Abstract
Purpose
This study aims to analyze the clinicopathological features, diagnostic steps, and therapeutic results of TSHomas and to reveal the effective factors on remission.
Methods
The clinical, radiological, and pathological features and surgical and endocrinological results of 41 TSHoma cases followed between 2005 and 2022 were retrospectively analyzed. The factors affecting the surgical cure were investigated by comparing the groups with and without remission.
Results
A total of 41 patients (23 male,18 female) were included in the study and the mean age was 42 (31.5–49). Palpitation and headache were the most common complaints. The time from the onset of symptoms to diagnosis was 8 (3–20) months. There were 8 patients with a preoperative clinical and biochemical diagnosis of TSH + GH co-secretion. In the TRH stimulation test, a blunted TSH response was obtained in 18 patients (90.0%). Complete suppression could not be obtained in any of the patients who underwent the T3 suppression test. The median maximum tumor diameter was 19.0 mm (6.8–41). There was microadenoma in 4 (9.8%) patients and macroadenoma in 37 patients (92.8%). Remission was achieved in 31 (75.6%) of 40 patients who underwent endoscopic transsphenoidal surgery (eTSS). The Ki-67 labeling index was 2% (1.00–4.00) in the entire patient group. Preoperative use of antithyroid drugs appears to be significantly associated with surgical cure.
Conclusion
Diagnosis of TSHoma is still full of challenges and dynamic tests remain important. Recognition and good management of inappropriate TSH secretion states affect subsequent surgical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyrotropin (TSH)-secreting pituitary adenoma (TSHoma) is the rarest type of pituitary adenoma, representing only 0.7–2.8% of all pituitary adenomas, with a reported incidence of 0.03–0.15/100 000 persons per year [1, 2]. Although the pathogenesis of TSHoma is unknown, transcription factors such as PIT-1 and GATA-2 may play an important role in the regulation of the TSH beta gene. Co-secretion of hormones (growth hormone (GH) and prolactin (PRL)) originating from the PIT-1 lineage factor may be seen in TSHomas [3].
TSHomas, which are the main cause of central hyperthyroidism, are often misdiagnosed as primary hyperthyroidism and are unnecessarily treated with antithyroid drugs, iodine-131 radiotherapy, and even with thyroidectomy [4]. In recent years, the diagnosis rate of TSHomas has increased significantly in parallel with the advances and accessibility of imaging technologies [5]. TSHomas are usually diagnosed as macroadenomas due to diagnostic delay and many tumors have an extrasellar extension, cavernous sinus invasion, and intralesional fibrosis, therefore total resection is not always possible. Transsphenoidal surgery is recommended as the first-line therapy [6, 7]. Gross total or near-total surgery is the most important factor determining remission, providing a remission rate of more than 90% [8].
This study aims to investigate the diagnosis and treatment methods, clinicopathological features, and postoperative follow-up results of TSHoma patients in our pituitary center.
Materials and methods
Patient population
In this study, we retrospectively analyzed the medical records of 41 patients with TSHoma who were diagnosed and treated at Kocaeli University Pituitary Research Center between 2005 and 2022.
The following criteria were used for the diagnosis of TSHoma [5, 9].
-
1.
High levels of free thyroid hormone and inappropriately non-suppressed TSH levels with or without clinical symptoms of hyperthyroidism.
-
2.
Pituitary adenoma (PA) detected by magnetic resonance imaging (MRI).
-
3.
Compliance with diagnostic criteria in further confirmation tests.
-
4.
Immunohistochemical diagnostic confirmation with TSH and/or PIT-1 in all patients undergoing pituitary surgery.
Endocrine evaluation
Basal anterior pituitary hormones (GH, PRL, TSH, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), adrenocorticotropic hormone (ACTH)) and target hormones such as insulin-like growth factor 1 (IGF-1), free thyroxine (fT4), free triiodothyronine (fT3), cortisol, testosterone and estradiol were measured in blood taken in the morning from 08.00 to 09.00 after 12 h fasting pre and postoperatively. In the postoperative period, follow-up was performed for either inappropriate antidiuretic hormone syndrome or diabetes insipidus, and treatment was given when necessary.
Preoperative sex hormone binding globulin (SHBG) and alpha-subunit were measured. The ratio between the alpha-subunit and TSH using the formula with (alpha-subunit [µg/l]/TSH [mUI/l])x10 [10]. Preoperative thyrotropin-releasing hormone (TRH) (200 mg i.v.) stimulation test were done. An increase in TSH of >50% and/or >4 IU/L in the TRH stimulation test was defined as a normal response [11]. If the TRH stimulation test results are inconclusive, T3 suppression test (50 mcg/day for days 1–3, followed by 100 mcg/day for days 4–6, and then 200 mcg/day for days 7–9) performed for the confirmation. In the T3 suppression test, complete suppression of TSH was considered as a normal response [10].
Co-secretion of GH was defined by elevated age and sex-adjusted IGF-1 levels and the absence of GH suppression in the 75 g oral glucose tolerance test (GH nadir <0.4 ng/ml) [12].
Based on our laboratory reference values, hyperprolactinemia was diagnosed when PRL levels were >20 ng/mL in men and >25 ng/mL in women.
Plasma cortisol levels ≥18 μg/dL were considered normal. Morning serum cortisol <5 μg/dL or peak serum cortisol <18 μg/dL after ACTH stimulation test was considered adrenal insufficiency.
TSH, fT4 and fT3 measurements were made using electrochemiluminescence immunoassay (Beckman CoulterUniCelDxI 800 Access Immunoassay System). Reference range: TSH 0.38–5.33 µIU/mL; fT4 0.61–1.20 ng/dL; fT3 2.60–4.37 pg/mL
Imaging studies
Preoperative, postoperative, and follow-up MRI scans were performed before and after the gadolinium injection. The tumor diameter was measured from all three planes and the maximum diameter was accepted as the tumor diameter. PA was categorized according to their size as microadenoma (<1 cm), and macroadenoma (≥1 cm). Cavernous and sphenoid sinus invasion was evaluated according to the Knosp classification [13]. The presence of residual tumor was evaluated with MRI at postoperative 3rd month.
Histological and Immunocytochemical Studies
Forty transsphenoidal surgical specimens were stained with hematoxylin–eosin after fixation in buffered formol at 10% dilution. In cases with a clinical diagnosis of adenoma, the diagnosis of adenoma was confirmed histochemically by reticulin staining. For immunohistochemical (IHC) studies, 6-micron-thick sections were studied with Benchmark XT (Ventana medical systems, AZ, USA). Polyclonal anti-GH, polyclonal anti-PRL, polyclonal anti-ACTH, polyclonal anti-PIT-1, monoclonal anti-TSH, and monoclonal anti-Ki-67 were applied as IHC. Histochemical and IHC findings were evaluated simultaneously by two pathologists. GH, PRL, ACTH, PIT-1, and TSH stainings were grouped as positive and negative, and focal weak staining was considered as non-specific and negative. PIT-1 and TSH stainings were evaluated by pathologists blindly. Ki-67 proliferation index was determined by dividing the stained cells into all cells under the microscope.
Criteria for Remission and Recurrence
Postoperative normalization of serum TSH, fT3, and fT4 levels without using any medication at least for 3 months and the absence of residual tumor in MRI was considered as complete remission. Normalization of serum TSH, fT3, and fT4 levels in patients who received radiotherapy was also considered remission independent of residual tumor. In cases of co-secretion, remission was defined according to individual criteria of each hormone hypersecretion separately. In the case of clinical and biochemical findings of central hyperthyroidism after complete remission, relapse was accepted regardless of whether there was a lesion detected by MRI or not. During the follow-up period patients’ evaluation was performed at regular intervals (every 6–12 months).
Statistical analysis
Statistical analysis was performed with the IBM SPSS 20.0 (IBM Corp., Armonk, NY, USA) program. Normal distribution was evaluated with the Shapiro-Wilk test. Numerical variables were given as mean ± standard deviation and median (25th–75th percentile). Categorical variables were given as frequency (percentage). The difference between the groups was determined by the Mann–Whitney U test. Wilcoxon signed-rank test was used for dependent group comparisons. Relationships between categorical variables were determined by the Chi-square test. P < 0.05 was considered sufficient for statistical significance in hypothesis tests.
Results
Patient Characteristics
A total of 41 patients, 23 male (56.1%) and 18 female (43.9%), were included in the study, and the mean age was 42 (31.5–49). According to this study, 39% (n = 16) of the patients were diagnosed before 2017, and 61% (n = 25) after 2017. It was observed that antithyroid drug (ATD) was given to 13 (31.7%) patients before due to misdiagnosis of primary hyperthyroidism. Palpitation and headache were the most common complaints of the patients with the same rate (51.2%). The mean time between the onset of symptoms and diagnosis was 8 (3–20) months. Multiple endocrine neoplasia syndrome was not considered in any patient due to a lack of other components but genetic testing was not performed. The clinical characteristics of the patients are shown in Table 1.
Baseline laboratory tests
Preoperative mean TSH was 3.50 mIU/L (0.38–5.33), mean fT4 was 2.05 ng/dL (0.61–1.20), and mean fT3 was 5.90 pg/mL (2.60–4.37). Preoperative evaluation revealed one patient with GH deficiency (2.4%), three patients with adrenocorticotropic hormone (ACTH) deficiency (7.3%), and four patients with gonadotropin deficiency (9.8%). Ten patients (24.4%) had evidence of slightly elevated prolactin (≤87 ng/mL), which was thought to be consistent with a pituitary stalk compression effect. 8 patients had plurihormonal secretion and all were TSH + GH co-secretion.
Differential diagnostic and confirmatory laboratory tests
SHBG levels were measured in 18 patients and were above the normal limit in 14 (77.7%). Median SHBG levels were 71 nmol/L (55.75–114.75). Alpha-subunit levels were measured in ten patients and were above normal in 8 (80%). Median alpha-subunit levels were 0.95 ng/ml (0.52–3.61). In addition, a positive correlation was found between adenoma size and alpha-subunit (p = 0.05, rho=0.632). The alpha-subunit/TSH ratio was calculated in ten patients and the median value was found to be 3.22 (1.42–9.82).
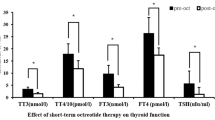
TRH stimulation test was performed in 20 patients (48.8%). A blunted TSH response was achieved in 18 patients (90.0%). The delta change in TSH was 0.58 mIU/L (0.08–1.42), which was only a 12.4% increase (Fig. 1).
T3 suppression test was performed in 13 patients (31.7%). Complete TSH suppression was not achieved in any of the patients. The delta change in TSH was −1.70 mIU/L ((−3.31)-(−0.93)) which was corresponded to 47.7% (Fig. 1).
Imaging studies
Only 4 (9.8%) patients had microadenomas and the median tumor diameter was 19.0 mm (6.8–26.0). Before 2017, only 1 patient had a microadenoma (6.25%). However, in the next 5 years, microadenoma was detected in three patients (12%). Other radiological features are given in Table 2.
Preoperative medical treatment
The preoperative treatment of the patients included in the study was selected according to the treatment modalities available in our country at the time of diagnosis and suitable for reimbursement conditions. 19 of the patients were asymptomatic with normal heart rate and operated on without any specific preoperative medication On the other hand, ATD (41.5%), dopamine agonist (DA) (2.4%), short-acting somatostatin analog (9.8%), long-acting somatostatin analog (2.4%), and B-blocker (39%) were the treatment options in symptomatic patients according to clinical situation.
Histopathological characteristics
Since a patient was primarly medically treated without any operation, histopathological evaluation of a total of 40 patients was performed. One out of 40 operated patients showed negative immunostaining for TSH. However PIT-1 immunostaining was observed positive in all patients (Fig. 2). TSH and GH immunostaining was observed in 10 patients. Among them, eight patients had confirmed clinical and laboratory evidence of TSH-GH co-secretion. The remaining two patients did not have any clinical or laboratory evidence of acromegaly. PRL immune positivity was observed in 12 patients, all without any clinical or laboratory evidence of hyperprolactinemia. ACTH immunostaining was not observed in any of the patients. The median Ki-67 labeling index was 2% (1.00–4.00) in the entire cohort. All immunohistochemical results were given in Table 2.
Pathological characteristics of thyrotropin-secreting pituitary adenomas. a Pituitary adenoma that forms large islands surrounded by thin fibrous septa. Although the tumor has small, hyperchromatic oval-shaped nuclei, it consists of cells with eosinophilic cytoplasm and indistinct borders (H&E; ×100). b Preserved reticular natural roof areas in the lower right corner. In other areas, adenoma in which the reticular natural roof is disrupted is noteworthy (Reticulin stain; ×200). c Cytoplasmic TSH immunoreactivity (×100). d PIT-1 nuclear staining (×100). e Cytoplasmic GH immunoreactivity (×100). f Diffuse cytoplasmic PRL immunoreactivity (×100)
Surgical Outcomes
A total of 40 patients underwent endoscopic transsphenoidal surgery (eTSS). Three of them were operated in another center after the diagnosis in our clinic and they applied for recurrence. 31 patients (75.6%) were in remission after the first eTSS. One of these patients relapsed ten months later. Recurrent surgery was performed in 7 of 10 patients who did not go into remission and relapsed. One of them (14.2%) went into remission after the second eTSS. Radiotherapy (RT) was applied in patient who relapsed because remission was still not achieved after the third eTSS. Normalization of thyroid function tests after RT was obtained at six months. Surgical remission rate was 77.5% and total remission rate was 80%. The remaining patients were followed under medical treatment (Fig. 3).
Postoperative Medical Treatment
Eight of the patients who could not achieve remission with eTSS are being followed up under medical treatment. The treatments of the eight patients were as follows; six only SSA, 1 SSA + DA, 1 SSA + ATD. The drug doses, treatment durations and the latest disease status of these patients are given in Table 3.
Postoperative Pituitary Function
In the postoperative period, 10 (24.4%) patients required levothyroxine (LT-4) replacement however, it was only needed temporarily in 6 of these cases. The remaining four patients required permanent LT-4 replacement. GH deficiency in one patient, ACTH deficiency in six patients, FSH/LH deficiency in four patients, and replacement therapy was given. In addition, DI was observed in seven patients. Of these, two were temporary and five were permanent, and these patients are currently continuing to receive desmopressin replacement.
Analysis of factors affecting surgical remission
To evaluate the factors affecting the surgical remission, the patients were divided into two groups as the remission group (31) and the non-remission group (9). According to univariate analysis data, preoperative ATD usage, fT4 and fT3 levels, postoperative TSH, fT4 and fT3 levels seem to be significantly associated with postoperative remission (Table 4).
Discussion
TSHoma is very rare compared to other types of pituitary adenomas. Due to the disease’s rare nature, there is less literatüre data than other pituitary adenoma types. Errors in the interpretation of inappropriate thyroid function tests and difficulties in differential diagnosis, causes diagnostic delay. In this process, the delay in diagnosis is an average of 19 months, and this period has been reported up to 133 months previously [3]. However with the widespread use of ultrasensitive TSH assays and MRI, diagnostic delay was reduced to 4.5 months [14]. Our series determined this period as 8 (3–20) months. In addition, only 9.8% of the patients in our study had microadenoma. However, this rate was found to be 39.3% in the Swedish TSHoma incidence and prevalence study, and the detection rate of microadenoma has been observed to increase gradually after the 2000 s [2]. Similar results have been shown in a study based in France, and the microadenoma detection rate has gradually increased in recent years [15]. In the current study, the diagnosis time decreased gradually and the microadenoma detecting ratio increased in the last years, similar to the literature. However, the higher macroadenoma rate may be related to the genetic background and the higher number of co-secreting adenomas.
The clinician faces some difficulties in the diagnosis of TSHoma. In 6.6–30% of cases, treatments such as ATD, thyroidectomy, or radioactive iodine ablation are performed due to inappropriate thyroid function tests [6, 11]. In our series, this rate was similarly quite high. A differential diagnosis should be made to prevent unnecessary surgery, especially with thyroid hormone resistance. Due to difficulties in diagnosis, besides thyroid function tests, serum alpha-subunit (α-SU) and SHBG measurement, TRH stimulation test, and T3 suppression test are recommended for differential diagnosis [5, 9]. The highly variable α-SU is elevated in 30–83% of TSHoma cases [11, 15]. SHBG, which increased in the circulation as a result of excessive thyroid hormone secretion, was found to be higher in TSHoma cases [16]. Our study results also support this, suggesting that alpha-subunit and SHBG may be useful markers in the differential diagnosis of TSHoma. On the other hand, dynamic tests continue to be the most important tools in the differential diagnosis. However, as with the two patients who had a normal response on the TRH stimulation test, all of these tests may contain misleading results. In the literature, normal TSH response to TRH stimulation was obtained in 13% of TSHoma patients [6]. Therefore, the combination of all these tests on the way to diagnosis continues to be the most rational approach.
It has not been clearly stated which patients should be treated in the preoperative period. The most common preoperative symptoms in TSHoma patients are those associated with hyperthyroidism, and preoperative control of hyperthyroidism symptoms is essential. In this current study, we did not use any medication before surgery in 47.5% of the operated patients. Neo-adjuvant therapy was used in 34.5% of patients in a meta-analysis. According to this study, SSA was preferred in 88% of cases, DA in 10% and a combination of both treatments in 2% of cases. Moreover, these treatments do not have a negative effect on surgical outcomes [3]. Due to the reimbursement conditions in our country, octreotide treatment was not the primary option for preoperative restoration of euthyroidism in the past, but it has become the preferred treatment in recent years. In our series, 31.7% of the patients had a history of previous ATD use. A negative effect of this condition on surgical remission was observed. In the study of Sanno et al., preoperative ATD use was observed in 68.7% of the patients. Interestingly, these tumors were found to be more rigid, and histopathological examination revealed abundant connective tissue between the tumor cells [17]. Treatments aimed at reducing thyroid hormone synthesis in the end organ may cause the growth of pituitary adenoma tissue and a change in its histopathological features, similar to the development of Nelson’s syndrome.
Co-secretory adenomas may occur as a result of the expression of common transcription factors such as Prop-1 and Pit-1 by thyrotroph, somatotroph, and lactotroph cells. About one-third of TSHoma cases are mixed adenomas that can co-secrete TSH and other anterior pituitary hormones (GH, PRL, and LH/FSH) [5]. A recent meta-analysis found the rate of co-secretory adenoma to be 42%, with the most frequent co-secretion of GH and PRL (53% and 40%, respectively) [3]. There was no difference in remission rate between monohormonal and plurihormonal TSHomas [18]. Malchiodi et al. found similar results [19]. However, in the study by Yamada et al., GH co-secretion was shown to be significantly associated with surgical outcomes [6]. In the current study, neither the preoperative clinical and biochemical diagnosis of acromegaly nor the postoperative immunohistochemical plurihormonal positivity were associated with surgical outcomes. This difference may be related to the location of the somatotroph and thyrotrope cells in the pituitary gland, tumor characteristics, and tumor invasion into the surrounding structures.
Our study has several limitations. First, the retrospective nature of the study, changes in treatment modalities and measurement methods over time, advances in surgical techniques, and clinical experience may cause some limitations. Second, due to the retrospective design, a standard preoperative approach could not be provided to all patients, and some data were missing. Third, the small population of the non-remission group made it difficult to have statistical significance. Fourth, Patients who could not be controlled with medical treatment could not continue their control examinations regularly during the pandemic period, as they came from far cities. These patients were called for examination recently and their treatment was reviewed.
Conclusion
TSHomas are one of the most important causes of inappropriate TSH secretion and still pose difficulties in the differential diagnosis. Currently, dynamic tests along with biochemical tests continue to be the most important diagnostic tools. Recognizing and treating inappropriate TSH secretion well is important for subsequent results. As shown in our study, approaches such as ATD for the end organ adversely affect the results of eTSS. Pituitary surgery is still the first-line treatment option and provides remission in three out of four patients.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
A. Tjörnstrand, K. Gunnarsson, M. Evert, E. Holmberg, O. Ragnarsson, T. Rosén et al. The incidence rate of pituitary adenomas in western Sweden for the period 2001–2011. Eur. J. Endocrinol. 171, 519–526 (2014). https://doi.org/10.1530/EJE-14-0144
L. Önnestam, K. Berinder, P. Burman, P. Dahlqvist, B.E. Engström, J. Wahlberg et al. National incidence and prevalence of TSH-secreting pituitary adenomas in Sweden. J. Clin. Endocrinol. Metab. 98, 626–635 (2013). https://doi.org/10.1210/JC.2012-3362
G. Cossu, R.T. Daniel, K. Pierzchala, M. Berhouma, N. Pitteloud, F. Lamine et al. Thyrotropin-secreting pituitary adenomas: a systematic review and meta-analysis of postoperative outcomes and management. Pituitary. 22, 79–88 (2019). https://doi.org/10.1007/S11102-018-0921-3/FIGURES/6
Luo P., Zhang L., Yang L., An Z., Tan H. (2020). Progress in the pathogenesis, diagnosis, and treatment of TSH-secreting pituitary neuroendocrine tumor. Front. Endocrinol. (Lausanne). 11 https://doi.org/10.3389/FENDO.2020.580264.
P. Beck-Peccoz, C. Giavoli, A. Lania, A 2019 update on TSH-secreting pituitary adenomas. J. Endocrinol. Invest. 42, 1401–1406 (2019). https://doi.org/10.1007/S40618-019-01066-X/TABLES/2
S. Yamada, N. Fukuhara, K. Horiguchi, M. Yamaguchi-Okada, H. Nishioka, A. Takeshita et al. Clinicopathological characteristics and therapeutic outcomes in thyrotropin-secreting pituitary adenomas: a single-center study of 90 cases: clinical article. J. Neurosurg. 121, 1462–1473 (2014). https://doi.org/10.3171/2014.7.JNS1471
N.C. Van Varsseveld, P.H.L.T. Bisschop, N.R. Biermasz, A.M. Pereira, E. Fliers, M.L. Drent, A long-term follow-up study of eighteen patients with thyrotrophin-secreting pituitary adenomas. Clin. Endocrinol. 80, 395–402 (2014). https://doi.org/10.1111/CEN.12290
H.E. Sen, E.C. Ceylan, S. Atayev, M. Sozen, B.Y. Bayrak, B. Cetinarslan et al. The endoscopic endonasal transsphenoidal approach for thyrotropin-secreting pituitary adenomas: single-center experience and clinical outcomes of 49 patients. World Neurosurg. 167, e1275–e1283 (2022)
A. Tjörnstrand, H.F. Nyström, DIAGNOSIS OF ENDOCRINE DISEASE: Diagnostic approach to TSH-producing pituitary adenoma. Eur. J .Endocrinol. 177, R183–R197 (2017). https://doi.org/10.1530/EJE-16-1029
P. Beck-Peccoz, F. Brucker-Davis, L. Persani, R.C. Smallridge, B.D. Weintraub, Thyrotropin-secreting pituitary tumors. Endocr. Rev. 17, 610–638 (1996). https://doi.org/10.1210/EDRV-17-6-610
V. Socin, P. Chanson, B. Delemer, A. Tabarin, V. Rohmer, J. Mockel et al. The changing spectrum of TSH-secreting pituitary adenomas: diagnosis and management in 43 patients. Eur. J. Endocrinol. 148, 433–442 (2023). www.eje.org. Accessed 25 Jan 2023
L. Katznelson, E.R. Laws, S. Melmed, M.E. Molitch, M.H. Murad, A. Utz et al. Acromegaly: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 99, 3933–3951 (2014). https://doi.org/10.1210/JC.2014-2700
E. Knosp, E. Steiner, K. Kitz, C. Matula, Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33, 610–617 (1993). https://doi.org/10.1227/00006123-199310000-00008. discussion 617
F. Gatto, L.F. Grasso, E. Nazzari, T. Cuny, P. Anania, C. Di Somma et al. Clinical outcome and evidence of high rate post-surgical anterior hypopituitarism in a cohort of TSH-secreting adenoma patients: might somatostatin analogs have a role as first-line therapy? Pituitary. 18, 583–591 (2015). https://doi.org/10.1007/S11102-014-0611-8/TABLES/2
O.M. Căpraru, C. Gaillard, A. Vasiljevic, H. Lasolle, F. Borson-Chazot, V. Raverot et al. Diagnosis, pathology, and management of TSH-secreting pituitary tumors.a single-center retrospective study of 20 patients from 1981 to 2014. Ann. Endocrinol. (Paris). 80, 216–224 (2019)
P. Beck Peccoz, R. Roncoroni, S. Mariotti, G. Medri, C. Marcocci, G. Brabant et al. Sex hormone-binding globulin measurement in patients with inappropriate secretion of thyrotropin (IST): Evidence against selective pituitary thyroid hormone resistance in nonneoplastic IST. J. Clin. Endocrinol. Metab. 71, 19–25 (1990)
N. Sanno, A. Teramoto, R.Y. Osamura, Long-term surgical outcome in 16 patients with thyrotropin pituitary adenoma. J. Neurosurg. 93, 194–200 (2000)
S.H. Kim, C.R. Ku, M. Na, J. Yoo, W. Kim, I.H. Jung et al. Immediate postoperative measurement of thyroid-stimulating hormone as an early predictor of remission in thyroid-stimulating hormone–secreting pituitary adenomas. J. Neurosurg. 1 (aop), 1–7 (2020). https://doi.org/10.3171/2020.1.JNS192787
E. Malchiodi, E. Profka, E. Ferrante, E. Sala, E. Verrua, I. Campi et al. Thyrotropin-secreting pituitary adenomas: outcome of pituitary surgery and irradiation. J. Clin. Endocrinol. Metab. 99, 2069–2076 (2014). https://doi.org/10.1210/JC.2013-4376
Funding
This project was supported by Kocaeli University Scientific Research Projects Coordination Unit with project number 2756.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by Kocaeli University Non-Interventional Clinical Research Ethics Committee with project number 2021/271.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sözen, M., Bayrak, B.Y., Selek, A. et al. A reference center study in thyrotropin-secreting pituitary adenomas: clinicopathological, therapeutic and long-term follow-up outcomes. Endocrine 82, 622–630 (2023). https://doi.org/10.1007/s12020-023-03480-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03480-x