Abstract
Purpose
To investigate the relationship between estimated glucose disposal rate (eGDR) and bone turnover markers in patients with type 2 diabetes mellitus (T2DM).
Materials and methods
This is a cross-sectional study, which recruited 549 patients with T2DM. The eGDRs of patients were calculated based on the presence of hypertension, glycated hemoglobin, and body mass index. All patients were divided into high-eGDR group and low-eGDR group using the median of eGDR as the boundary. The patients were further divided into two subgroups: males and postmenopausal females.
Results
The lower the eGDR, the more severe was insulin resistance. The levels of osteocalcin (OC), type I collagen carboxyl-terminal peptide (β-CTX), and type I procollagen amino-terminal peptide (PINP) were significantly lower in the low-eGDR group than those in the high-eGDR group. The eGDR was positively correlated with OC, β-CTX, and PINP in all patients, and in the male subgroups. In the postmenopausal female subgroup, there was no correlation between eGDR and OC, β-CTX, or PINP. In addition, this positive correlation remained after adjusting for other factors in multilinear regression analysis.
Conclusion
Our study was the first to demonstrate that eGDR is positively correlated with bone turnover markers in patients with T2DM. This correlation was observed among the male patients with T2DM but not among postmenopausal female patients with T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes has reached pandemic proportions worldwide. The International Diabetes Federation estimated that there are approximately 425 million patients with diabetes worldwide, including 115 million of those in China [1]. Type 2 diabetes mellitus (T2DM) is the most common type of diabetes. It is one of the most common chronic metabolic diseases affecting human health. Persistent hyperglycemia can cause various complications, such as osteoporosis, diabetic retinopathy, diabetic nephropathy, and coronary heart disease. The primary pathophysiology in T2DM patients is insulin resistance (IR). All patients with T2DM have IR [2]. Identifying IR and quantifying its severity is a challenge for clinicians. Currently, the hyperinsulinemic-euglycemic clamp is the standard for measuring IR [3]. However, it is invasive and expensive, and unsuitable for large-scale clinical use. Homeostasis model assessment of IR (HOMA-IR) is currently most commonly used in clinical practice to assess IR; however, the use of exogenous insulin can cause some errors in this index. Therefore, an estimated glucose disposal rate (eGDR) has been developed to assess IR in patients with diabetes, based on readily available clinical factors [4]. Previously, eGDR was used to assess IR in type 1 diabetes mellitus (T1DM). The low levels of eGDR are associated with increased diabetic microvascular complications, macrovascular complications, and increased mortality in patients with T1DM [3, 5, 6]. Recently, Zabala et al. demonstrated that eGDR can also be used to assess IR in patients with T2DM. Moreover, they observed that the low levels of eGDR were associated with increased cardiovascular complications and all-cause mortality in patients with T2DM [7, 8].
Patients with T2DM are highly susceptible to osteoporosis. Patient with T2DM have a normal or increased bone mineral density (BMD) but are at an increased risk for fractures [9]. Therefore, early markers are needed to identify fracture risk in patients with T2DM. A meta-analysis revealed an impairment of bone turnover in patients with T2DM [10]. Bone turnover involves a complex process of bone formation and resorption, which can be evaluated by the presence of bone turnover markers (BTMs) in blood and urine. The BTMs can identify fracture risk and osteoporosis earlier than BMD.
Although there is a clear association between T2DM and osteoporosis, their pathogenesis has not been elucidated. Insulin receptors are present on the surface of both osteoblasts and osteoclasts; insulin can regulate their proliferation, survival, and differentiation [11, 12]. However, the relationship between IR and bone turnover is unclear. Therefore, we investigated the relationship between eGDR and BTMs to explore whether eGDR can assess the risk of osteoporosis in patients with T2DM.
Materials and methods
Study population
This was a hospital-based cross-sectional study, conducted in the Department of Endocrinology, Hebei General Hospital, from December 2018 to December 2019. Five hundred forty-nine adult male and postmenopausal female patients with T2DM complying with the WHO diagnostic criteria for diabetes, 1999, were included. Patients with acute complications of diabetes, pregnant or lactating female patients, patients with hepatic or renal dysfunction or malignant tumor, patients with diseases affecting bone metabolism, and patients using drugs that could affect bone metabolism, such as calcitriol, calcitonin, bisphosphonates, RANKL inhibitors, anabolics, and selective estrogen receptor modulators, and active vitamin D were excluded. This study was approved by the Medical Ethics Committee of the Hebei General Hospital, and an informed consent form was signed by all patients before enrollment. This study was conducted per the Declaration of Helsinki.
Patient demographics
All participants completed a recorded questionnaire for the collection of basic information, including sex, age, duration of diabetes, history of hypertension, etc. Height, weight, systolic blood pressure, and diastolic blood pressure (DBP) were measured twice by a professional, and the average value was recorded.
Biomarkers
Blood samples were collected from the patients following 8 h of fasting. All blood biomarkers were determined in the same laboratory. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), serum creatinine (Scr), blood urea nitrogen (BUN), uric acid (UA), fasting blood glucose (FBG) were analyzed by professionals using a fully automatic biochemical analyzer (Hitachi 7600, Japan). Osteocalcin (OC), type I collagen carboxyl-terminal peptide (β-CTX), type I procollagen amino-terminal peptide (PINP), Vitamin D, and glycosylated hemoglobin (HbA1c) were all determined by the laboratory physician using an electrochemical luminescence method (Roche Cobase 601, Germany).
Calculation of parameters
eGDR was calculated using a composite of BMI, HbA1c, and hypertensive status using the following formulae: eGDR = 19.02 – [0.22 × BMI (kg/m2)] – (3.26 × HTN) – (0.61 × HbA1c (%)], wherein HTN is hypertension (1 = yes, 0 = no) [8].
Statistical analysis
All analyses were conducted using the SPSS software version 25.0. We tested the distribution normality using the Kolmogorov–Smirnov test. Data that conformed to the normal distribution were presented as mean ± standard deviation, and comparisons between groups were made using Student’s t-test. Data that did not conform to the normal distribution were presented as median (interquartile range: 25–27), and comparisons between groups were made using the Mann–Whitney U test. For categorical variables, data were expressed as numbers (percentages), and the χ2 test was used to compare groups. Spearman or Pearson correlation analysis was used for determining the correlation between eGDR and BTMs. Multiple linear regression analysis was used to investigate the independent correlation between eGDR and BTMs. A P value <0.05 was considered statistically significant.
Result
Clinical characteristics of all participants
For 549 patients with T2DM (352 males and 197 postmenopausal females) included in this study, the mean age was 59.32 years and the median duration of diabetes was 10 years. The mean eGDR level was 6.26, with 274 patients in the low-eGDR group and 275 patients in the high-eGDR group. The mean OC level was 11.80 ng/mL, the mean β-CTX level was 0.32 ng/mL, and the mean PINP level was 37.29 ng/mL.
Comparison of basic parameters between the low-eGDR group and high-eGDR group
eGDR is an indicator of IR. The lower the eGDR level, the more severe is the IR. The levels of OC, β-CTX, and PINP were significantly lower in the low-eGDR group than those in the high-eGDR group (P < 0.05). The difference in BTMs between low- and high-eGDR groups is demonstrated in Fig. 1a.
Compared to the high-eGDR group, the low-eGDR group had higher TG, UA, FBG, and HbA1c levels and lower vitamin D levels (P < 0.05). The baseline anthropometric and biochemical characteristics of the low- and high-eGDR groups are presented in Table 1.
Comparison of basic parameters between the male group and postmenopausal female group
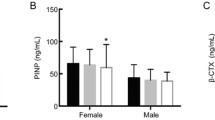
Compared with the postmenopausal female group, the levels of OC, β- CTX, and PINP were lower in the male group (P < 0.05). The difference in BTMs between male and postmenopausal female groups is presented in Fig. 1b.
The mean age, duration of diabetes, and levels of TC, LDL, and HDL were lower in the male group than in the postmenopausal female group (P < 0.05). However, the levels of DBP, ALT, Scr, BUN, UA, and vitamin D were higher in the male subgroup compared with those in the postmenopausal female subgroup (P < 0.05). There was no significant difference in eGDR and FBG between the two groups (P > 0.05). The baseline anthropometric and biochemical characteristics of the male group and postmenopausal female group are presented in Table 2.
Correlation analysis of eGDR and bone turnover marker levels in patients with T2DM
eGDR was positively correlated with OC, β-CTX, and PINP in all patients with T2DM and the male subgroup. However, there was no correlation among them in the postmenopausal female subgroup (Fig. 2).
The correlation between eGDR and bone turnover markers levels. a The correlation between eGDR and bone turnover markers levels in all participants. b The correlation between eGDR and bone turnover markers levels in male participants. c The correlation between eGDR and bone turnover markers levels in postmenopausal female participants
Multivariate linear correlation analysis of eGDR and BTMs in patients with T2DM
For all patients, eGDR was positively correlated with OC, β-CTX, and PINP in the crude models 1 and 2 (adjusted for age, duration of diabetes, and vitamin D) (Table 3).
eGDR was positively correlated with OC, β-CTX, and PINP levels in male patients with T2DM, regardless of whether an unadjusted or adjusted model was used (Table 3).
In contrast, eGDR was not associated with OC, β-CTX, and PINP levels in postmenopausal female patients with T2DM, regardless of the model used (Table 3).
Discussion
T2DM and osteoporosis are public health concerns, which require close attention in the aging society. With aging, people become more susceptible to osteoporosis and diabetes, which further increases the risk of fracture. Therefore, early markers are needed to clinically evaluate bone turnover and osteoporosis risk for early intervention.
eGDR is an indicator of IR that can be calculated based on clinical factors. Our study revealed that among all patients with T2DM, bone formation and resorption markers were reduced in the low-eGDR group. eGDR was positively correlated with BTMs, and this correlation was not affected by risk factors for osteoporosis, such as age, disease duration, and vitamin D levels. In addition, the correlation between eGDR and bone turnover was sex-specific. eGDR positively correlated with BTMs in male patients with T2DM albeit did not correlate with those in postmenopausal female patients with T2DM. Conclusively, this study suggests that IR is associated with low bone turnover in patients with T2DM, especially in male patients with T2DM, and low eGDR is a good indicator of impaired bone turnover.
Bone is a dynamic tissue where osteoclasts resorb bone tissue and are renewed by osteoblasts, which undergo a self-balancing cycle of renewal and repair in coordination with osteoclasts, osteoblasts, and osteocytes [13]. Insulin can influence bone metabolism [14], and it has a critical role in the regulation of skeletal development and bone integrity. Insulin acts on insulin receptors on the surface of osteoblasts and osteoclasts to regulate insulin signaling, which is essential for cell proliferation, survival, and differentiation [15, 16].
Insulin plays a crucial role in bone turnover, however, the effect of IR on bone turnover is not yet definitively established. Huang et al. observed that IR inhibited bone turnover in mice [17]. Currently, almost all analyses of the correlation between IR and bone turnover in patients with T2DM arise from observational studies and have conflicting results. Kunutsor et al. observed that HOMA-IR, an indicator of IR, was negatively correlated with OC [18], consistent with our findings. A similar result has been observed in several studies [19,20,21,22]. However, Mori et al. observed no correlation between OC and IR measured by the hyperinsulinemic-euglycemic clamp in patients with T2DM [23], and some studies have also reported no correlation between OC and HOMA-IR [24,25,26]. Limited studies have been conducted on the relationship between IR, PINP, and β-CTX. Guo et al. reported that PINP and β-CTX were negatively correlated with HOMA-IR [27]. However, Feldbrin et al. observed no correlation between PINP and HOMA-IR [28].
In this study, eGDR was positively correlated with bone turnover and was not affected by confounding factors. This study suggests that the lower the eGDR level, the more severe will be the IR and the more impaired will be the bone turnover. The mechanism is still unclear; however, the following mechanisms may exist: First, in IR there is impaired binding of insulin to its receptors and impairment of distal insulin signaling pathways on the cell surface [29,30,31]. Owing to IR, the expression of insulin receptors on the surface of osteoblasts is downregulated, the activity of osteoblasts is inhibited, and the survival time of osteoblasts is shortened [32], resulting in impaired bone formation. Bilotta et al. reported that IR can reduce OC gene promoter activity during osteoblast differentiation, negatively affecting OC gene expression and thus, impairing bone formation [33].
Second, to overcome IR, pancreatic β-cells initially increase insulin secretion but are followed by relative progressive hyposecretion resulting in hyperglycemia. Hyperglycemia can directly damage osteoblasts and inhibit osteoclast growth, mineralization, and expression of osteocalcin [34, 35]. Persistent hyperglycemia can reduce the viability of mesenchymal stem cells. In addition, hyperglycemia can deplete or alter the differentiation of mesenchymal stem cells into osteoblasts, causing the inhibition of osteogenesis [36] and inducing the production of advanced glycosylation products (AGEs), which inhibit osteoblast differentiation and stimulate apoptosis [37]. AGE-modified collagen fibers affect the proliferation and function of osteoblasts and osteoclasts. Hyperglycemia can also cause increased production of reactive oxygen species, which inhibits the osteoblast formation by suppressing the Runx2 gene, causing low bone formation [38]. Glycosylated collagen interacts with bone cells, which further fail to adhere to collagen and produce a new matrix, ultimately causing low bone turnover [39].
IR is a chronic inflammatory process. Ma et al. reported that insulin-resistant individuals have more pro-inflammatory cytokines than insulin-sensitive individuals, particularly interleukin-1, interleukin-6, and tumor necrosis factor (TNF-α) [40]. These pro-inflammatory cytokines can cause osteoblast dysfunction and inhibit the production of osteoblast alkaline phosphatase [41]. It can cause impairment of bone turnover and a reduction in bone strength. Baek et al. observed that TNF-α upregulates sclerostin, which has an antianabolic effect on bone formation [42, 43]. In addition, several studies have reported that inflammatory factors can promote osteoclast differentiation and increase osteoclast survival, thereby, increasing bone resorption [40, 44]. However, our study demonstrated that IR impairs bone resorption. The reason for this contradictory result may be that IR causes bone turnover in various ways.
The main mechanism of IR is ectopic lipid accumulation in the liver and skeletal muscle, accompanied by adipocyte dysfunction [45]. The patients with IR have more visceral fat [13]. Bao et al. reported that visceral fat was negatively correlated with OC in Chinese male patients with T2DM [46]. Adipokines promote the differentiation of mesenchymal stem cells into adipocytes, inhibit the expression of intracellular osteogenic signals, and ultimately reduce bone formation [47].
In addition, this study revealed that the level of BTMs, age, and duration of disease was higher in the postmenopausal female subgroup than in the male subgroup. The eGDR was positively correlated with BTMs in male patients with T2DM whereas there was no correlation between eGDR and BTMs in postmenopausal female patients with T2DM. To exclude the effects of age, disease duration, and vitamin D confounders, we performed multiple linear analyses in the follow-up study. However, the levels of eGDR still did not correlate with BTMs in postmenopausal female patients with T2DM. This indicates that the reason for the correlation between eGDR and bone turnover was sex difference. This sex difference may be secondary to the difference in levels of serum sex hormones in the patients, which, however, were not examined in this study.
Testosterone is the most important androgen in adult males. It plays a significant role in maintaining bone mass and anti-osteoporosis. In adulthood, testosterone promotes bone formation and inhibits bone resorption. Testosterone can be converted to estradiol, which also plays a role in bone metabolism. Bone tissue is an important target tissue for estrogen, which can bind to the estrogen receptors on the surface of bone tissue and regulate bone turnover through various pathways. Estrogen can promote osteoblast proliferation, bone formation, protein synthesis, and bone mineralization and inhibit osteoblast apoptosis. In addition, it can inhibit osteoclast activity and promote osteoclast apoptosis [48].
Im et al. observed a negative correlation between OC and HOMA-IR in postmenopausal female patients with T2DM [49], consistent with the results of several other studies [50, 51]. Consistent with our study, Zhou et al. observed no correlation between OC and IR in postmenopausal female patients with T2DM [52]. Currently, there are no studies on the correlation between PINP, β-CTX, and IR in postmenopausal female patients with T2DM. These conflicting results may be owing to differences in study populations, regions, and sex hormone levels. Therefore, large-scale clinical data analysis and meta-analysis are needed to analyze the effects of IR on bone turnover among all sexes.
The strength of our study is that we are the first to demonstrate a positive correlation between eGDR and BTMs in patients with T2DM. The lower the level of eGDR, the more impaired is the bone turnover. This study suggested that eGDR is a good indicator for assessing bone turnover and fracture risk in patients with T2DM, especially male patients with T2DM. More importantly, the levels of eGDR are simple and easy to measure and thus, can be widely measured clinically.
This study has some limitations. First, it was a cross-sectional study, and the causal relationship of positive correlation between eGDR and BTMs could not be confirmed. Second, the duration of outdoor exercise and dietary factors were not considered in this study. These should be considered in future studies examining bone turnover. Last, we did not examine the levels of serum sex hormones in this study. It cannot be clarified whether the sex difference between eGDR and bone turnover was caused by the difference in the levels of serum sex hormones.
Conclusively, we observed that eGDR was positively correlated with BTMs, and this correlation was different based on sex. It indicates that low eGDR is a significant clinical indicator for identifying low bone turnover or fracture risk in patients with T2DM, especially in male patients with T2DM.
References
F. Alaei-Shahmiri, M.E. Khamseh, K. Manhoei, H. Yadegari, H. Kazemi et al. The optimal vitamin D cut-off value associated with hyperglycemia in an Iranian population. J. Diabetes Metab. Disord. 19, 5–12 (2020)
H. Chang-Quan, D. Bi-Rong, H. Ping, L. Zhen-Chan, Insufficient renal 1-alpha hydroxylase and bone homeostasis in aged rats with insulin resistance or type 2 diabetes mellitus. J. Bone Miner. Metab. 26, 561–568 (2008)
R. Nishtala, N. Kietsiriroje, Estimated glucose disposal rate demographics and clinical characteristics of young adults with type 1 diabetes mellitus: a cross-sectional pilot study. Diab. Vasc. Dis. Res. 17, 1479164120952321 (2020)
K.V. Williams, J.R. Erbey, D. Becker, S. Arslanian, T.J. Orchard, Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes 49, 626–632 (2000)
L.L. O’Mahoney, N. Kietsiriroje, Estimated glucose disposal rate as a candidate biomarker for thrombotic biomarkers in T1D: a pooled analysis. J. Endocrinol. Invest. 44, 2417–2426 (2021)
J.J. Chillarón, A. Goday, J.A. Flores-Le-Roux, D. Benaiges, M.J. Carrera et al. Estimated glucose disposal rate in assessment of the metabolic syndrome and microvascular complications in patients with type 1 diabetes. J. Clin. Endocrinol. Metab. 94, 3530–3534 (2009)
A. Zabala, V. Darsalia, M. Lind, A.M. Svensson, S. Franzén et al. Estimated glucose disposal rate and risk of stroke and mortality in type 2 diabetes: a nationwide cohort study. Cardiovascular Diabetol. 20, 202 (2021)
T. Nyström, M.J. Holzmann, B. Eliasson, A.M. Svensson, J. Kuhl et al. Estimated glucose disposal rate and long-term survival in type 2 diabetes after coronary artery bypass grafting. Heart Vessels 32, 269–278 (2017)
P. Vestergaard, Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes–a meta-analysis. Osteoporos. Int. 18, 427–444 (2007)
K. Hygum, J. Starup-Linde, T. Harsløf, P. Vestergaard, B.L. Langdahl, MECHANISMS IN ENDOCRINOLOGY: diabetes mellitus, a state of low bone turnover – a systematic review and meta-analysis. Eur. J. Endocrinol. 176, R137–r157 (2017)
J.Y. Lee, N.K. Lee, Up-regulation of cyclinD1 and Bcl2A1 by insulin is involved in osteoclast proliferation. Life Sci. 114, 57–61 (2014)
K. Fulzele, R.C. Riddle, D.J. DiGirolamo, X. Cao, C. Wan et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 142, 309–319 (2010)
K.T. Tonks, C.P. White, J.R. Center, D. Samocha-Bonet, J.R. Greenfield, Bone turnover is suppressed in insulin resistance, independent of adiposity. J. Clin. Endocrinol. Metab. 102, 1112–1121 (2017)
M. Tencerova, M. Okla, M. Kassem, Insulin signaling in bone marrow adipocytes. Curr. Osteoporos. Rep. 17, 446–454 (2019)
V.V. Shanbhogue, J.S. Finkelstein, M.L. Bouxsein, E.W. Yu, Association between insulin resistance and bone structure in nondiabetic postmenopausal women. J. Clin. Endocrinol. Metab. 101, 3114–3122 (2016)
E.M. Dennison, H.E. Syddall, A. Aihie Sayer, S. Craighead, D.I. Phillips et al. Type 2 diabetes mellitus is associated with increased axial bone density in men and women from the Hertfordshire Cohort Study: evidence for an indirect effect of insulin resistance? Diabetologia 47, 1963–1968 (2004)
S. Huang, M. Kaw, M.T. Harris, N. Ebraheim, M.F. McInerney et al. Decreased osteoclastogenesis and high bone mass in mice with impaired insulin clearance due to liver-specific inactivation to CEACAM1. Bone 46, 1138–1145 (2010)
S.K. Kunutsor, T.A. Apekey, J.A. Laukkanen, Association of serum total osteocalcin with type 2 diabetes and intermediate metabolic phenotypes: systematic review and meta-analysis of observational evidence. Eur. J. Epidemiol. 30, 599–614 (2015)
S. Yano, T. Nabika, A. Nagai, T. Hamano, M. Yamasaki et al. Interrelationship between glucose metabolism and undercarboxylated osteocalcin: a cross-sectional study in a community-dwelling population. Asia Pac. J. Clin. Nutr. 24, 489–495 (2015)
S.W. Lee, H.H. Jo, M.R. Kim, J.H. Kim, Y.O. You, Association between osteocalcin and metabolic syndrome in postmenopausal women. Arch. Gynecol. Obstet. 292, 673–681 (2015)
W.W. Hu, Y.H. Ke, J.W. He, W.Z. Fu, Y.J. Liu et al. Serum osteocalcin levels are inversely associated with plasma glucose and body mass index in healthy Chinese women. Acta Pharmacol. Sin. 35, 1521–1526 (2014)
G.S. Kim, Y. Jekal, H.S. Kim, J.A. Im, J.Y. Park et al. Reduced serum total osteocalcin is associated with central obesity in Korean children. Obes. Res. Clin. Pract. 8, e201–e298 (2014)
K. Mori, M. Emoto, K. Motoyama, E. Lee, S. Yamada et al. Undercarboxylated osteocalcin does not correlate with insulin resistance as assessed by euglycemic hyperinsulinemic clamp technique in patients with type 2 diabetes mellitus. Diabetol. Metab. Syndr. 4, 53 (2012)
X.Y. Ma, F.Q. Chen, H. Hong, X.J. Lv, M. Dong et al. The relationship between serum osteocalcin concentration and glucose and lipid metabolism in patients with type 2 diabetes mellitus – the role of osteocalcin in energy metabolism. Ann. Nutr. Metab. 66, 110–116 (2015)
K.M. Bador, L.D. Wee, S.A. Halim, M.F. Fadi, P. Santhiran et al. Serum osteocalcin in subjects with metabolic syndrome and central obesity. Diabetes Metab. Syndr. 10, S42–S45 (2016)
Q. Wang, B. Zhang, Y. Xu, H. Xu, N. Zhang, The relationship between serum osteocalcin concentration and glucose metabolism in patients with type 2 diabetes mellitus. Int. J. Endocrinol. 2013, 842598 (2013)
H. Guo, C. Wang, B. Jiang, S. Ge, J. Cai et al. Association of insulin resistance and β-cell function with bone turnover biomarkers in dysglycemia patients. Front. Endocrinol. 12, 554604 (2021)
Z. Feldbrin, M. Shargorodsky, Bone remodelling markers in hypertensive patients with and without diabetes mellitus: link between bone and glucose metabolism. Diabetes Metab. Res. Rev. 31, 752–757 (2015)
G.M. Reaven, Pathophysiology of insulin resistance in human disease. Physiological Rev. 75, 473–486 (1995)
A. Fraser, R. Harris, N. Sattar, S. Ebrahim, G. Davey Smith et al. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: the British Women’s Heart and Health Study and meta-analysis. Diabetes Care 32, 741–750 (2009)
A.J. Hanley, L.E. Wagenknecht, A. Festa, R.B. D’Agostino Jr., S.M. Haffner, Alanine aminotransferase and directly measured insulin sensitivity in a multiethnic cohort: the Insulin Resistance Atherosclerosis Study. Diabetes Care 30, 1819–1827 (2007)
L. Aguirre, N. Napoli, D. Waters, C. Qualls, D.T. Villareal et al. Increasing adiposity is associated with higher adipokine levels and lower bone mineral density in obese older adults. J. Clin. Endocrinol. Metab. 99, 3290–3297 (2014)
F.L. Bilotta, B. Arcidiacono, S. Messineo, M. Greco, E. Chiefari et al. Insulin and osteocalcin: further evidence for a mutual cross-talk. Endocrine 59, 622–632 (2018)
L. Wang, T. Li, J. Liu, X. Wu, H. Wang et al. Association between glycosylated hemoglobin A1c and bone biochemical markers in type 2 diabetic postmenopausal women: a cross-sectional study. BMC Endocr. Disord. 19, 31 (2019)
W. Wang, X. Zhang, J. Zheng, J. Yang, High glucose stimulates adipogenic and inhibits osteogenic differentiation in MG-63 cells through cAMP/protein kinase A/extracellular signal-regulated kinase pathway. Mol. Cell. Biochem. 338, 115–122 (2010)
P. Lakatos, B. Szili, B. Bakos, I. Takacs, Z. Putz et al. Thyroid hormones, glucocorticoids, insulin, and bone. Handb. Exp. Pharmacol. 262, 93–120 (2020)
M. Alikhani, Z. Alikhani, C. Boyd, C.M. MacLellan, M. Raptis et al. Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone 40, 345–353 (2007)
L.F. Liu, W.J. Shen, Z.H. Zhang, L.J. Wang, F.B. Kraemer, Adipocytes decrease Runx2 expression in osteoblastic cells: roles of PPARγ and adiponectin. J. Cell. Physiol. 225, 837–845 (2010)
P. Vestergaard, Diabetes and osteoporosis – cause for concern? Front. Endocrinol. 5, 53 (2014)
C. Ma, K.T. Tonks, J.R. Center, D. Samocha-Bonet, J.R. Greenfield, Complex interplay among adiposity, insulin resistance and bone health. Clin. Obes. 8, 131–139 (2018)
S. Rathinavelu, C. Guidry-Elizondo, J. Banu, Molecular modulation of osteoblasts and osteoclasts in type 2. Diabetes 2018, 6354787 (2018)
K. Baek, H.R. Hwang, H.J. Park, A. Kwon, A.S. Qadir et al. TNF-α upregulates sclerostin expression in obese mice fed a high-fat diet. J. Cell. Physiol. 229, 640–650 (2014)
V. Boschert, M. van Dinther, S. Weidauer, K. van Pee, E.M. Muth et al. Mutational analysis of sclerostin shows importance of the flexible loop and the cystine-knot for Wnt-signaling inhibition. PloS One 8, e81710 (2013)
Z. Yao, L. Xing, C. Qin, E.M. Schwarz, B.F. Boyce, Osteoclast precursor interaction with bone matrix induces osteoclast formation directly by an interleukin-1-mediated autocrine mechanism. J. Biol. Chem. 283, 9917–9924 (2008)
C. Conte, S. Epstein, N. Napoli, Insulin resistance and bone: a biological partnership. Acta Diabetol. 55, 305–314 (2018)
Y. Bao, X. Ma, R. Yang, F. Wang, Y. Hao et al. Inverse relationship between serum osteocalcin levels and visceral fat area in Chinese men. J. Clin. Endocrinol. Metab. 98, 345–351 (2013)
S. Muruganandan, C.J. Sinal, The impact of bone marrow adipocytes on osteoblast and osteoclast differentiation. IUBMB Life 66, 147–155 (2014)
X.G. Han, D.W. Wang, Z.G. Bi, F. Gao, Regulatory effect of estrogen receptor-α-mediated Wnt/β-catenin signaling pathway on osteoblast proliferation. J. Biol. Regul. Homeost. Agents 30, 381–387 (2016)
J.A. Im, B.P. Yu, J.Y. Jeon, S.H. Kim, Relationship between osteocalcin and glucose metabolism in postmenopausal women. Clin. Chim. Acta 396, 66–69 (2008)
Y.C. Hwang, I.K. Jeong, K.J. Ahn, H.Y. Chung, Circulating osteocalcin level is associated with improved glucose tolerance, insulin secretion and sensitivity independent of the plasma adiponectin level. Osteoporos. Int. 23, 1337–1342 (2012)
P.D. Sarkar, A.B. Choudhury, Relationships between serum osteocalcin levels versus blood glucose, insulin resistance and markers of systemic inflammation in central Indian type 2 diabetic patients. Eur. Rev. Med. Pharmacol. Sci. 17, 1631–1635 (2013)
M. Zhou, X. Ma, H. Li, X. Pan, J. Tang et al. Serum osteocalcin concentrations in relation to glucose and lipid metabolism in Chinese individuals. Eur. J. Endocrinol. 161, 723–729 (2009)
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Z.L., X.P., Y.J., X.Z., and C.D. The first draft of the manuscript was written by Z.L. Writing (review and editing) was performed by S.C. and C.Q.; all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Hebei General Hospital (Date May 8, 2020/No. 202027).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Qi, C., Pan, X. et al. The relationship between estimated glucose disposal rate and bone turnover markers in type 2 diabetes mellitus. Endocrine 77, 242–251 (2022). https://doi.org/10.1007/s12020-022-03090-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03090-z