tables 2 and 3. Symptom and QoL ascertainment were done using validated and internally generated scales (Supplemental Digital Content Table S1).
Impact of interventions on hypothyroid symptoms and QoL
Supplements
Ashraf et al. evaluated the effect of LT4 + ginger supplementation (500 mg twice daily) vs. LT4 + placebo on hypothyroid symptoms as assessed by the Thyroid Symptom Rating Questionnaire (ThySRQ scale) [10]. After 30 days, ginger supplementation resulted in a clinically significant improvement of tiredness, weight gain, cold intolerance, constipation, dry skin, appetite, memory loss, concentration, and feeling “giddy or dizzy.” No significant improvements were observed in hair loss, nail fragility, hearing, hoarseness, speech, depression, or feeling down (Table 4). Similarly, Hyun An et al. studied the impact of LT4 + L-carnitine vs. LT4 + placebo on fatigue [11]. L-carnitine, administered at 990 mg twice daily, did not significantly reduce disabling or physical fatigue at 12 weeks. However, the group treated with L-carnitine showed significant improvement in “mental fatigue” (problems concentrating, thinking clearly, finding the correct words, and memory). In a subgroup analysis, both physical and mental fatigue significantly improved in patients younger than 50 years. The mental score was also more markedly improved among patients with postsurgical hypothyroidism compared to patients with other causes of hypothyroidism [11].
Combined LT4/LT3 therapy
Rodriguez et al. showed that combination therapy with LT4 and LT3 daily at a ratio of 5:1 (LT4:LT3) for 6 weeks did not lead to significant differences in fatigue (assessed by the Piper Fatigue Scale), depression (assessed by the Beck Depression Inventory-II), feeling sad or nervous, forgetfulness, slow thinking and attention problems (assessed by visual analog scaling) when compared to LT4 monotherapy [15]. On the other hand, Michaelsson et al. showed that combined LT4 and LT3 (given twice daily) at a ratio of 17:1 did not change tiredness, weight loss, sensitivity to cold, dry/itchy skin (assessed by ThyPRO-39) at 3 and 12 months when compared with data from a reference population [14]. However, they showed that overall ThyPRO-39 scores (inversely proportional to the QoL) significantly decreased on combined LT4/LT3 therapy despite stable TSH. This improvement in QoL occurred across several domains, including emotional susceptibility, impaired social and daily life, and cognitive complaints. Another study conducted by Fadeyev et al. using the 36-Item Short Form Survey (SF-36) showed improved QoL in patients taking LT4 and LT3, especially in social functioning and mental health [16]. Also, in the combination therapy group, there was a significant reduction in the severity of depression using the Hospital Anxiety and Depression Scale (HADS) [16].
Surgery
Guldvog et al. randomized patients with serum antithyroid peroxidase (TPO Ab) titers greater than 1000 IU/ml to total thyroidectomy or medical management with thyroid hormone replacement to maintain euthyroidism [12]. They demonstrated that chronic fatigue scores, as measured by a standardized fatigue scale, decreased at 6, 12, and 18 months in the surgical intervention group. Furthermore, general health scores on the SF-36 were higher at 18 months in the surgical group compared to the medical therapy group across most domains, including physical functioning, emotional score, mental health, and vitality. In contrast, an observational study by Thatipamala et al. showed that patients with Hashimoto’s thyroiditis (with an average preoperative TPO Ab of 260 IU/ml) who underwent surgery had similar total SF-36 scores compared with normative data of age-matched healthy controls after a mean follow-up of 18 months [13].
Adverse events
Overall, 31 (12%) adverse events were observed in the intervention group, while 18 (10.8%) events were reported in the control group. Studies assessing thyroidectomy as the intervention had the highest rate of adverse events (e.g., transient, or permanent hypocalcemia, recurrent laryngeal nerve palsy) [12, 13]. A study assessing L-carnitine + LT4 as the intervention had nausea as the highest adverse event [11]. The other interventions resulted in fewer adverse events (Table 5).
Discussion
Around 10-15% of patients with hypothyroidism report persistent hypothyroid symptoms despite adequate LT4 replacement and achievement of biochemical euthyroidism. The most common symptoms are fatigue, weight gain, neurocognitive impairment, and cold intolerance [4]. Their presence is tightly correlated with a perceived lower QoL as assessed by validated tools. Although this is a well-described clinical problem, the optimal management of persistent symptoms remains unclear. To complement pharmacological treatments for hypothyroidism and address persistent symptoms or enhance QoL, patients and clinicians often explore non-pharmacological interventions, including dietary supplements. These supplements, such as ginger and L-carnitine, are chosen for their potential to support thyroid function and improve overall energy metabolism, offering a natural adjunct to traditional therapy. While these supplements are popular among patients seeking alternative or supplementary treatments, their effectiveness and safety must be evaluated with careful consideration, guided by evidence-based recommendations.
Non-pharmacological interventions explored include ginger supplementation, L-carnitine supplementation, and surgical management with thyroidectomy. Ashraf et al. randomized 53 patients to either ginger supplementation or placebo for 1 month [10]. At the end of follow-up, the intervention group had significant improvement in several hypothyroid symptoms and QoL scores, and no side effects were reported [10]. Previously, Mahassni et al. had shown that ginger supplementation lowers TSH levels in patients with hypothyroidism [17]. A potential mechanism is ginger’s antioxidant properties, conferring a protective effect against endocrine-disruptive chemicals such as bisphenol A (BPA) [18]. On the other hand, Hyun An et al. showed improvement in fatigue severity, physical fatigue, and mental fatigue scores in patients treated with L-carnitine for 12 weeks compared to placebo [11]. L-carnitine is essential for mitochondrial fatty acid oxidation and energy production and supplementation reduces fatigue in cancer patients [19]. Of note, 36.6% of patients in the intervention group developed side effects, mostly gastrointestinal [11].
Regarding thyroidectomy as a therapeutic option for persistent hypothyroid symptoms, two studies have shown conflicting results [12, 13]. The RCT conducted by Guldvog et al. showed that, in patients with Hashimoto’s thyroiditis with high TPO Ab titers, total thyroidectomy plus thyroid hormone replacement resulted in significantly higher health related QoL scores and lower fatigue scores compared to continued thyroid hormone replacement alone after 18 months [12]. The resulting dramatic decline in TPO Ab titers suggests that thyroidectomy may modulate the immune response and reduce inflammatory mediators responsible for non-thyroidal symptoms [20]. Nonetheless, the non-blinded nature of the study (no placebo surgery performed) raises concern about a placebo effect, and the strict inclusion criteria argue against this study’s generalizability. On the contrary, a retrospective study conducted by Thatipamala et al. evaluating the impact of thyroidectomy on persistent hypothyroid symptoms showed no significant difference in any of the eight domains assessed by the SF-36 questionnaire compared to a healthy cohort [13]. The major limitations of this study were the lack of pre-operative SF-36 responses in the intervention group, the small sample size (19 patients) and the low survey response rate. Importantly, total thyroidectomy was associated with surgical complications in both studies, including transient or permanent hypocalcemia and recurrent laryngeal nerve palsy.
In the pharmacologic arena, three studies evaluating the effect of combination LT4 and LT3 therapy on persistent hypothyroid symptoms despite biochemical euthyroidism were included; patients in the control group remained on LT4 monotherapy. Two of them were mainly positive and showed improvement in QoL measures: the open label prospective cohort study by Michaelsson et al. [14] and the cross-over RCT by Fadeyev et al. [16] both from Europe. Conversely, the RCT conducted by Rodriguez et al. [15] in the U.S. did not show a significant difference in depression scores, fatigue, working memory and concentration disturbance between groups.
Some clinical implications from this systematic review include consideration of a ginger supplementation trial in patients reporting non-remitting hypothyroid symptoms despite adequate LT4 replacement, given its potential benefit and favorable safety profile. Despite conflicting data, a combination of LT4 and LT3 may improve symptoms and QoL in some patients. Finally, there may be a role for thyroidectomy in patients with Hashimoto’s thyroiditis, very high TPO Ab titers, and persistent symptoms.
From a research perspective, the lack of large, high-quality studies prevents clinicians from providing definitive recommendations to this group of patients. Moreover, further bench and translational research efforts are critical to better understand the mechanism behind these manifestations and ultimately look for more specific therapeutic targets. Due to our limited understanding of the mechanisms and best strategies to improve the quality of life of patients with persistent hypothyroid symptoms, it is important for clinicians to validate and listen to their patients’ symptoms, evaluate patients for non-thyroid explanations for ongoing symptoms, and provide support.
Limitations and strengths
Our study has some limitations, such that some observations should be interpreted with caution. The main limitation of this review is that it can only draw an overview of alternatives for refractory hypothyroidism, as well, some of the included studies were retrospective in nature. Significant heterogeneity among studies existed in terms of inclusion/exclusion criteria, type of intervention and explored outcomes limited our ability to conduct a comprehensive meta-analysis, Similarly, sample sizes were small in most of the studies, particularly the RCTs. In addition, some RCTs were not blinded and open. Finally, the short intervention period and follow-up duration of the included studies did not allow us to assess the long-term effects and durability of these interventions.
The strengths of our study derive from an in-depth database search, which allowed us to include a broad array of studies from a methodological, geographical, and interventional standpoint. Interventions span from supplements to surgery, with attention to the increasingly popular combination LT4/LT3 therapy. Finally, a wide variety of difficult-to-measure outcomes such as fatigue, QoL, depression, social life impairment and cognitive dysfunction are explored using validated questionnaires, providing clinicians with objective data to support treatment recommendations.
The substantial prevalence of patients enduring persistent symptoms despite standard hypothyroidism treatment underscores an urgent necessity for further research. This gap, accentuated by a scarcity of studies comparing potential interventions, demands a multifaceted research approach. Key to this endeavor is the comprehensive examination of the underlying mechanisms contributing to these persistent symptoms. Such investigations, spanning both bench and translational research, are vital for uncovering new therapeutic targets and paving the way for more effective treatment options.
Moreover, the development of a systematic framework to facilitate comparative effectiveness research is imperative. This framework should encompass the identification of patients grappling with persistent hypothyroid symptoms, along with the establishment of the most appropriate scales for evaluating the efficacy of various interventions. Additionally, there is a pressing need for the infrastructure capable of supporting multicenter clinical trials. These trials must not only be well-designed but also inclusive of a diverse patient population to ensure the generalizability of findings.
Conclusion
There is no high-quality evidence supporting any intervention for persistent symptoms in hypothyroidism. Available evidence, limited by the risk of bias, inconsistency, and heterogeneity, suggests that some persistent symptoms, particularly fatigue, could improve with ginger and thyroidectomy. There is urgent need for the evaluation of interventions to address persistent symptoms in patients with hypothyroidism.
References
B. Biondi, F.S. Celi, E.A. McAninch. Critical Approach to Hypothyroid Patients With Persistent Symptoms. J Clin Endocrinol Metab 2023;108. https://doi.org/10.1210/clinem/dgad224.
N. Patil, A. Rehman, I. Jialal, A.D. Saathoff. Hypothyroidism (Nursing). StatPearls 2022. https://pubmed.ncbi.nlm.nih.gov/33760505/ (accessed July 24, 2023).
L. Chaker, A.C. Bianco, J. Jonklaas, R.P. Peeters, Hypothyroidism. Lancet 390, 1550–1562 (2017). https://doi.org/10.1016/S0140-6736(17)30703-1.
E.A. McAninch, A.C. Bianco. The history and future of treatment of hypothyroidism. Ann Intern Med 2016;164. https://doi.org/10.7326/M15-1799.
M.H. Samuels, L.J. Bernstein, Brain fog in hypothyroidism: what is it, how is it measured, and what can be done about it. Thyroid 32, 752–763 (2022). https://doi.org/10.1089/thy.2022.0139.
S.J. Peterson, E.A. McAninch, A.C. Bianco, Is a normal TSH synonymous with “euthyroidism” in levothyroxine monotherapy? J Clin Endocrinol Metab. 101, 4964–4973 (2016). https://doi.org/10.1210/jc.2016-2660.
A. Liberati, D.G. Altman, J. Tetzlaff, C. Mulrow, P.C. Gøtzsche, J.P.A. Ioannidis et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339. https://doi.org/10.1136/bmj.b2700.
J.P. Higgins, J. Savović, M.J. Page, J.A.C. Sterne. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). The Cochrane Collaboration 2019.
G. Wells, B. Shea, D. O’Connell, J. Peterson, V. Welch, M. Losos et al. The Newcastle–Ottawa Scale (NOQAS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. The Ottawa Hospital 2004. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed July 24, 2023).
H. Ashraf, M. Heydari, M. Shams, M.M. Zarshenas, A. Tavakoli, M. Sayadi. Efficacy of Ginger Supplementation in Relieving Persistent Hypothyroid Symptoms in Patients with Controlled Primary Hypothyroidism: A Pilot Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Evidence-Based Complementary and Alternative Medicine 2022;2022. https://doi.org/10.1155/2022/5456855.
J.H. An, Y.J. Kim, K.J. Kim, S.H. Kim, N.H. Kim, H.Y. Kim, et al. L-carnitine supplementation for the management of fatigue in patients with hypothyroidism on levothyroxine treatment: A randomized, double-blind, placebo-controlled trial. Endocr J 2016;63. https://doi.org/10.1507/endocrj.EJ16-0109.
I. Guldvog, L.C. Reitsma, L. Johnsen, A. Lauzike, C. Gibbs, E. Carlsen, et al. Thyroidectomy versus medical management for euthyroid patients with hashimoto disease and persisting symptoms: A randomized trial. Ann Intern Med 2019;170. https://doi.org/10.7326/M18-0284.
P. Thatipamala, J.E. Noel, L. Orloff. Quality of Life After Thyroidectomy for Hashimoto Disease in Patients With Persistent Symptoms. Ear Nose Throat J 2020. https://doi.org/10.1177/0145561320967332.
L.F. Michaelsson, J.L. La Cour, B.B. Medici, T. Watt, J. Faber & B. Nygaard. Levothyroxine/Liothyronine Combination Therapy and Quality of Life: Is It All about Weight Loss? Eur Thyroid J 2018;7. https://doi.org/10.1159/000490383.
T. Rodriguez, V.R. Lavis, J.C. Meininger, A.S. Kapadia & L.F. Stafford. Substitution of liothyronine at a 1:5 ratio for a portion of levothyroxine: Effect on fatigue, symptoms of depression, and working memory versus treatment with levothyroxine alone. Endocrine Practice 2005;11. https://doi.org/10.4158/EP.11.4.223.
M. Fadeyev, M. Madiyarova & T. Morgunova. Comparative effectiveness of replacement monotherapy with l-thyroxine and combination with l-thyroxine and triiodothyronine in women with postoperative and autoimmune hypothyroidism. Eur Thyroid J. 2014:150–151.
S.H. Mahassni & O.A. Bukhari. Beneficial effects of an aqueous ginger extract on the immune system cells and antibodies, hematology, and thyroid hormones in male smokers and non-smokers. J Nutr Intermed Metab. 2019;15. https://doi.org/10.1016/j.jnim.2018.10.001.
E.T. Mohammed, K.S. Hashem, A.E. Ahmed, M.T. Aly, L. Aleya & M.M. Abdel-Daim. Ginger extract ameliorates bisphenol A (BPA)-induced disruption in thyroid hormones synthesis and metabolism: Involvement of Nrf-2/HO-1 pathway. Science of the Total Environment 2020;703. https://doi.org/10.1016/j.scitotenv.2019.134664.
A.V. Plioplys & S. Plioplys. Amantadine and L-Carnitine treatment of chronic fatigue syndrome. Neuropsychobiology 1997;35. https://doi.org/10.1159/000119325.
A.P. Weetman, A.M. McGregor, J.H. Lazarus & R. Hall. Thyroid antibodies are produced by thyroid-derived lymphocytes. Clin Exp Immunol 1982;48.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Author Jessica Hidalgo: Writing of protocol. Screening the titles, abstracts, and full text articles for eligibility. Extracting the study data into a spreadsheet. Analysis and interpretation of the data. Writing original draft. Approval of the version to be published. Author Eddy P. Lincango: Extracting the study data into a spreadsheet. Analysis and interpretation of the data. Approval of the version to be published. Author Sandra Cordova-Madera: Screening the titles, abstracts, and full text articles for eligibility. Approval of the version to be published. Author Kim Ruiz. Screening the titles, abstracts, and full text articles for eligibility. Approval of the version to be published. Author Camila Wenczenovicz: Screening the titles, abstracts, and full text articles for eligibility. Approval of the version to be published. Author Oscar Ponce: Analysis and interpretation of the data. Approval of the version to be published. Author Neri Álvarez-Villalobos: Analysis and interpretation of the data. Approval of the version to be published. Author Arbaaz: Review draft and editing. Approval of the version to be published. Author Naykky M Singh Ospina: Review draft and editing. Approval of the version to be published. Author Spyridoula Maraka: Review draft and editing. Approval of the version to be published. Author Gonzalo J. Acosta: Review draft and editing. Approval of the version to be published. Author Juan Pablo Brito: Writing of protocol. Writing original draft. Review draft and editing. Approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hidalgo, J., Lincango, E.P., Cordova-Madera, S. et al. Interventions to improve symptomatology in patients with hypothyroidism and persistent symptoms: A systematic review. Endocrine 84, 864–873 (2024). https://doi.org/10.1007/s12020-024-03816-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-024-03816-1