Abstract
Purpose
The primary aim of this systematic review and meta-analysis was to determine the association between gestational diabetes mellitus (GDM) and metabolic syndrome (MetS) in women and children. Our secondary aim was to assess the development of MetS with respect to the elapsed time postpartum at which MetS was diagnosed.
Methods
This review is registered with PROSPERO (CRD42020173319). PubMed, CINHAL, SCOPUS, and EMBASE databases were searched. Studies reporting on the rate of MetS in pregnant women with GDM, the rate of MetS in women with a history of GDM, and the rate of MetS in offspring exposed to GDM in utero compared to healthy controls were selected.
Results
We identified 588 articles from the literature search. Fifty-one studies were included in the review and of those 35 were included in the meta-analysis. Quantitative summary measures showed that women with a history of GDM had an increased risk of developing MetS compared to those without a history of GDM (RR 2.36, 95% CI 1.77–3.14, 29 studies, 13,390 participants; heterogeneity: χ2 p < 0.00001; I2 = 93%). Offspring exposed to GDM in utero have an increased risk of developing MetS compared to those not exposed to GDM in utero. (RR 2.07, 95% CI 1.26–3.42, three studies, 4,421 participants; heterogeneity: χ2 p = 0.33; I2 = 12%). Women diagnosed with GDM have an increased risk of developing MetS during pregnancy (RR 20.51, 95% CI 5.04–83.55; three studies, 406 participants; heterogeneity: χ2 p = 0.96; I2 = 0%). Subgroup analysis revealed that MetS is diagnosed as early as <1 year postpartum in women with a history of GDM.
Conclusions/interpretation
Women with GDM have an increased risk of developing MetS during pregnancy. Women with a history of GDM and offspring exposed to GDM in utero have higher risks of developing MetS compared to those with no history of GDM. Metabolic syndrome in women with a history of GDM is seen as early as <1 year postpartum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) is impairment of glucose that is first diagnosed during pregnancy, hence different from both type I and II diabetes mellitus. GDM is estimated to affect one in seven pregnancies [1]. Women with a history of GDM are more likely to be obese, have dyslipidaemia and hypertension during the postpartum period [2]. These women also have an approximately sevenfold increased risk of developing type II diabetes mellitus (T2DM) later in life [3]. The diagnostic criteria for GDM have changed as of recent, being defined as fasting glycaemia ≥5.1 mmol/l, or 1-h plasma glucose ≥10.0 mmol/l and 2-h plasma glucose: ≥8.5 mmol/l with a 75 g oral glucose tolerance test [4].
Metabolic syndrome (MetS) is defined as a cluster of metabolic disorders, conventionally defined as three or more of the following: central obesity, reduced high-density lipoprotein cholesterol, hypertriglyceridemia, hyperglycemia, and hypertension. However, the cut-offs for these individual components of MetS are different between definitions [5, 6]. Both GDM and MetS share a similar etiology and both increase the risk of chronic diseases such as T2DM and cardiovascular disease (CVD) [3, 7,8,9].
GDM is promoted by an inability of β-cells to undergo expansion. Therefore, β-cells are unable to compensate for the highly insulin resistant state leading to the subsequent elevation of glucose during pregnancy [10]. Development of pregnancy complications, such as GDM, is influenced by prepregnancy lifestyle and metabolic characteristics [11]. Women with MetS are already in a state of pro-inflammation and insulin resistance [12], therefore it is possible that when they become pregnant, they are more susceptible to developing GDM [13]. This association has not been explored in a systematic review and meta-analysis. Furthermore, GDM increases the risk of developing CVD in later life and ~50% of women who develop GDM go on to develop T2DM later in life [14]. Therefore, women who may not have MetS in pregnancy or only present with one or two components of MetS may be at risk of developing MetS postpartum. A meta-analysis in 2014 showed that women who experience GDM have a higher risk of developing MetS than women with a normal pregnancy [15]. However, the studies included in the above meta-analysis were conducted before the implementation of the new International Association of Diabetes in Pregnancy Study Group (IADPSG) guidelines that recommended a lowering of the glucose threshold for the diagnosis of GDM [16]. As the new guidelines are known to increase the number of women diagnosed with GDM, it is possible that the number of metabolic risk factors in women who had GDM will also increase. Children exposed to GDM in utero may also be more susceptible to developing MetS, as it has been shown that they have higher systolic blood pressure (SBP), body mass index (BMI), and blood glucose than those not exposed to GDM in utero [17]. To our knowledge, no systematic review has assessed the risk for MetS among children born to pregnancies complicated by GDM. Even small improvements in the components of MetS such as hypertension and dyslipidaemia can significantly reduce the risk of ischemic heart disease in young and middle age adults [18,19,20] and reducing childhood adiposity can reduce the risk of CVD later in life [21].
Therefore, the objective of our systematic review and meta-analysis was to evaluate the association between GDM and MetS by determining (1) the risk of MetS in pregnancy among women who are diagnosed with GDM, (2) the risk for postpartum MetS among women who experienced GDM, and (3) the risk of developing MetS in children born to pregnancies complicated by GDM.
Methods
The review protocol is registered in PROSPERO (CRD42020173319). The review was undertaken with reference to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline [22].
Search strategy
All studies describing the association between GDM and MetS were identified by searching the following electronic databases: PubMed, CINAHL, SCOPUS, and EMBASE with an end search date of February 18, 2020. The search was conducted by Z.S.L. The search strategy included the terms (“gestational diabetes*” OR “pregnancy induced diabetes”) AND (“metabolic syndrome” OR “insulin resistance syndrome” OR “syndrome X”) and is detailed in Appendix S1. We included observational studies (case-control, cross-sectional, and cohort). Bibliographies of previously conducted systematic reviews and meta-analyses on closely related topics, and eligible studies were checked for additional studies. All identified studies were independently assessed for relevance by two authors (M.M.P. and A.A.). Two authors (M.M.P. and A.A.) independently extracted data, and discrepancies were resolved by discussion with Z.S.L. and P.H.A.
Studies were eligible for inclusion if they reported the number of cases of MetS in (1) pregnant women diagnosed with GDM, (2) women with a history of GDM, compared to women who did not experience/have a history of GDM, and (3) those exposed to GDM in utero compared to those not exposed to GDM in utero. We included studies that defined GDM based on the IADPSG guidelines [23]. However, since the diagnostic criteria have been revised recently, we included studies that used prior recommended diagnostic criteria of GDM including the 1999 World Health Organization (WHO) definition [5], and other regional and study-specific definitions as detailed in Table S1 [5, 24,25,26,27,28,29,30,31]. MetS was defined based on the definitions of the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP-III) [6], International Diabetes Federation (IDF) [32], the WHO [5], or the American Heart Association [33]. Because there is no validated definition of MetS in children and pregnant women, we accepted variations of current guidelines and study-specific definitions. The definitions of GDM and MetS of included studies are detailed in Table S1. Studies that did not include a definition of GDM or MetS, those that did not define the case and control groups, and those that compared women with GDM in pregnancy/postpartum, and those exposed to GDM in utero to another risk group were excluded.
Statistical analysis
Data were extracted independently and in duplicate for the number of MetS cases. We analyzed all studies collectively as an overall analysis, and subsequently stratified into subgroups based on the time of follow up postpartum as: <1 year, 1–5 years, 5–10 years, and 10+ years from the index pregnancy. Some studies analyzed the rate of MetS based on the multiple definitions. Therefore, when assessing data from those studies, the NCEP-ATP-III definition was used in the overall analysis as the majority of studies used this definition. However, we conducted subgroup analyses based on the rate of MetS defined according to the NCEP-ATP-III, IDF, and WHO guidelines. We performed an ad hoc analysis based on ethnicity, but only for Asian and Caucasian ethnicities, as these were the most commonly reported ethnicities. When the same cohort was assessed multiple times during the postpartum period, the study with the largest sample size was used in the overall analysis. For the analysis on offspring exposed to GDM in utero, the oldest cohort was used in the meta-analysis. We considered studies published in English. We did not need to contact any authors for additional information, as only one dichotomous outcome was evaluated, and only studies reporting on the outcome were eligible.
The following data were collected from each included study: definition of GDM, definition of MetS, time of postpartum follow up (number of years since index pregnancy for both women and children), or gestational age (week) at which MetS and GDM were diagnosed during pregnancy, number of cases (those who experienced GDM) and controls (those who did not experience GDM), birthweight of offspring and gestational age at delivery for both cases and controls.
The meta-analysis was performed using RevMan software (Review Manager Version 5.3) based on an inverse variance method. As per protocol, the random effects model was selected to account for the differences in diagnostic criteria of GDM. For each outcome measure, the number of events and the total number of participants were used in the meta-analysis to analyze the risk difference. If the number was only reported as a percentage, then the number of participants/events was calculated based on the total sample size for each group. The analysis was cross-checked and discrepancies were resolved by discussion (P.H.A. and M.M.P.).
Substantial heterogeneity was considered when I2 statistic exceeded 50%, and the Chi² p value was <0.1. Data from eligible studies that could not be included in the meta-analysis are included in Table S2. To assess publication bias, funnel plots were used for the primary outcome. The methodological quality was assessed using the National Heart, Lung and Blood Institute (NHLBI) Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies and are presented in the Supplementary data (Table S3) [34]. Sensitivity analysis was performed to evaluate heterogeneity for outcomes after excluding low-moderate quality studies (i.e., studies that were considered of low-moderate quality in the NHLBI Quality assessment tool after discussion with authors).
Results
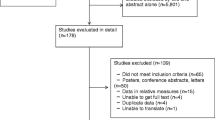
The literature search identified 588 articles. One hundred and ninety articles were eligible for full text review. Of these, 51 were included in the review and 35 were included in the meta-analyses (Table S1). The reasons for excluding 139 studies are detailed in Fig. 1. The quality assessment showed that all studies were of moderate to high quality (Table S3).
Risk of MetS in pregnancy among women diagnosed with GDM
Eight studies were included in the assessment of this outcome [13, 35,36,37,38,39,40,41], of which three studies were included in the meta-analysis [35,36,37]. All three studies assessed GDM and MetS at the same time (i.e., ~24–32 weeks gestation). Pooled analysis showed that women diagnosed with GDM had an increased risk of MetS in pregnancy (RR 20.51, 95% CI 5.04–83.55; three studies, 406 participants; heterogeneity: χ2 p = 0.96; I2 = 0%) (Fig. 2a). Five studies were not included in the meta-analysis [13, 38,39,40,41], with four showing an increased risk of developing GDM in women who are diagnosed with MetS during pregnancy [39, 40, 42, 43] (Table S2).
Risk of MetS in women with a history of GDM
Thirty-five studies were included in the assessment of this outcome [42, 44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78], of which 29 studies were included in the meta-analysis [30, 42, 44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71]. Pooled analysis showed that women with a history of GDM had a significantly increased risk of developing MetS (RR 2.36, 95% CI 1.77–3.14; 29 studies, 13,390 participants; heterogeneity: χ2 p < 0.00001; I2 = 93%) (Fig. 2b). Of the six studies that were not included in the meta-analysis [72,73,74,75,76,77,78], one showed an increase in prevalence of MetS among women with a history of GDM compared to controls [51] (Table S2). Sensitivity analysis after excluding the studies of moderate quality resulted in a slight reduction in heterogeneity (χ2 p < 0.00001; I2 = 78%) (Fig. S1). Assessment of the funnel plot of the meta-analysis revealed moderate publication bias (Fig. S2).
Risk of MetS in offspring exposed to GDM in utero
Four studies were included in the assessment of this outcome [79,80,81,82], of which three studies were included in the meta-analysis [79,80,81]. Pooled analysis showed that offspring exposed to GDM in utero had a significantly increased risk of developing MetS (RR 2.07, 95% CI 1.26–3.42; three studies, 4421 participants; heterogeneity: χ2 p 0.33; I2 = 12%) (Fig. 2c). The study that was not included in the meta-analysis showed an increased MetS severity Z-score in those exposed to GDM in utero compared to controls [82] (Table S2).
Subgroup analyses
We conducted subgroup analyses based on the time of postpartum follow up among women with a history of GDM. The results are shown in Table S4. The risk of developing MetS was significantly increased in women with a history of GDM at <1 year postpartum (RR 1.95, 95% CI 1.15–3.28, three studies, 850 participants; heterogeneity χ2 p = 0.09; I2 = 59%), 1–5 years postpartum (RR 2.99, 95% CI 2.14–4.18, 18 studies, 7.328 participants; heterogeneity χ2 p < 0.00001; I2 = 70%), 5–10 years postpartum (RR 2.29, 95% CI 1.62–3.25, nine studies, 4518 participants; heterogeneity χ2 p < 0.0001; I2 = 79%), and >10 years postpartum (RR 2.07 95% CI 1.22–3.50, six studies, 3037 participants; heterogeneity χ2 p < 0.00001; I2 = 94%).
We conducted a subgroup analysis to evaluate the risk of developing MetS in women with a history of GDM based on the three most common definitions of MetS (i.e., NCEP-ATP-III, IDF, and WHO). A significantly increased risk of MetS was demonstrated for women with a history of GDM compared to women without a history of GDM, irrespective of the definition used to diagnose MetS (NCEP-ATP-III: RR 2.58 95% CI 1.72–3.87, 20 studies, 8768 participants; heterogeneity χ2 p < 0.00001; I2 = 94%; IDF: RR 2.15 95% CI 1.60–2.90, 11 studies, 5615 participants; heterogeneity χ2 p < 0.00001; I2 = 79%; WHO: RR 2.99 95% CI 2.51–3.57, five studies, 3433 participants; heterogeneity χ2 p = 0.69; I2 = 0%) (Table S5). We performed an ad hoc analysis based on ethnicity (Asian and Caucasian) and found that there was a similar increased risk of MetS for women with a history of GDM for both ethnicities (Table S6).
Discussion
Main findings
Our meta-analysis revealed that women with a history of GDM are at a significantly increased risk of developing MetS later in life, and that this risk is seen as early as <1 year postpartum. Our results also demonstrate that the risk for MetS in pregnancy is higher among women diagnosed with GDM and that children born to women who experience GDM have an increased risk of developing MetS in later life.
Strengths and limitations
This systematic review and meta-analysis was a comprehensive review of the literature on the association between GDM and MetS, among women and their offspring. There has not been a systematic review and meta-analysis that investigated the association between GDM and MetS in pregnant women and offspring, and no review has evaluated the association between GDM and MetS in women with a history of GDM after the change of guidelines in 2013 [15].
Many environmental and genetic factors contribute to the risk for GDM. There are certain candidate genes that are associated with T2DM and GDM that mainly influence insulin secretion [83]. Obesity and GDM share the same causal pathway, through elevation of free fatty acids and dysregulation of cytokines to promote insulin resistance [84, 85]. Common risk factors such as advanced maternal age, familial history of T2DM or GDM in a first-degree relative (either mother or sister) also contribute to a higher risk for GDM [86]. Therefore, it is unclear whether MetS in overweight/obese women with a history of GDM is due to the disease phenotype, or due to a preexisting predisposition. Asian ethnicity is a significant risk factor for GDM [86] and diagnosis of MetS can also vary based on ethnicity. Therefore, we assessed the influence of ethnicity through an ad hoc analysis and found that both Caucasian and Asian ethnicities conferred similar increased risks for MetS in women with a history of GDM (Table S6). Women and men have different CVD risks, particularly with regard to obesity, as men generally have greater muscle mass and women have higher fat mass. Research into a modified female definition of MetS may be important, considering the differences in body composition and conventional risk factors between males and females and the higher risk of CVD among women who experience major pregnancy complications [87].
Our results on the risk for MetS among women with a history of GDM showed substantial heterogeneity. However, when we performed subgroup analyses based on the time of diagnosis of MetS, definition of MetS and ethnicity, heterogeneity was substantially reduced (Tables S4 and S5). Sensitivity analysis also showed a reduction in heterogeneity after removing studies of moderate quality. Funnel plot assessment revealed a moderate degree of publication bias (Fig. S2). It is difficult to elucidate the reason for heterogeneity in aggregate data, but it is typically due to differences in study design, differences in definitions (i.e., MetS and GDM definitions), years of postpartum follow-up, and study populations. The heterogeneity that was observed in our analysis could also be attributed to genetic and environmental factors. Large, well-characterized longitudinal cohort studies will contribute to further evidence and help reduce overall heterogeneity.
Interpretation in light of other evidence
Our meta-analysis revealed that women with a history of GDM are at significantly increased risk for developing MetS later in life (RR 2.48). Women who experience GDM have a reduction in insulin sensitivity in the third trimester, to support an increase in glucose transfer to the fetus. This is promoted by an increase in fetal and placental factors [84, 88]. However, if women are insulin resistant prior to pregnancy and fail to increase β-cell capacity during pregnancy, maternal glucose levels are unlikely to return to normal after pregnancy [89]. Considering the increased risk for cardiovascular risk factors and T2DM in women with a history of GDM [3, 11], it is not surprising that these women are at a higher risk for developing MetS later in life. Intervention trials to reduce the development of T2DM are known to be successful during the early period after pregnancy, but compliance in exercise and weight loss are shown to decrease over time [90,91,92]. This is likely due to the difficulty in changing behavioral patterns and individual circumstances. It may be more beneficial to intervene before a diagnosis of GDM, as both diet and physical activity changes have been shown to result in an 18% reduction in the risk for GDM among women with a prepregnancy BMI <25 kgm2 as well as ≥25 kgm2; and this intervention was shown to be most effective before 15 weeks’ gestation [93]. The prevalence of obesity in women of reproductive age is around 15–18% in Australian women [94]. Therefore, it is necessary to identify women who are at increased risk of developing GDM and implement interventions as soon as practical (either during preconception planning or in early pregnancy) with the aim of reducing the risk of development of GDM. This is especially important, as our results showed that women who experience GDM are at increased risk of being diagnosed with MetS, as early as <1 year postpartum.
Our study also demonstrated that offspring exposed to GDM in utero have a twofold increased risk of developing MetS. GDM promotes a hyperinsulinemic environment to allow increased nutrient delivery to the fetus, thereby increasing fetal growth and body mass resulting in macrosomia which may persist as obesity throughout childhood and adolescence [88]. This idea pertains to “The Barker Hypothesis” which states that adverse nutrition in early life increases the likelihood of developing metabolic risk factors [95]. We have recently shown in a meta-analysis that those exposed to GDM in utero have higher SBP, BMI z-score, and blood glucose compared to those not exposed to GDM in utero [17]. Previous studies have also shown that juvenile T2DM is significantly associated with exposure to GDM in utero [96, 97], therefore highlighting the need for weight management and lifestyle guidance throughout childhood and adolescence for this group. It is important to note that there were only four eligible studies for the meta-analysis on offspring of pregnancies complicated by GDM. We believe this is influenced by the lack of consensus on a definition of MetS in childhood. An IDF recommended definition for the diagnosis of MetS in children older than 6 years of age does exist, but this definition is not universally used [98]. Furthermore, obesity as measured by BMI is not an accurate measure, as BMI varies greatly based on the muscle mass and fat mass, hence it is accurate for fatter children but not for those who are lean. BMI z-score is a more appropriate measure as it adjusts for age and gender [99]. Only one study assessed the MetS z-score, which adjusts for age and gender [82]. Considering the increasing rate of childhood obesity, a clear definition of MetS is required that can accurately account for childhood adiposity and adjust for important factors such as age, gender, weight distribution, and puberty.
We also observed that the risk for MetS in pregnancy was increased among women who were diagnosed with GDM compared to normoglycaemic women (RR 20.51). There are studies that have investigated the association between individual components of MetS including dyslipidaemia and obesity and the risk of developing GDM [100,101,102]. Gunderson et al. showed that BMI and waist circumference were associated with increased risks for GDM after adjusting for lipids, fasting glucose, and insulin [102]. Studies by Grieger and Chatzi showed a threefold increased risk of GDM for women diagnosed with MetS in early pregnancy [13, 39]. It is difficult to diagnose MetS in pregnancy due to hemodynamic and inflammatory changes that occur during the first trimester of pregnancy, as SBP and maternal lipids decrease during this time [43, 103]. Furthermore, placental and maternal hormones during pregnancy promote weight gain and also result in altered fat distribution in both healthy pregnancies and those complicated by GDM [104]. Therefore, these results signify a need for further research in large pregnancy cohorts.
Conclusion
Pregnant women with GDM are at a higher risk of developing MetS during pregnancy. Furthermore, women who experience GDM have an increased risk of developing MetS later in life. They may develop MetS as early as <1 year postpartum. Children born to pregnancies complicated by GDM are also at increased risk of developing MetS in later life. This review signifies the importance of considering GDM in CVD risk stratification, thus allowing an opportunity for primordial prevention. Based on our findings, pre-conceptional management of cardiometabolic risk factors may be useful to reduce the risk of both GDM and MetS. Furthermore, it will be beneficial to screen women who experience GDM and children born to pregnancies complicated by GDM to detect modifiable CVD risk factors.
Abbreviations
- MetS:
-
metabolic syndrome
- CVD:
-
cardiovascular disease
- GDM:
-
gestational diabetes mellitus
References
International Diabetes Federation, IDF Diabetes Atlas (International Diabetes Federation, Belgium, 2017)
P.H. Andraweera, G.A. Dekker, M. Arstall, T. Bianco-Miotto, C.T. Roberts, Complications of pregnancy and future cardiovascular risk. in Encylopedia of Cardiovascular Research and Medicine, (eds D.B. Sawyer, R.S. Vasan) vol. 1 (Oxford, The Netherlands, 2018), pp. 643–650
L. Bellamy, J.P. Casas, A.D. Hingorani, D. Williams, Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 373(9677), 1773–1779 (2009). https://doi.org/10.1016/s0140-6736(09)60731-5
A. Lorenzo-Almorós, T. Hang, C. Peiró, L. Soriano-Guillén, J. Egido, J. Tuñón, Ó. Lorenzo, Predictive and diagnostic biomarkers for gestational diabetes and its associated metabolic and cardiovascular diseases. Cardiovasc. Diabetol. 18(1), 140 (2019). https://doi.org/10.1186/s12933-019-0935-9
K.G. Alberti, P.Z. Zimmet, Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 15(7), 539–553 (1998). https://doi.org/10.1002/(sici)1096-9136(199807)15:7<539::Aid-dia668>3.0.Co;2-s
Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP), Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 285(19), 2486–2497 (2001). https://doi.org/10.1001/jama.285.19.2486
C.K. Kramer, S. Campbell, R. Retnakaran, Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia 62(6), 905–914 (2019). https://doi.org/10.1007/s00125-019-4840-2
J. Fan, Y. Song, Y. Chen, R. Hui, W. Zhang, Combined effect of obesity and cardio-metabolic abnormality on the risk of cardiovascular disease: a meta-analysis of prospective cohort studies. Int. J. Cardiol. 168(5), 4761–4768 (2013). https://doi.org/10.1016/j.ijcard.2013.07.230
P.W. Wilson, W.B. Kannel, H. Silbershatz, R.B. D’Agostino, Clustering of metabolic factors and coronary heart disease. Arch. Intern. Med. 159(10), 1104–1109 (1999). https://doi.org/10.1001/archinte.159.10.1104
J.F. Plows, J.L. Stanley, P.N. Baker, C.M. Reynolds, M.H. Vickers, The pathophysiology of gestational diabetes mellitus. Int. J. Mol. Sci. 19(11) (2018). https://doi.org/10.3390/ijms19113342
P. Andraweera, G. Dekker, M. Arstall, T. Bianco-Miotto, C. Roberts, Complications of pregnancy and future cardiovascular risk. in Encyclopedia of Cardiovascular Research and Medicine, (eds D.B. Sawyer, R.S. Vasan) vol. 1 (Elsevier, Oxford The Netherlands, 2018)
F.K. Welty, A. Alfaddagh, T.K. Elajami, Targeting inflammation in metabolic syndrome. Transl. Res. 167(1), 257–280 (2016). https://doi.org/10.1016/j.trsl.2015.06.017
J.A. Grieger, T. Bianco-Miotto, L.E. Grzeskowiak, S.Y. Leemaqz, L. Poston, L.M. McCowan, L.C. Kenny, J.E. Myers, J.J. Walker, G.A. Dekker, C.T. Roberts, Metabolic syndrome in pregnancy and risk for adverse pregnancy outcomes: a prospective cohort of nulliparous women. PLoS Med. 15(12), e1002710 (2018). https://doi.org/10.1371/journal.pmed.1002710
S.L. Kjos, R.K. Peters, A. Xiang, O.A. Henry, M. Montoro, T.A. Buchanan, Predicting future diabetes in Latino women with gestational diabetes. Utility of early postpartum glucose tolerance testing. Diabetes 44(5), 586–591 (1995). https://doi.org/10.2337/diab.44.5.586
Y. Xu, S. Shen, L. Sun, H. Yang, B. Jin, X. Cao, Metabolic syndrome risk after gestational diabetes: a systematic review and meta-analysis. PLoS ONE 9(1), e87863 (2014). https://doi.org/10.1371/journal.pone.0087863
D.R. Coustan, L.P. Lowe, B.E. Metzger, A.R. Dyer; International Association of Diabetes and Pregnancy Study Groups, The hyperglycemia and adverse pregnancy outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. Am. J. Obst. Gynecol. 202(6), 654.e651–654.e6546 (2010). https://doi.org/10.1016/j.ajog.2010.04.006
M.M. Pathirana, Z.S. Lassi, C.T. Roberts, P.H. Andraweera, Cardiovascular risk factors in offspring exposed to gestational diabetes mellitus in utero: systematic review and meta-analysis. J. Dev. Orig. Health Dis. 1–18 (2020). https://doi.org/10.1017/s2040174419000850
E. Di Angelantonio, N. Sarwar, P. Perry, S. Kaptoge, K.K. Ray, A. Thompson, A.M. Wood, S. Lewington, N. Sattar, C.J. Packard, R. Collins, S.G. Thompson, J. Danesh, Major lipids, apolipoproteins, and risk of vascular disease. JAMA 302(18), 1993–2000 (2009). https://doi.org/10.1001/jama.2009.1619
Y. Yano, J. Stamler, D.B. Garside, M.L. Daviglus, S.S. Franklin, M.R. Carnethon, K. Liu, P. Greenland, D.M. Lloyd-Jones, Isolated systolic hypertension in young and middle-aged adults and 31-year risk for cardiovascular mortality: the Chicago Heart Association Detection Project in Industry study. J. Am. Coll. Cardiol. 65(4), 327–335 (2015). https://doi.org/10.1016/j.jacc.2014.10.060
J.S. Son, S. Choi, K. Kim, S.M. Kim, D. Choi, G. Lee, S.M. Jeong, S.Y. Park, Y.Y. Kim, J.M. Yun, S.M. Park, Association of Blood Pressure Classification in Korean Young Adults According to the 2017 American College of Cardiology/American Heart Association Guidelines With Subsequent Cardiovascular Disease Events. JAMA 320(17), 1783–1792 (2018). https://doi.org/10.1001/jama.2018.16501
A. Umer, G.A. Kelley, L.E. Cottrell, P. Giacobbi Jr., K.E. Innes, C.L. Lilly, Childhood obesity and adult cardiovascular disease risk factors: a systematic review with meta-analysis. BMC Public Health 17(1), 683 (2017). https://doi.org/10.1186/s12889-017-4691-z
D. Moher, A. Liberati, J. Tetzlaff, D.G. Altman, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535 (2009). https://doi.org/10.1136/bmj.b2535
B.E. Metzger, S.G. Gabbe, B. Persson, T.A. Buchanan, P.A. Catalano, P. Damm, A.R. Dyer, Ad. Leiva, M. Hod, J.L. Kitzmiler, L.P. Lowe, H.D. McIntyre, J.J.N. Oats, Y. Omori, M.I. Schmidt, International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33(3), 676 (2010). https://doi.org/10.2337/dc09-1848
National Diabetes Data Group, Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 28(12), 1039–1057 (1979). https://doi.org/10.2337/diab.28.12.1039
M.W. Carpenter, D.R. Coustan, Criteria for screening tests for gestational diabetes. Am. J. Obstet. Gynecol. 144(7), 768–773 (1982). https://doi.org/10.1016/0002-9378(82)90349-0
American Diabetes Association, Standards of medical care in diabetes-2007. Diabetes Care 30(Suppl 1), S4–S41 (2007). https://doi.org/10.2337/dc07-S004
P.R. Rani, J. Begum, Screening and diagnosis of gestational diabetes mellitus, where do we stand. J. Clin. Diagn. Res. 10(4), QE01–QE04 (2016). https://doi.org/10.7860/jcdr/2016/17588.7689
J.B. O’Sullivan, C.M. Mahan, Criteria for the oral glucose tolerance test in pregnancy. Diabetes 13, 278–285 (1964)
Current Care Guidelines, Working group set up by the Finnish Medical Society Duodecim, the Medical Advisory Board of the Finnish Diabetes Association and the Finnish Gynecological Association. 2013. www.kaypahoito.fi
E. Wender-Ozegowska, M. Sporna, A. Sporna, J. Brazert, A. Zawiejska, Components of metabolic syndrome (MS) in women after gestational diabetes (GDM). J. Diabetes 1, A286–A287 (2009). https://doi.org/10.1111/j.1753-0407.2009.00020.x
T. Hansen, H. Vestergaard, Increasing incidence of diabetes after gestational diabetes. Diabetes Care 27(5), 1194–1199 (2004). https://doi.org/10.2337/diacare.27.5.1194
K.G.M.M. Alberti, P. Zimmet, J. Shaw, The metabolic syndrome—a new worldwide definition. The Lancet 366(9491), 1059–1062 (2005). https://doi.org/10.1016/S0140-6736(05)67402-8
S.M. Grundy, J.I. Cleeman, S.R. Daniels, K.A. Donato, R.H. Eckel, B.A. Franklin, D.J. Gordon, R.M. Krauss, P.J. Savage, S.C. Smith Jr., J.A. Spertus, F. Costa, Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112(17), 2735–2752 (2005). https://doi.org/10.1161/circulationaha.105.169404
National Institutes of Health, Quality assessment tool for observational cohort and cross-sectional studies (2014), https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort. Accessed 14 Apr 2020
S. Bo, G. Menato, M.L. Gallo, C. Bardelli, A. Lezo, A. Signorile, R. Gambino, M. Cassader, M. Massobrio, G. Pagano, Mild gestational hyperglycemia, the metabolic syndrome and adverse neonatal outcomes. Acta Obstet. Gynecol. Scand. 83(4), 335–340 (2004). https://doi.org/10.1111/j.0001-6349.2004.00314.x
B. Dane, F. Ustaoǧlu, Y. Yildirim, Y. Döventaş, C. Dane, A. Çetin, M. Yenigün, Are the criteria of metabolic syndrome associated with pregnancy complications? Turk Jinekoloji ve Obstetrik Dernegi Dergisi 8(2), 100–106 (2011)
C.A. Negrato, L. Jovanovic, A. Rafacho, M.A. Tambascia, B. Geloneze, A. Dias, M.V. Rudge, Association between different levels of dysglycemia and metabolic syndrome in pregnancy. Diabetol. Metab. Syndr. 1(1), 3–3 (2009). https://doi.org/10.1186/1758-5996-1-3
F. Zaman, S. Nouhjah, H. Shahbazian, N. Shahbazian, S.M. Latifi, A. Jahanshahi, Risk factors of gestational diabetes mellitus using results of a prospective population-based study in Iranian pregnant women. Diabetes Metab. Syndr. 12(5), 721–725 (2018). https://doi.org/10.1016/j.dsx.2018.04.014
L. Chatzi, E. Plana, A. Pappas, D. Alegkakis, P. Karakosta, V. Daraki, C. Tsatsanis, A. Kafatos, A. Koutis, M. Kogevinas, Metabolic syndrome in early pregnancy and risk of gestational diabetes mellitus. Diabetologia 52(S1), S457 (2009). https://doi.org/10.1007/s00125-009-1445-1
M. Migda, M.S. Migda, B. Migda, P. Krzyżanowska, E. Wender-Ożegowska, Components of metabolic syndrome in the first trimester of pregnancy as predictors of adverse perinatal outcome. Ginekol. Pol. 87(9), 644–650 (2016). https://doi.org/10.5603/GP.2016.0060
R. Retnakaran, S.W. Wen, H. Tan, C. Ye, M. Shen, G.N. Smith, M.C. Walker, Pregravid metabolic syndrome and risk of adverse outcomes in pregnancy: a preconception cohort study. Diabetes 68 (2019). https://doi.org/10.2337/db19-1419-P
T. Costacou, Z. Bosnyak, G.F. Harger, N. Markovic, N. Silvers, T.J. Orchard, Postpartum adiponectin concentration, insulin resistance and metabolic abnormalities among women with pregnancy-induced disturbances. Prev. Cardiol. 11(2), 106–115 (2008). https://doi.org/10.1111/j.1751-7141.2008.07512.x
P. Soma-Pillay, C. Nelson-Piercy, H. Tolppanen, A. Mebazaa, Physiological changes in pregnancy. Cardiovasc. J. Afr. 27(2), 89–94 (2016). https://doi.org/10.5830/CVJA-2016-021
B. Akinci, A. Celtik, S. Genc, S. Yener, T. Demir, M. Secil, L. Kebapcilar, S. Yesil, Evaluation of postpartum carbohydrate intolerance and cardiovascular risk factors in women with gestational diabetes. Gynecol. Endocrinol. 27(5), 361–367 (2011). https://doi.org/10.3109/09513590.2010.492885
M. Albareda, A. Caballero, G. Badell, J. Rodríguez-Espinosa, J. Ordóñez-Llanos, A. de Leiva, R. Corcoy, Metabolic syndrome at follow-up in women with and without gestational diabetes mellitus in index pregnancy. Metabolism 54(8), 1115–1121 (2005). https://doi.org/10.1016/j.metabol.2005.03.017
B. Edalat, F. Sharifi, Z. Badamchizadeh, A. Hossein-Nezhad, B. Larijani, M. Mirarefin, H. Fakhrzadeh, Association of metabolic syndrome with inflammatory mediators in women with previous gestational diabetes mellitus. J. Diabetes Metab. Disord. 12(1), 8 (2013). https://doi.org/10.1186/2251-6581-12-8
S. Bo, G. Menato, C. Botto, I. Cotrino, C. Bardelli, R. Gambino, M. Cassader, M. Durazzo, A. Signorile, M. Massobrio, G. Pagano, Mild gestational hyperglycemia and the metabolic syndrome in later life. Metab. Syndr. Relat. Disord. 4(2), 113–121 (2006). https://doi.org/10.1089/met.2006.4.113
D.B. Carr, K.M. Utzschneider, R.L. Hull, J. Tong, T.M. Wallace, K. Kodama, J.B. Shofer, S.R. Heckbert, E.J. Boyko, W.Y. Fujimoto, S.E. Kahn, Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care 29(9), 2078–2083 (2006)
A. Derbent, A. Kargili, C. Koca, I.I. Gümüş, S. Sevgili, S. Smavli, F. Karakurt, N.O. Turhan, Serum platelet-activating factor acetylhydrolase activity: relationship with metabolic syndrome in women with history of gestational diabetes mellitus. Gynecol. Endocrinol. 27(2), 128–133 (2011). https://doi.org/10.3109/09513590.2010.487612
G. Di Cianni, C. Lencioni, L. Volpe, A. Ghio, I. Cuccuru, G. Pellegrini, L. Benzi, R. Miccoli, S. Del Prato, C-reactive protein and metabolic syndrome in women with previous gestational diabetes. Diabetes Metab. Res. Rev. 23(2), 135–140 (2007). https://doi.org/10.1002/dmrr.661
E.P. Gunderson, D.R. Jacobs Jr, V. Chiang, C.E. Lewis, J. Feng, C.P. Quesenberry Jr, S. Sidney, Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status: a 20-year prospective study in CARDIA (Coronary Artery Risk Development in Young Adults). Diabetes 59(2), 495–504 (2010). https://doi.org/10.2337/db09-1197
H. Hakkarainen, H. Huopio, H. Cederberg, R. Voutilainen, S. Heinonen, Future risk of metabolic syndrome in women with a previous LGA delivery stratified by gestational glucose tolerance: a prospective cohort study. BMC Pregnancy Childbirth 18(1), N.PAG (2018). https://doi.org/10.1186/s12884-018-1958-z
H. Ijäs, L. Morin-Papunen, A.K. Keränen, R. Bloigu, A. Ruokonen, K. Puukka, T. Ebeling, T. Raudaskoski, M. Vääräsmäki, Pre-pregnancy overweight overtakes gestational diabetes as a risk factor for subsequent metabolic syndrome. Eur. J. Endocrinol. 169(5), 605–611 (2013). https://doi.org/10.1530/EJE-13-0412
E. Kousta, Z. Efstathiadou, N.J. Lawrence, J.A.R. Jeffs, I.F. Godsland, S.C. Barrett, C.J. Doré, A. Penny, V. Anyaoku, B.A. Millauer, E. Cela, S. Robinson, M.I. McCarthy, D.G. Johnston, The impact of ethnicity on glucose regulation and the metabolic syndrome following gestational diabetes. Diabetologia 49(1), 36–40 (2006). https://doi.org/10.1007/s00125-005-0058-6
G.V. Krishnaveni, J.C. Hill, S.R. Veena, S. Geetha, M.N. Jayakumar, C.L.S. Karat, C.H.D. Fall, Gestational diabetes and the incidence of diabetes in the 5 years following the index pregnancy in South Indian women. Diabetes Res. Clin. Pract. 78(3), 398–404 (2007). https://doi.org/10.1016/j.diabres.2007.06.002
J. Lauenborg, E. Mathiesen, T. Hansen, C. Glümer, T. Jørgensen, K. Borch-Johnsen, P. Hornnes, O. Pedersen, P. Damm, The prevalence of the metabolic syndrome in a Danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J. Clin. Endocrinol. Metab. 90(7), 4004–4010 (2005). https://doi.org/10.1210/jc.2004-1713
L.J. Li, I.M. Aris, L.L. Su, Y.S. Chong, T.Y. Wong, K.H. Tan, J.J. Wang, Effect of gestational diabetes and hypertensive disorders of pregnancy on postpartum cardiometabolic risk. Endocr. Connect. 7(3), 433–442 (2018). https://doi.org/10.1530/EC-17-0359
E. Madarász, G. Tamás, A.G. Tabák, Z. Kerényi, Carbohydrate metabolism and cardiovascular risk factors 4 years after a pregnancy complicated by gestational diabetes. Diabetes Res. Clin. Pract. 85(2), 197–202 (2009). https://doi.org/10.1016/j.diabres.2009.05.001
Z. Maghbooli, A. Hossein-Nezhad, K. Mirzaei, F. Karimi, A. Besharati, K. Omidfar, B. Larijani, Association between retinol-binding protein 4 concentrations and gestational diabetes mellitus and risk of developing metabolic syndrome after pregnancy. Reprod. Sci. 17(2), 196–201 (2010). https://doi.org/10.1177/1933719109351097
C. Mai, M. Hou, R. Chen, D. Duan, H. Xu, X. Lin, J. Wen, L. Lv, Q. Lei, J. Niu, Cardiovascular risk factors in Chinese women with a history of gestational diabetes mellitus. Int. J. Clin. Exp. Med. 8(11), 21694–21698 (2015)
E. Noctor, C. Crowe, L.A. Carmody, B. Kirwan, A. O’Dea, L.G. Glynn, B.E. McGuire, P.M. O’Shea, F.P. Dunne, ATLANTIC-DIP: prevalence of metabolic syndrome and insulin resistance in women with previous gestational diabetes mellitus by International Association of Diabetes in Pregnancy Study Groups criteria. Acta Diabetol. (2014). https://doi.org/10.1007/s00592-014-0621-z
S. Nouhjah, H. Shahbazian, N. Shahbazian, S. Jahanfar, A. Jahanshahi, B. Cheraghian, Z.D. Mohammadi, N. Ghodrati, S. Houshmandi, Early postpartum metabolic syndrome in women with or without gestational diabetes: results from Life after Gestational Diabetes Ahvaz cohort study. Diabetes Metab. Syndr. 12(3), 317–323 (2018). https://doi.org/10.1016/j.dsx.2017.12.027
R. Retnakaran, Y. Qi, P.W. Connelly, M. Sermer, B. Zinman, A.J.G. Hanley, Glucose intolerance in pregnancy and postpartum risk of metabolic syndrome in young women. J. Clin. Endocrinol. Metab. 95(2), 670–677 (2010). https://doi.org/10.1210/jc.2009-1990
M.M. Roca-Rodríguez, C. López-Tinoco, A. Fernández-Deudero, M. Murri, M.V. García-Palacios, M.A. García-Valero, F.J. Tinahones-Madueño, M. Aguilar-Diosdado, Adipokines and metabolic syndrome risk factors in women with previous gestational diabetes mellitus. Diabetes Metab. Res. Rev. 28(6), 542–548 (2012). https://doi.org/10.1002/dmrr.2313
R. Ruksasakul, T. Tharavanij, P. Sritipsukho, Metabolic syndrome in Thai women previously diagnosed with gestational diabetes. J. Med. Assoc. Thai. 99(Suppl 4), S195–S202 (2016)
Y. Shen, W. Li, J. Leng, S. Zhang, H. Liu, W. Li, L. Wang, H. Tian, J. Chen, L. Qi, X. Yang, Z. Yu, J. Tuomilehto, G. Hu, High risk of metabolic syndrome after delivery in pregnancies complicated by gestational diabetes. Diabetes Res. Clin. Pract. 150, 219–226 (2019). https://doi.org/10.1016/j.diabres.2019.03.030
W.H. Tam, X.L. Yang, J.C. Chan, G.T. Ko, P.C. Tong, R.C. Ma, C.S. Cockram, D. Sahota, M.S. Rogers, Progression to impaired glucose regulation, diabetes and metabolic syndrome in Chinese women with a past history of gestational diabetes. Diabetes Metab. Res. Rev. 23(6), 485–489 (2007)
A. Verma, C.M. Boney, R. Tucker, B.R. Vohr, Insulin resistance syndrome in women with prior history of gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 87(7), 3227–3235 (2002). https://doi.org/10.1210/jcem.87.7.8684
T. Vilmi-Kerälä, O. Palomäki, M. Vainio, J. Uotila, A. Palomäki, The risk of metabolic syndrome after gestational diabetes mellitus—a hospital-based cohort study. Diabetol Metab. Syndr. 7, 43–43 (2015). https://doi.org/10.1186/s13098-015-0038-z
A. Zawiejska, E. Wender-Ozegowska, J. Brazert, K. Sodowski, Components of metabolic syndrome and their impact on fetal growth in women with gestational diabetes mellitus. J. Physiol. Pharmacol. 59(Suppl 4), 5–18 (2008)
C.N. Wijeyaratne, R. Waduge, D. Arandara, A. Arasalingam, A. Sivasuriam, S.H. Dodampahala, A.H. Balen, Metabolic and polycystic ovary syndromes in indigenous South Asian women with previous gestational diabetes mellitus. BJOG 113(10), 1182–1187 (2006). https://doi.org/10.1111/j.1471-0528.2006.01046.x
B. Akinci, A. Celtik, S. Yener, S. Yesil, Prediction of developing metabolic syndrome after gestational diabetes mellitus. Fertil. Steril. 93(4), 1248–1254 (2010). https://doi.org/10.1016/j.fertnstert.2008.12.007
B. Akinci, A. Celtik, F. Yuksel, S. Genc, S. Yener, M. Secil, M.A. Ozcan, S. Yesil, Increased osteoprotegerin levels in women with previous gestational diabetes developing metabolic syndrome. Diabetes Res. Clin. Pract. 91(1), 26–31 (2011). https://doi.org/10.1016/j.diabres.2010.09.028
E.W. Dehmer, M.A. Phadnis, E.P. Gunderson, C.E. Lewis, K. Bibbins-Domingo, S.M. Engel, M. Jonsson Funk, H. Kramer, A.V. Kshirsagar, G. Heiss, Association between gestational diabetes and incident maternal CKD: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am. J. Kidney Dis. 71(1), 112–122 (2018). https://doi.org/10.1053/j.ajkd.2017.08.015
T.B. Ferraz, R.S. Motta, C.L. Ferraz, D.M. Capibaribe, A.C. Forti, A.R. Chacra, C-reactive protein and features of metabolic syndrome in Brazilian women with previous gestational diabetes. Diabetes Res. Clin. Pract. 78(1), 23–29 (2007). https://doi.org/10.1016/j.diabres.2007.01.025
E.P. Gunderson, D.R. Jacobs, V. Chiang, C.E. Lewis, J. Feng, C.P. Quesenberry, S. Sidney, Lactation and lower incidence of the metabolic syndrome in women of reproductive age by gestational diabetes mellitus: a 20-year prospective study of CARDIA women. Diabetes 58, 495–504 (2009)
C. Mai, B. Wang, J. Wen, X. Lin, J. Niu, Lipoprotein-associated phospholipase A2 and AGEs are associated with cardiovascular risk factors in women with history of gestational diabetes mellitus. Gynecol. Endocrinol. 30(3), 241–244 (2014). https://doi.org/10.3109/09513590.2013.871522
W.H. Tam, R.C.W. Ma, X. Yang, G.T.C. Ko, T.T.H. Lao, M.H.M. Chan, C.W.K. Lam, C.S. Cockram, J.C.N. Chan, Cardiometabolic risk in Chinese women with prior gestational diabetes: a 15-year follow-up study. Gynecol. Obstet. Invest. 73(2), 168–176 (2012). https://doi.org/10.1159/000329339
C.M. Boney, A. Verma, R. Tucker, B.R. Vohr, Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115(3), e290–e296 (2005). https://doi.org/10.1542/peds.2004-1808
T.D. Clausen, E.R. Mathiesen, T. Hansen, O. Pedersen, D.M. Jensen, J. Lauenborg, L. Schmidt, P. Damm, Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J. Clin. Endocrinol. Metab. 94(7), 2464–2470 (2009). https://doi.org/10.1210/jc.2009-0305
M. Vääräsmäki, A. Pouta, P. Elliot, P. Tapanainen, U. Sovio, A. Ruokonen, A.-L. Hartikainen, M. McCarthy, M.-R. Järvelin, Adolescent manifestations of metabolic syndrome among children born to women with gestational diabetes in a general-population birth cohort. Am. J. Epidemiol. 169(10), 1209–1215 (2009). https://doi.org/10.1093/aje/kwp020
E. Maslova, S. Hansen, L.G. Grunnet, M. Strøm, A.A. Bjerregaard, L. Hjort, F.B. Kampmann, C.M. Madsen, A.C.B. Thuesen, B.H. Bech, T.I. Halldorsson, A.A. Vaag, C. Zhang, S.F. Olsen, Maternal glycemic index and glycemic load in pregnancy and offspring metabolic health in childhood and adolescence—a cohort study of 68,471 mother–offspring dyads from the Danish National Birth Cohort. Eur. J. Clin. Nutr. 73(7), 1049–1062 (2019). https://doi.org/10.1038/s41430-018-0316-6
L. Wu, L. Cui, W.H. Tam, R.C. Ma, C.C. Wang, Genetic variants associated with gestational diabetes mellitus: a meta-analysis and subgroup analysis. Sci. Rep. 6, 30539 (2016). https://doi.org/10.1038/srep30539
S.K. Abell, B. De Courten, J.A. Boyle, H.J. Teede, Inflammatory and other biomarkers: role in pathophysiology and prediction of gestational diabetes mellitus. Int. J. Mol. Sci. 16(6), 13442–13473 (2015). https://doi.org/10.3390/ijms160613442
J.F. Plows, J.L. Stanley, P.N. Baker, C.M. Reynolds, M.H. Vickers, The pathophysiology of gestational diabetes mellitus. Int. J. Mol. Sci. 19(11), 3342 (2018). https://doi.org/10.3390/ijms19113342
R.S. Pons, F.C. Rockett, B. de Almeida Rubin, M.L.R. Oppermann, V.L: Bosa, Risk factors for gestational diabetes mellitus in a sample of pregnant women diagnosed with the disease. Diabetol. Metab. Syndr. 7(1), A80 (2015). https://doi.org/10.1186/1758-5996-7-S1-A80
E. Jarosz, Lifestyle behaviours or socioeconomic characteristics? Gender differences in covariates of BMI in Hungary. Obes. Sci. Pract. 4(6), 591–599 (2018). https://doi.org/10.1002/osp4.316
D. Mitanchez, C. Yzydorczyk, B. Siddeek, F. Boubred, M. Benahmed, U. Simeoni, The offspring of the diabetic mother-short- and long-term implications. Best Pract. Res. Clin. Obstet. Gynaecol. 29(2), 256–269 (2015). https://doi.org/10.1016/j.bpobgyn.2014.08.004
T.A. Buchanan, A.H. Xiang, R.K. Peters, S.L. Kjos, K. Berkowitz, A. Marroquin, J. Goico, C. Ochoa, S.P. Azen, Response of pancreatic beta-cells to improved insulin sensitivity in women at high risk for type 2 diabetes. Diabetes 49(5), 782–788 (2000). https://doi.org/10.2337/diabetes.49.5.782
N.W. Shek, C.S. Ngai, C.P. Lee, J.Y. Chan, T.T. Lao, Lifestyle modifications in the development of diabetes mellitus and metabolic syndrome in Chinese women who had gestational diabetes mellitus: a randomized interventional trial. Arch. Gynecol. Obstet. 289(2), 319–327 (2014). https://doi.org/10.1007/s00404-013-2971-0
A. Ferrara, M.M. Hedderson, C.L. Albright, S.F. Ehrlich, C.P. Quesenberry Jr, T. Peng, J. Feng, J. Ching, Y. Crites, A pregnancy and postpartum lifestyle intervention in women with gestational diabetes mellitus reduces diabetes risk factors: a feasibility randomized control trial. Diabetes Care 34(7), 1519–1525 (2011). https://doi.org/10.2337/dc10-2221
R.E. Ratner, C.A. Christophi, B.E. Metzger, D. Dabelea, P.H. Bennett, X. Pi-Sunyer, S. Fowler, S.E. Kahn, Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J. Clin. Endocrinol. Metab. 93(12), 4774–4779 (2008). https://doi.org/10.1210/jc.2008-0772
C. Song, J. Li, J. Leng, R.C. Ma, X. Yang, Lifestyle intervention can reduce the risk of gestational diabetes: a meta-analysis of randomized controlled trials. Obes. Rev. 17(10), 960–969 (2016). https://doi.org/10.1111/obr.12442
AIHW, A picture of overweight and obesity in Australia (2017)
D.J. Barker, The developmental origins of adult disease. J. Am. Coll. Nutr. 23(6 Suppl), 588S–595S (2004). https://doi.org/10.1080/07315724.2004.10719428
A.L. Blotsky, E. Rahme, M. Dahhou, M. Nakhla, K. Dasgupta, Gestational diabetes associated with incident diabetes in childhood and youth: a retrospective cohort study. CMAJ 191(15), E410–E417 (2019). https://doi.org/10.1503/cmaj.181001
J. Halipchuk, B. Temple, A. Dart, D. Martin, E.A.C. Sellers, Prenatal, obstetric and perinatal factors associated with the development of childhood-onset type 2 diabetes. Can. J. Diabetes 42(1), 71–77 (2018). https://doi.org/10.1016/j.jcjd.2017.04.003
International Diabetes Federation, The IDF consensus definition of the metabolic syndrome in children and adolescents (2007). Accessed 5 May 2020
D.S. Freedman, B. Sherry, The validity of BMI as an indicator of body fatness and risk among children. Pediatrics 124(Suppl 1), S23–S34 (2009). https://doi.org/10.1542/peds.2008-3586E
E.S. Han, R.M. Krauss, F. Xu, S.B. Sridhar, A. Ferrara, C.P. Quesenberry, M.M. Hedderson, Prepregnancy adverse lipid profile and subsequent risk of gestational diabetes. J. Clin. Endocrinol. Metab. 101(7), 2721–2727 (2016). https://doi.org/10.1210/jc.2015-3904
Y.M. Wei, H.X. Yang, W.W. Zhu, X.Y. Liu, W.Y. Meng, Y.Q. Wang, L.X. Shang, Z.Y. Cai, L.P. Ji, Y.F. Wang, Y. Sun, J.X. Liu, L. Wei, Y.F. Sun, X.Y. Zhang, T.X. Luo, H.X. Chen, L.J. Yu, Risk of adverse pregnancy outcomes stratified for pre-pregnancy body mass index. J. Matern. Fetal. Neonatal. Med. 29(13), 2205–2209 (2016). https://doi.org/10.3109/14767058.2015.1081167
E.P. Gunderson, C.P. Quesenberry Jr., D.R. Jacobs Jr, J. Feng, C.E. Lewis, S. Sidney, Longitudinal study of prepregnancy cardiometabolic risk factors and subsequent risk of gestational diabetes mellitus: the CARDIA study. Am. J. Epidemiol. 172(10), 1131–1143 (2010). https://doi.org/10.1093/aje/kwq267
N.F. Butte, Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am. J. Clin. Nutr. 71(5 Suppl), 1256S–1261S (2000). https://doi.org/10.1093/ajcn/71.5.1256s
D.E. Smith, C.E. Lewis, J.L. Caveny, L.L. Perkins, G.L. Burke, D.E. Bild, Longitudinal changes in adiposity associated with pregnancy. The CARDIA Study. Coronary Artery Risk Development in Young Adults Study. JAMA 271(22), 1747–1751 (1994)
Funding
M.M.P. is funded by Faculty of Health and Medical Sciences Divisional Scholarship from the University of Adelaide. Z.S.L. is supported by an NHMRC Public Health Early Career Fellowship (GNT1141382).
Author information
Authors and Affiliations
Contributions
M.M.P., Z.S.L., M.A.A., C.T.R., and P.H.A. designed and conceptualized this particular study. Z.S.L. designed and performed the literature search. M.M.P., Z.S.L., A.A., and P.H.A. were involved in screening and selecting the included studies. M.M.P. performed the meta-analysis with expert advice from Z.S.L. The original manuscript was drafted by M.M.P. All authors critically reviewed and revised the manuscript and approved the final version. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pathirana, M.M., Lassi, Z.S., Ali, A. et al. Association between metabolic syndrome and gestational diabetes mellitus in women and their children: a systematic review and meta-analysis. Endocrine 71, 310–320 (2021). https://doi.org/10.1007/s12020-020-02492-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02492-1