Abstract
Purpose
Immune checkpoint inhibitor (ICI) induced type 1 diabetes (T1D) and pituitary dysfunction are life-threatening adverse events, yet there is little clinical data available. We aimed to investigate the clinical characteristics of patients with these adverse events and report their human leukocyte antigen (HLA) profile to determine its relevance.
Methods
This is a single-center prospective study. We enrolled patients with cancers who were administered ICI and diagnosed as ICI induced T1D (ICI-T1D) and pituitary dysfunction (ICI-PD). Clinical data and extracted DNA from blood samples were collected. HLA typing was performed using next-generation sequencing. We compared our results with those previously reported in healthy controls and investigated the correlation between HLA and the occurrence of ICI-T1D and ICI-PD.
Results
We identified 914 patients treated with ICI in our facility from 1st September, 2017 to 30th June, 2022. Six of these patients developed T1D and 15 developed pituitary dysfunction. The duration from the initiation of ICI treatment to the onset of T1D or pituitary dysfunction averaged 492 ± 196 days and 191 ± 169 days. Among the six patients with T1D, two were positive for anti-GAD antibody. The frequencies of HLA-DR11, -Cw10, -B61, -DRB1*11:01, and -C*03:04 were significantly higher in patients with ICI-T1D than in controls. The frequencies of HLA-DR15 and -DRB*15:02 were significantly higher in patients with ICI-PD than in controls.
Conclusion
This study revealed the clinical characteristics of ICI-T1D and ICI-PD and the association between specific HLAs and these adverse events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune checkpoint inhibitors (ICIs) have shown great promise for the treatment of several malignancies which are frequently resistant to conventional therapies [1]. ICIs enhance anti-tumor immunity by inhibiting endogenous immune down-regulators such as cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death 1 (PD-1) receptors or their respective ligands, CD 80/86 and programmed cell death ligand 1 (PD-L1). Several immune checkpoint-targeted antibodies prolong overall survival in patients with various types of cancers. However, increasing the activity of the immune system by ICI treatment is associated with various immune-related adverse events (irAEs), which can cause damage to the gastrointestinal tract, liver, skin, and endocrine glands [2]. Among these irAEs, endocrine dysfunctions are life-threatening.
The frequencies of different types of endocrine dysfunction vary by disease, with a prevalence of 6.2% for hypothyroidism, 2.6% for hyperthyroidism, 1.3% for hypophysitis, 0.7% for primary adrenal insufficiency, and 0.2% for insulin-dependent diabetes [3]. Insulin-dependent diabetes is also named type 1 diabetes (T1D) according to the Japanese Clinical Practice Guideline for Diabetes 2019 [4]. Specific variants of human leukocyte antigens (HLAs) are associated with autoimmune disease development. Furthermore, specific HLA alleles and haplotypes are associated with the development of endocrine irAEs such as T1D and pituitary and thyroid dysfunction [5,6,7,8]. For instance, the frequency of HLA-DR4 genotypes was higher in Caucasian patients with ICI-induced T1D (ICI-T1D) than that in patients with T1D which was not an irAE of treatment with ICIs and that in the general population [5]. Additionally, HLA-DR15 might be a predictive marker of ICI-induced secondary adrenal insufficiency in Japanese patients [6]. The allele and haplotype frequencies of HLA-DPA1:01:03 and -DPB1*02:01 were significantly higher in patients with ICI-induced thyroid dysfunction than those in controls [8]. However, these reports are limited by the small number of patients studied. Considering the known racial differences in HLAs [9], more reports are needed to increase the certainty of HLAs as a generally applicable predictor of irAE development. Herein, we aim to investigate the clinical characteristics of patients with ICI-T1D and ICI-induced pituitary dysfunction (ICI-PD) at our hospital and demonstrate that each of these complications is associated with specific HLA serotypes or alleles.
Methods
Patients
We identified inpatients who consulted the Department of Diabetes, Metabolism, and Endocrinology at Tokyo Medical University Hospital. Patients who met the following criteria were included in this prospective study:
-
1.
Aged 20 years or older and having a diagnosis of cancer for which there were treated with ICI from 1st September, 2017 to 30th June, 2022.
-
2.
Undergoing therapy with the following ICIs: anti PD-1 antibodies (nivolumab or pembrolizumab), anti PD-L1 antibodies (atezolizumab or durvalumab), anti-CTLA-4 antibody (ipilimumab), or combination therapy with nivolumab and ipilimumab.
-
3.
Having new-onset diabetes or pituitary dysfunction after ICI treatment initiation.
-
4.
Having given written consent for this study.
The follow-up period was 1–46 months. We identified six patients who presented with T1D and 14 who developed pituitary dysfunction. One patient presented with both T1D and pituitary dysfunction.
Clinical assessments
Each endocrine irAE was diagnosed and treated according to the clinical guidelines of the Japan Endocrine Society [10]. Clinical symptoms were recorded during each hospital visit. Blood samples were collected from patients every 3–4 weeks after the first ICI treatment, on an occasional basis. Blood tests and adrenocorticotropic hormone (ACTH) stimulation tests to diagnose pituitary dysfunction were performed early in the morning, under fasting conditions. The criteria for the diagnosis of fulminant T1D were: (1) occurrence of diabetic ketosis or ketoacidosis soon (approximately 7 days) after the onset of hyperglycemic symptoms (elevation of urinary and/or serum ketone bodies at first visit); (2) plasma glucose level ≥16.0 mmol/L ( ≥ 288 mg/dL) and glycated hemoglobin level <8.7% at the first visit; and (3) urinary C-peptide excretion <10 μg/day, basal serum C-peptide level <0.3 ng/mL (<0.10 nmol/L), or stimulated C-peptide level (glucagon or after a meal) <0.5 ng/mL (<0.17 nmol/L, respectively), at symptom onset [11]. We calculated the C-peptide index using the following equation:
we tested for the presence of anti-GAD antibodies in all patients with ICI-T1D and measured the serum levels of amylase, lipase, and elastase-1.
HLA genotype
DNA was extracted from blood. HLA typing of HLA-A, HLA-B, HLA-C, HLA-DRB1, HLA-DQA1, HLA-DQB1, HLA-DPA1, and HLA-DPB1 was performed using next-generation sequencing at the HLA Foundation Laboratory (Kyoto, Japan). We compared the carrier frequency of HLA serotypes and alleles in the study patients with those of the general Japanese population (controls) and investigated the association between the HLA serotypes and alleles and the occurrence of endocrine irAEs [12].
Statistical analysis
The continuous patient characteristic variables are expressed as mean ± standard deviation (SD). The median is shown in parenthesis where relevant. All statistical analyses were performed using IBM SPSS Statistics (version 28 RRID:SCR_016479). The carrier frequency of HLA alleles was compared between groups using Fisher’s exact test. Statistical significance was set at p < 0.05.
Results
Patient characteristics
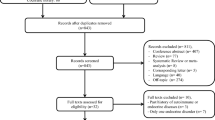
We identified 914 patients treated with ICI from 1st September, 2017 to 30th June, 2022 at our facility. Among these, six patients developed ICI-T1D and 15 patients developed ICI-PD. The incidence rates of ICI-T1D and ICI-PD in this cohort were 0.66% and 1.64%, respectively. All patients were hospitalized and all except one ICI-PD patient gave consent for their participation in the study. Therefore, a total of 14 ICI-PD patients were involved in the study. The characteristics of each patient are shown in Tables 1 and 2 for ICI-T1D and ICI-PD, respectively. One patient developed both T1D and pituitary dysfunction. All patients were Japanese, Asian. None of the patients had a history of diabetes or Pituitary dysfunction prior to ICI treatment. Treatments with ICI included the anti PD-1 antibodies pembrolizumab and nivolumab, the anti PD-L1 antibodies atezolizumab and durvalumab, the anti CTLA-4 antibody ipilimumab, or combination therapy with ipilimumab and nivolumab (Fig. 1). Among the six patients who developed ICI-T1D, five received anti PD-1 antibodies and one received PD-L1 antibodies. Among the 14 patients with ICI-PD, eight underwent therapy with anti PD-1 antibodies, two with anti PD-L1 antibodies, three received combination therapy with ipilimumab and nivolumab, and one was treated with atezolizumab followed by pembrolizumab. Primary diseases varied from patient to patient and included lung cancer (both adenocarcinoma and small cell lung cancer), malignant melanoma, Hodgkin’s lymphoma, or renal cell, gastric, hypopharyngeal, hepatocellular, gingival, nasal, bladder, or salivary gland cancer. Patients with ICI-T1D were significantly younger than those with ICI-PD (p = 0.032). We did not find any sex-related differences in the incidences of these adverse effects. The number of days from the initiation of therapy with ICI until diagnosis was significantly longer in ICI-T1D group than that in ICI-PD group (p = 0.009) and the number of cycles of treatment was significantly higher (p = 0.007). No difference in terms of mortality was observed for the two diseases across the study period. The last day of observation was 30th June, 2022. A comparison of clinical characteristics between ICI-T1D and ICI-PD is given in Table 3.
Flow diagram of patients. We enrolled 19 patients who developed type 1 diabetes or pituitary dysfunction and were referred to the department of diabetes, metabolism, and endocrinology in 914 cancer patients who have been treated with Immune checkpoint inhibitors between September 1st, 2017 and June 30th, 2022. *Included one case which developed both type 1 diabetes and pituitary dysfunction
Clinical and biochemical characteristics of patients with ICI-T1D
Six patients were diagnosed with acute-onset T1D or fulminant T1D. Three of these patients developed typical hyperglycemic symptoms, such as thirst, five patients complained of fatigue, and three presented weight loss. Two patients developed ketoacidosis. Their clinical and biological characteristics are summarized in Table 4. Anti-GAD antibodies were detected in two patients with antibody titers of 266 U/mL and 15.6 U/mL, respectively. The average plasma glucose concentration was 633.5 mg/dL and all patients presented with glycemia values above 288 mg/dL at symptom onset. The average HbA1c level was 7.3%, and all of were below 8.7%. The fasting serum C-peptide level of one patient was lower than 0.3 ng/mL at symptom onset. In the two cases in which we were able to determine serum C-peptide, levels had fallen to below 0.3 ng/mL at 48 days from the onset of symptoms and remained at that level until day 58. Elevated pancreatic enzyme levels, a clinical feature of fulminant T1D, was observed in two of the six patients. One patient who did not meet the diagnostic criteria for fulminant T1D also had elevated levels of all three enzymes (amylase, lipase, and elastase-1). All patients but one survived the study period.
Clinical characteristics of ICI-PD
The mean duration of time from the initiation of treatment with ICI to the diagnosis of ICI-PD was 191.3 days and the median was 125.5 days. Eleven patients developed isolated ACTH deficiency, and three developed partial pituitary deficiency. Fatigue, loss of appetite, and weakness were the most common symptoms at onset, reported in 12, eight, and six patients, respectively. Magnetic resonance imaging was performed in 11 cases, none of which showed pituitary enlargement. At the end of the follow-up period, five deaths and nine survivors were registered Fig. 2.
Comparison of HLA serotype frequencies of patients with type1 diabetes induced by Immune checkpoint inhibitors. Circles show odds ratios. The lines show 95% confidence intervals. This forest plot shows that HLA-DR11, HLA-B61 and Cw10 frequencies of our cases with type1 diabetes induced by Immune checkpoint inhibitors were significantly higher than those of the controls in Japan. (p = 0.033, OR: 9.47, 95% CI: 1.732–51.769; p = 0.036, OR: 6.128, 95% CI: 1.122–33.469; p = 0.034, OR: 6.251, 95% CI: 1.145–34.138, respectively)
HLA typing in patients who developed ICI-T1D
HLA analysis was performed in all six patients. HLA-B61 and HLA-Cw10 were observed in four (67%) of the six patients with ICI-T1D. Statistically, the frequencies of serotypes HLA-DR11, -B61, and -Cw10 were significantly higher in patients with ICI-T1D than in controls [12] (p = 0.033, OR: 9.47, 95% CI: 1.732–51.769; p = 0.036, OR: 6.128, 95% CI: 1.122–33.469; p = 0.034, OR: 6.251, 95% CI: 1.145–34.138, respectively) (Fig. 3).
Comparison of HLA serotype frequencies of patients with pituitary dysfunction induced by immune checkpoint inhibitors. Circles show odds ratios. The lines show 95% confidence intervals. This forest plot shows that HLA-DR15 frequency was significantly higher in patients with pituitary dysfunction than in the controls in Japan [Ikeda, 2015, Determination of HLA-A‘, -C‘, -B‘, -DRB1 allele and haplotype frequency in Japanese population based on family study]. (p = 0.004, OR: 4.97, 95 % CI: 1.558–15.853)
According to the results of next-generation sequencing, HLA-DRB1*11:01:01 and HLA-C*03:04:01 were identified in two and four of the six patients with ICI-T1D, respectively (Supplementary Table 1). The allele frequencies of HLA-DRB1*11:01 and HLA-C*03:04 were found to be significantly higher in patients with ICI-T1D than in controls [12].
HLA typing in patients who developed ICI-PD
HLA analysis was performed in all 14 patients with ICI-PD. HLA- DR15 was identified in 10 (71%) of the 14 patients. The frequency of the HLA-DR15 serotype was found to be significantly higher in patients with ICI-PD than that in controls [12] (p = 0.004, OR: 4.97, 95% CI: 1.558–15.853) (Fig. 3). Four and six of the 14 patients carried HLA-DRB1*15:01:01 and HLA-DRB1*15:02:01 alleles, respectively, and 11 out of 14 carried HLA-A*24:02:01 (Supplementary Table 1). The frequency of the HLA-DRB1*15:02 allele was found to be significantly higher in cases with ICI-PD than that in controls (p = 0.046, OR: 2.97, 95% CI: 1.03–8.563).
Discussion
This study provides new insights into the development of ICI-T1D and ICI-PD through the detailed analysis of clinical data. Using high-resolution sequence-based HLA typing (3-field), we identified HLA alleles in patients with ICI-T1D and ICI-PD. Our results are in agreement with those previously reported, reinforcing the certainty of the research. In this study, 33.3% of patients with ICI-T1D were positive for autoantibodies, while 94% of T1D patients who had not been treated with ICI had at least one autoantibody at new onset [13]. Previous literatures have also reported that around 40% of patients who developed ICI-T1D were positive for at least one T1D related autoantibody [5, 14]. These results demonstrate the difference between ICI-T1D and T1D which was not treated with ICI.
This study found that HLA-DR15 and HLA-DR*15:02 were most common in patients who developed ICI-PD. A small number of studies have previously reported an association between HLA alleles/serotypes and ICI-PD. For example, Yano et al. and Kobayashi T et al. found a correlation between HLA -DR 15 and ICI-PD [6, 7]. Yano et al. examined 11 patients with ICI-PD and reported an association between HLA-DR*15:02 and increased risk of ICI-PD [6]. Our findings are consistent with these previous results, thus strengthening the idea that HLA typing might be useful as a predictive marker for identifying those at highest risk of developing these complications.
This study revealed correlations between the occurrence of ICI-T1D and the presence of HLA-B61 and HLA-Cw10. In this way, our results relating to ICI-T1D differ from previous literatures, which reported that DR4, DR3, DR9, and A2 were the dominant HLA serotypes in patients who developed ICI-T1D [5, 14,15,16]. Recently, similar studies have also been reported in Japan, where the presence of HLA-DR4 was also found to be correlated with the development of ICI-T1D [17]. However, in our study, we identified the presence of the serotypes HLA-B61 and HLA-Cw10 in four out of six patients who developed ICI-T1D. HLA-B61 has also been shown to have a significant association with T1D which was not treated with ICI in a Japanese population [18].
Although there are still no reliable predictors of irAEs for asymptomatic patients with ICIs, identifying biomarkers that predict them is important and of interest for the optimal management of patients treated with ICIs [19]. Among these, recent studies focused on HLA typing have presented the results as indicating potential predictive biomarkers of irAEs. Associations between irAEs and specific HLA alleles have not only been reported for endocrine irAEs but also for the other irAEs. For example, Hasan Ali et al. reported a significant association between the alleles HLA-DRB1*11:01 I and HLA-DQB1:03:01 and pruritus and colitis induced by ICI, respectively [20]. In addition, Cappelli et al. identified a correlation between HLA-DRB1*04:05 and inflammatory arthritis induced by ICI [21]. The cost of HLA typing has fallen in recent years and is expected to continue decreasing, making such methods more accessible.
The principal limitation of the study was that the data used were obtained from a single center. Hence, our report involved a limited number of cases and all patients were Japanese. Considering the known racial differences in HLA profiles [9], it is not yet possible to conclude which HLA serotypes or alleles induce susceptibility to ICI-T1D, and racial differences may determine the differences in the results observed across different studies with different patient cohorts.
In conclusion, universal HLA typing might be an effective predictor of the occurrence of endocrine irAEs in cancer patients for whom ICI therapy is being considered. Further studies including more patients and conducting an evaluation of the costs within the context of the quality-adjusted life-years are needed to consolidate the position of HLA typing as a predictive biomarker.
References
S.L. Topalian, F.S. Hodi, J.R. Brahmer, S.N. Gettinger, D.C. Smith, D.F. McDermott et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med 366(26), 2443–2454 (2012)
M.A. Postow, M.D. Hellmann, Adverse events associated with immune checkpoint blockade. N. Engl. J. Med 378(12), 1165 (2018)
R. Barroso-Sousa, W.T. Barry, A.C. Garrido-Castro, F.S. Hodi, L. Min, I.E. Krop et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 4(2), 173–182 (2018)
E. Araki, A. Goto, T. Kondo, M. Noda, H. Noto, H. Origasa et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int 11(3), 165–223 (2020)
A.M. Stamatouli, Z. Quandt, A.L. Perdigoto, P.L. Clark, H. Kluger, S.A. Weiss et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes. 67(8), 1471–80. (2018)
S. Yano, K. Ashida, R. Sakamoto, C. Sakaguchi, M. Ogata, K. Maruyama et al. Human leucocyte antigen DR15, a possible predictive marker for immune checkpoint inhibitor-induced secondary adrenal insufficiency. Eur. J. Cancer. 130, 198–203 (2020)
T. Kobayashi, S. Iwama, D. Sugiyama, Y. Yasuda, T. Okuji, M. Ito et al. Anti-pituitary antibodies and susceptible human leukocyte antigen alleles as predictive biomarkers for pituitary dysfunction induced by immune checkpoint inhibitors. J. Immunother. Cancer 9(5), e002493 (2021)
H. Inaba, H. Ariyasu, H. Iwakura, C. Kurimoto, K. Takeshima, S. Morita et al. Distinct clinical features and prognosis between persistent and temporary thyroid dysfunctions by immune-checkpoint inhibitors. Endocr. J. 68(2), 231–241 (2021)
K.E. King, P.M. Ness, H.G. Braine, K.S. Armstrong, Racial differences in the availability of human leukocyte antigen-matched platelets. J. Clin. Apher 11(2), 71–77 (1996)
H. Arima, S. Iwama, H. Inaba, H. Ariyasu, N. Makita, M. Otsuki et al. Management of immune-related adverse events in endocrine organs induced by immune checkpoint inhibitors: clinical guidelines of the Japan Endocrine Society. Endocr. J. 66(7), 581–586 (2019)
A. Imagawa, T. Hanafusa, T. Awata, H. Ikegami, Y. Uchigata, H. Osawa et al. Report of the Committee of the Japan Diabetes society on the research of fulminant and acute-onset type 1 diabetes mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus (2012). J. Diabetes Investig 3(6), 536–539 (2012)
N. Ikeda, H. Kojima, M. Nishikawa, K. Hayashi, T. Futagami, T. Tsujino et al. Determination of HLA-A, -C, -B, -DRB1 allele and haplotype frequency in Japanese population based on family study. Tissue Antigens 85(4), 252–259 (2015)
J.M. Wenzlau, K. Juhl, L. Yu, O. Moua, S.A. Sarkar, P. Gottlieb et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc. Natl. Acad. Sci. USA. 104(43), 17040–17045 (2007)
Lo, V. Preiato, S. Salvagni, C. Ricci, A. Ardizzoni, U. Pagotto, C. Pelusi, Diabetes mellitus induced by immune checkpoint inhibitors: type 1 diabetes variant or new clinical entity? Review of the literature. Rev Endocr Metab Disord 22(2), 337–349 (2021)
J.M.K. de Filette, J.J. Pen, L. Decoster, T. Vissers, B. Bravenboer, B.J. Van der Auwera et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur. J. Endocrinol 181(3), 363–374 (2019)
Y. Shi, M. Shen, X. Zheng, Y. Chen, R. Zhao, Y. Gu et al. ICPis-induced autoimmune polyendocrine syndrome type 2: a review of the literature and a protocol for optimal management. J. Clin. Endocrinol. Metab. 105(12), e4208–e4218 (2020)
H. Inaba, Y. Kaido, S. Ito, T. Hirobata, G. Inoue, T. Sugita et al. Human leukocyte antigens and biomarkers in type 1 diabetes mellitus induced by immune-checkpoint inhibitors. Endocrinol. Metab. (Seoul). 37(1), 84–95 (2022)
K. Hamaguchi, A. Kimura, N. Seki, T. Higuchi, S. Yasunaga, M. Takahashi et al. Analysis of tumor necrosis factor-alpha promoter polymorphism in type 1 diabetes: HLA-B and -DRB1 alleles are primarily associated with the disease in Japanese. Tissue Antigens 55(1), 10–16 (2000)
I. Les, M. Martínez, I. Pérez-Francisco, M. Cabero, L. Teijeira, V. Arrazubi et al. Predictive biomarkers for checkpoint inhibitor immune-related adverse events. Cancers (Basel). 15(5), 1629 (2023)
Hasan, O. Ali, F. Berner, D. Bomze, M. Fässler, S. Diem, A. Cozzio et al. Human leukocyte antigen variation is associated with adverse events of checkpoint inhibitors. Eur. J. Cancer 107, 8–14 (2019)
L.C. Cappelli, M.T. Dorak, M.P. Bettinotti, C.O. Bingham, A.A. Shah, Association of HLA-DRB1 shared epitope alleles and immune checkpoint inhibitor-induced inflammatory arthritis. Rheumatology (Oxford) 58(3), 476–480 (2019)
Acknowledgements
We thank Ms. Yoshiko Togawa (Tokyo Medical University) for her technical assistance.
Author contributions
The study was designed by H. S. and F. Y.. Data analysis was performed by N. H., who also wrote the manuscript. H. S., K. I., H. I., H. A., H. S., J. S., T. M., and R. S. contributed to the discussion and reviewed and edited the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by JSPS KAKENHI (Grant number JP22K07430) and Manda Memorial Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Financial interests: H.Suwanai receives personal fees from Nippon Boehringer Ingelheim Co., Ltd. R.S. receives personal fees from Ono Pharmaceutical CO., Ltd, and MSD K.K. N.H., F.Y., K.I., H.I., H.A., H.Sakai., J.S. and T.M. have no financial interests to disclose. All authors have no relevant no non-financial interests to disclose.
Ethical approval
The study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the institutional review board of Tokyo Medical University (Approval No. T2021-0005). The design of this prospective study is in accordance with STROBE guidelines.
Consent to participate
Written informed consents were obtained from all individual patients included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hara, N., Suwanai, H., Yakou, F. et al. Clinical characteristics and human leukocyte antigens in patients with immune checkpoint inhibitor-induced type 1 diabetes and pituitary dysfunction: a single center prospective study. Endocrine 81, 477–483 (2023). https://doi.org/10.1007/s12020-023-03394-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03394-8