Abstract
Purpose
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have been suggested to be associated with an increased risk of pancreatitis and pancreatic cancer. The aim of this meta-analysis was to collect data from large-scale cardiovascular outcome trials (CVOTs) to assess the effect of GLP-1RAs on the incidence of acute pancreatitis and pancreatic cancer.
Methods
Database of Medline, Embase, and the Cochrane Central Register of Controlled Trials were extensively searched up to October 10, 2019. Randomized controlled trials were eligible if they compared GLP-RA with placebo as add-on therapy to standard care in T2DM patients, and reported outcomes required for cardiovascular safety studies and events of acute pancreatitis and/or pancreatic cancer. Peto odds ratio (OR) with 95% confidence interval (CI) was calculated for acute pancreatitis and pancreatic cancer.
Results
Seven CVOTs enrolling 56,004 patients with T2DM were identified, with a median follow-up time ranging from 1.3 to 5.4 years. A total of 180 cases of acute pancreatitis and 108 cases of pancreatic cancer were reported. The risk of either acute pancreatitis or pancreatic cancer with GLP-1-RA treatment was not significantly different from that observed in placebo arm (Peto OR [95% CI] 1.05 [0.78–1.40], P = 0.76, and 1.12 [0.77–1.63], P = 0.56, respectively), and the results remained robust to sensitivity analyses.
Conclusion
Pooled analysis of CVOTs did not suggest any increased risk of either acute pancreatitis or pancreatic cancer with GLP-1RA treatment in T2DM patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is currently a global epidemic affecting 463 million people worldwide [1], and is found to be more prevalent among patients with pancreatic diseases compared with those without pancreatic diseases [2]. Moreover, patients with type 2 diabetes mellitus (T2DM) are at high risk of developing pancreatic events including pancreatitis and pancreatic cancer, with high mortality rates [3,4,5,6]. The management of diabetes will get more complex and challenging in the presence of coexisting pancreatic diseases, so it is important that antidiabetic agents did not increase the risk of developing pancreatic events.
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) mimic the action of native GLP-1 as an incretin hormone. In response to meal challenges, GLP-1RAs exert a glucose-dependent hypoglycemic activity via potentiation of insulin secretion, suppression of postprandial glucagon excursions, inhibition of gastric emptying and intestinal mobility, induction of satiety, and promotion of weight loss. GLP-1RAs show clinical advantages in improving glycemic control and weight management with minimal risks of hypoglycemia and fluid retention. Furthermore, encouraging results from several large-scale cardiovascular outcome trials (CVOTs) indicated that GLP-1RAs could reduce the risk of major adverse cardiovascular events (MACE) in T2DM patients who were at high cardiovascular risk [7,8,9,10,11,12,13]. However, some unfavorable findings and a lack of adequate evidence on their long-term safety caused concerns about their risks of pancreatic events [14,15,16,17,18]. Postmarketing reports and case-control studies indicated excessive risks of pancreatitis and pancreatic cancer in patients using exenatide compared with nonusers [14, 16], while other studies reported no increased risks of pancreatic events [19,20,21]. The inconsistent findings and methodological limitations underlying observational studies impaired the strength of the evidence.
Data derived from randomized controlled trials (RCTs) is considered a favorable source of information with minimal confounding bias. However, given the relatively low incidence of pancreatic cancer in patients T2DM (though more frequent than in the general population), no individual trial has the statistical power to disclose the difference, if any. Therefore, meta‐analyses pooling data from RCTs provide an alternative. However, prior meta-analyses of incretin-based therapy did not address the question consistently [22, 23]. Moreover, the included trials in some meta-analyses were of relatively small sample sizes and short follow-up durations.
CVOTs of GLP-1RAs enrolled a large number of participants with prolonged follow-up duration. Moreover, in most CVOTs, the adverse events of acute pancreatitis and pancreatic cancer were adjudicated by an external safety committee blinded to the treatment assignment. A previous meta-analysis of four CVOTs, LEADER [8], ELIXA [10], EXSCEL [11], and SUSTAIN-6 [7], showed that GLP-1RA therapy was not associated with an elevated risk of pancreatic events, but the most recently released data of Harmony Outcomes [9], REWIND [13], and PIONEER 6 [12], which would provide another substantial body of data, were not included in that study [24]. Therefore, an updated meta-analysis aimed at evaluating the risks of acute pancreatitis and pancreatic cancer associated with GLP‐1RAs in patients with type 2 diabetes, was performed by collecting data from all these available CVOTs.
Methods
Data sources and searches
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [25]. The PRISMA checklist and protocol are provided in the supplementary data. All available randomized clinical trials of GLP-1RAs in T2DM patients were searched in Medline/Embase/ Cochrane Central Register of Controlled Trials, up to October 10, 2019, using Medical Subject Heading terms and keywords related to “glucagon-like peptide-1 receptor agonist” (“exenatide”, “liraglutide”, “lixisenatide”, “albiglutide”, “dulaglutide”, “semaglutide”, and “taspoglutide”), “major adverse cardiovascular events” (“myocardial infarction”, “stroke”, “cardiovascular mortality”, “heart failure”, and “death”), and “type 2 diabetes mellitus”. Detailed search strategies were provided in Supplementary Table 1. The reference lists of eligible trials, prior reviews, and meta-analyses were manually searched for eligibility to supplement the computer search.
Study selection
Studies meeting the following inclusion criteria were eligible: (1) randomized clinical trials that compared the efficacy of GLP-1RAs with that of placebo; (2) enrolling only adults with T2DM; (3) reporting primary outcomes required by regulatory agencies for cardiovascular safety studies in diabetes (cardiovascular mortality, nonfatal myocardial infarction or nonfatal stroke); (4) providing an estimate on adverse events of acute pancreatitis and/or pancreatic cancer; (5) published in English. Editorials, commentaries, letters, articles with no aim outcome, and animal studies were excluded.
Data extraction and quality assessment
Results of trials reported in the publications and their supplementary materials served as the primary source of information. When multiple reports describing the same population were identified, the most informative one was selected. The data extracted from each study were as follows: the first author, year of publication, trial duration, baseline characteristics of subjects [sample size, mean age, percentage of females, diabetes duration, and glycated hemoglobin (HbA1c)], intervention, and the number of outcomes of interest (acute pancreatitis and/or pancreatic cancer).
The Cochrane Collaboration’ tool was used for assessing the risk of bias of the included RCTs, based on the following domains: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other source of bias. The risk of bias was rated as low, high, or unclear.
Quality of evidence was assessed using the grading of recommendations assessment, development, and evaluation (GRADE) system for the two pancreatic safety outcomes respectively, rated as high, moderate, low, or very low [26]. RCTs starts with high quality, but can be downgraded for risk of bias, imprecision, inconsistency, indirectness, and publication bias, or can also be rated up for a large magnitude of effect, a dose–response gradient, or presence of plausible confounders or other biases that would reduce a demonstrated effect.
Two reviewers (C.C. and S.Y.) independently extracted relevant data using a standardized format, and evaluated the risk of bias and the methodological quality of the included studies. Any discrepancy was resolved by consensus or by the third investigator (Z.Z.).
Data synthesis and analysis
The principal outcome of this analysis was the effect of GLP-1RAs, compared with placebo, on acute pancreatitis and pancreatic cancer. The outcome data were pooled using Peto’s method to account for low event rates [27], and therefore, the risks of these pancreatic events were reported with Peto odds ratios (ORs) and 95% confidence interval (CIs). Chi-square test and I2 statistic were used to assess the heterogeneity between studies, with I2 values of <25%, ≥25% and <75%, and ≥75% described as low, moderate, and high heterogeneity, respectively [28]. For each principle outcome, we carried out sensitivity analyses by using alternative effect measures (OR vs. risk ratio [RR]), pooling methods (Peto vs. Mantel–Haenszel method), and analysis models (random vs. fixed effect) to ensure the reliability of the results. We also performed a post hoc sensitivity analysis excluding one trial (Harmony Outcomes) [9] without adjudication of events of pancreatic cancer. Publication bias was assessed using Egger’s regression test. Data analyses were performed using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK).
Results
Search results
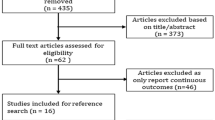
A total of 1435 citations were retrieved from the online database search. After automatic and manual deduplication, 1085 trials were screened for eligibility. After reviewing the titles and abstracts, 150 potentially eligible studies remained and their full texts were retrieved. Finally, we identified seven eligible studies (Fig. 1) [7,8,9,10,11,12,13]. The characteristics of the included studies were summarized in Table 1. The trials have been published between 2015 and 2019, with two studies published in 2019. All trials were of multicenter, parallel-group, double-blind design, and their median follow-up time ranged from 1.3 to 5.4 years. The average age of participants ranged from 60.3 to 66.2 years, and the mean diabetes duration and baseline HbA1c level ranged from 9.3 to 14.9 years and from 7.3% to 8.7%, respectively.
Intervention and the risk of bias
A total of 56,004 patients with T2DM who were on a standard-care regimen were randomly assigned to receive GLP-1RA or placebo. In all trials included, three- or four-point MACE was the primary outcome, whereas acute pancreatitis were reported as a prespecified adverse event of interest based on the assessment of an independent safety adjudication committee in a blinded manner, and pancreatic cancer was reported to be adjudicated in five of the included trials. Subcutaneous semaglutide [7], once-weekly exenatide [11], albiglutide [9], dulaglutide [13], liraglutide [8], and lixisenatide [10] were evaluated in six studies, while one trial compared once-daily oral semaglutide with placebo [12].
According to the Cochrane Collaboration’s tool, no major risk of bias was detected in any of the included study (Supplementary Fig. 1).
Acute pancreatitis
All of the seven trials fulfilling the inclusion criteria reported information on acute pancreatitis, with a safety population of 27,948 and 27,984 patients in GLP-1-RA and placebo groups, respectively. The number of reported cases of acute pancreatitis was 92 for GLP-1RAs and 88 for placebo. The definition of acute pancreatitis adopted in each CVOT was provided in Supplementary Table 2.
I2 was 5%, indicating low heterogeneity among studies in terms of the report of acute pancreatitis. No obvious publication bias for acute pancreatitis was revealed by Egger’s test (P = 0.24). The risk of acute pancreatitis associated with GLP-1RA treatment was comparable to that observed in placebo group (Peto OR [95% CI] 1.05 [0.78–1.40], P = 0.76) (Fig. 2). The result was confirmed in the sensitivity analyses (Supplementary Table 3). Using the GRADE system, we rated the quality of evidence as moderate, mainly owing to imprecision, shown in the summary of findings table (Supplementary Table 4).
Pancreatic cancer
Out of the seven eligible trials, one reported no information on pancreatic cancer, and the principal analysis was performed in the remaining six trials. Moderate heterogeneity was observed among trials regarding the report of pancreatic cancer (I2, 49%; P = 0.08) (Fig. 3). However, we did not perform any subgroup analysis because of the small number of the studies included. There was no relevant publication bias as the Egger’s test showed P = 0.27. Respectively, 57 and 51 cases of pancreatic cancer were reported for GLP-1RA and placebo treatment. The pooled analysis showed that GLP-RA use was not associated with an elevated risk of pancreatic cancer (Peto OR 1.12 [0.77–1.63], P = 0.56). In the prespecified sensitivity analyses, the result remained robust to different meta-analysis methods, including alternative effect measures, pooling methods, and analysis models (Supplementary Table 3). Similar results were obtained in the post hoc analysis excluding one trial (Harmony Outcomes) [9] without adjudication of events of pancreatic cancer (Peto OR 1.11 [0.75–1.65], P = 0.61). Using the GRADE system, we rated the quality of evidence as low, due to heterogeneity and imprecision (Supplementary Table 4).
Discussion
This meta-analysis of seven CVOTs enrolling 56,004 patients with T2DM suggested that treatment with GLP-1RA was not associated with an increased risk of either acute pancreatitis or pancreatic cancer, compared with treatment with placebo.
A β-cell-preserving effect of GLP-1 was observed in preliminary studies, via promotion of β-cell survival, stimulation of β-cell proliferation, and enhancement of β-cell neogenesis from ductal precursors [29, 30]. The possibility of sustained proproliferative actions of GLP-1RAs raised safety concerns about the potentially unintended effects on tumor formations in both the endocrine and exocrine pancreas. One of the early alarming signals came from a pharmacovigilance report based on the Food and Drug Administration adverse event reporting system (AERS) database, which claimed the ORs for reported pancreatitis and pancreatic cancer were 10.7-fold and 2.9-fold greater, respectively, in patients treated with exenatide as compared with those treated with other antidiabetic agents, from 2004 to 2009 [14]. After that, a population-based case-control study indicated that incretin-based therapies, exenatide and sitagliptin, were associated with a higher OR of hospitalization for acute pancreatitis after adjusted for potential confounding factors [16]. However, several cohort studies did not detect a signal of risk of either pancreatitis or pancreatic cancer with GLP-1RA use [19, 20]. A retrospective population-based cohort study based on the UK Clinical Practice Research Datalink reported a 1.7-fold increased risk of pancreatic cancer (hazard ration [HR] 1.67; 95% CI 1.01–2.77) in new users of incretin agents compared with users of other noninsulin antidiabetic drugs, which dropped to nonsignificance with prolonged use [18]. The authors claimed that the elevated risk was probably attributed to protopathic bias or distortions, and the presence of potential residual confounding after statistical adjustment and a lack of duration-of-use relationship prevented any definite conclusion of causality [18]. Notably, in an international, multicenter, nested case-control cohort study, the risk of pancreatic cancer tended to be lower with prolonged use of incretin-based therapy (HR 1.53, 1.07, and 0.62 for duration of use <1 year, 1–1.9 years, and ≥2 years, respectively), although the difference was not statistically significant [20].
Evidence from observational studies should be interpreted with caveat. First, when time axis was incorporated into the AERS database, regulatory authorities’ safety warnings showed a remarkable influence on the adverse events reporting (notoriety bias) [21]. In addition, GLP-1RAs are especially recommended to T2DM patients with obesity or as an add-on therapy to uncontrolled hyperglycemia in clinical practice, which might lead to between-group disequilibrium in the severity of diabetes and other risk factors in observational studies. Obesity per se, associated with higher rates of hypertriglyceridemia, gallstones, and biliary diseases, is a risk factor for pancreatitis and pancreatic cancer [6, 31]. Moreover, GLP-1RAs are usually prescribed with the exacerbation of glycemic control, which could be caused by occult pancreatic diseases. The process of treatment selection is an intrinsic source of imbalance and confounding, and adjustment for confounding factors, with statistical complexity, might result in mismatching samples between groups. Finally, pharmacy claims did not actually reflect real-world medication practice [32].
In this regard, RCTs provide a superior source of evidence because the process of randomization and a parallel-group setting minimize the impacts of confounders, and their inclusion of new users precludes the bias of combining prevalent and incident users. Considering the low incidence of pancreatic events, an individual RCT might not be powered enough to assess the risk of pancreatic issues sufficiently. Several prior meta-analyses which pooled data from RCTs did not link pancreatic safety issues with GLP-1RA therapies [23, 33, 34]. However, the included trials in some meta-analyses were of relatively small sample sizes with the possibility of chance finding not excluded, and were not long enough in follow-up durations to evaluate drug safety profiles, especially those related to cancers. Importantly, quite a proportion of trials had no clear information on the adjudication of pancreatic events and/or reported zero events, thus the possibility of misdiagnosis and underreporting being not eliminated. Though no RCT of GLP-1RAs included pancreatic safety issues as the primary outcome, reports of the pancreatic events in most CVOTs were relatively reliable because they were adjudicated in a blinded manner by an independent safety monitoring committee. Besides, with MACE defined as the primary endpoint instead of antihyperglycemic efficacy, a large set of patients were followed up for longer periods of time and were managed with a similar glycemic goal in CVOTs, providing a more parallel metabolic status for the assessment of pancreatic events. Our meta-analysis of seven CVOTs did not associate an increased risk of acute pancreatitis or pancreatic cancer with GLP-1RA treatment, in agreement with a prior meta-analysis based on only four CVOTs enrolling 33,457 patients with type 2 diabetes [24]. Importantly, we assessed the quality of the body of evidence instead of individual studies, based on the framework of the GRADE system, which was more relevant and informative to clinicians and stakeholders.
This study had several limitations. First, individuals enrolled in RCTs are highly selected and may not be well representative of T2DM patients in the general population, to some extent limiting the extrapolation of the results. Second, patients with a history of pancreatitis who are at higher risk of developing recurrent pancreatitis and/or pancreatic cancer, and patients with alcohol abuse (a well-known risk factor for pancreatitis) who usually have low adherence to treatment, are usually excluded from RCTs, and therefore selection bias could not be easily ruled out for pancreatic adverse events. Third, the durations of CVOTs were still not long enough for the evaluation of pancreatic safety profile especially for cancer surveillance. Indeed, it took nearly 12 years for initiated pancreatic intraepithelial neoplasias to develop metastatic capacity and initiate an infiltrating pancreatic carcinoma in human [35]. Finally, the number of cases who reported the outcome of pancreatitis or pancreatic cancer is relatively small when compared with that in observational studies, attributed to the nature of RCTs. Therefore, the negative finding in this meta-analysis should still be interpreted with caution, until further studies with larger sample size and longer duration provide more conclusive information about the pancreatic safety of GLP-1RA therapies in diabetic patients.
In conclusion, pooled analysis of CVOTs suggested that treatment with GLP-1RAs, compared with treatment with placebo, was not associated with an increased risk of either acute pancreatitis or pancreatic cancer in T2DM patients.
References
International Diabetes Federation. IDF diabetes atlas, 9th edn. (2019). http://www.diabetesatlas.org/across-the-globe.html. Accessed 25 Nov 2019
R. Pannala, J.B. Leirness, W.R. Bamlet, A. Basu, G.M. Petersen, S.T. Chari, Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology 134(4), 981–987 (2008). https://doi.org/10.1053/j.gastro.2008.01.039
F. Bragg, M.V. Holmes, A. Iona, Y. Guo, H. Du, Y. Chen, Z. Bian, L. Yang, W. Herrington, D. Bennett, I. Turnbull, Y. Liu, S. Feng, J. Chen, R. Clarke, R. Collins, R. Peto, L. Li, G. Z. Chen, China Kadoorie Biobank collaborative: association between diabetes and cause-specific mortality in rural and urban areas of China. JAMA 317(3), 280–289 (2017). https://doi.org/10.1001/jama.2016.19720
A. Gonzalez-Perez, R.G. Schlienger, L.A. Rodriguez, Acute pancreatitis in association with type 2 diabetes and antidiabetic drugs: a population-based cohort study. Diabetes Care 33(12), 2580–2585 (2010). https://doi.org/10.2337/dc10-0842
C.J. Girman, T.D. Kou, B. Cai, C.M. Alexander, E.A. O’Neill, D.E. Williams-Herman, L. Katz:, Patients with type 2 diabetes mellitus have higher risk for acute pancreatitis compared with those without diabetes. Diabetes Obes. Metab. 12(9), 766–771 (2010). https://doi.org/10.1111/j.1463-1326.2010.01231.x
R.A. Noel, D.K. Braun, R.E. Patterson, G.L. Bloomgren, Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care 32(5), 834–838 (2009). https://doi.org/10.2337/dc08-1755
S.P. Marso, S.C. Bain, A. Consoli, F.G. Eliaschewitz, E. Jodar, L.A. Leiter, I. Lingvay, J. Rosenstock, J. Seufert, M.L. Warren, V. Woo, O. Hansen, A.G. Holst, J. Pettersson, T. Vilsboll; S.-. Investigators, Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375(19), 1834–1844 (2016). https://doi.org/10.1056/NEJMoa1607141
S.P. Marso, G.H. Daniels, K. Brown-Frandsen, P. Kristensen, J.F. Mann, M.A. Nauck, S.E. Nissen, S. Pocock, N.R. Poulter, L.S. Ravn, W.M. Steinberg, M. Stockner, B. Zinman, R.M. Bergenstal, J.B. Buse; L.S. Committee, L.T. Investigators, Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375(4), 311–322 (2016). https://doi.org/10.1056/NEJMoa1603827
A.F. Hernandez, J.B. Green, S. Janmohamed, R.B. D’Agostino, Sr,C.B. Granger, N.P. Jones, L.A. Leiter, A.E. Rosenberg, K.N. Sigmon, M.C. Somerville, K.M. Thorpe, J.J.V. McMurray, S.Del Prato; c. Harmony Outcomes, investigators, Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 392(10157), 1519–1529 (2018). https://doi.org/10.1016/S0140-6736(18)32261-X
M.A. Pfeffer, B. Claggett, R. Diaz, K. Dickstein, H.C. Gerstein, L.V. Kober, F.C. Lawson, L. Ping, X. Wei, E.F. Lewis, A.P. Maggioni, J.J. McMurray, J.L. Probstfield, M.C. Riddle, S.D. Solomon, J.C. Tardif; E. Investigators, Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 373(23), 2247–2257 (2015). https://doi.org/10.1056/NEJMoa1509225
R.R. Holman, M.A. Bethel, R.J. Mentz, V.P. Thompson, Y. Lokhnygina, J.B. Buse, J.C. Chan, J. Choi, S.M. Gustavson, N. Iqbal, A.P. Maggioni, S.P. Marso, P. Ohman, N.J. Pagidipati, N. Poulter, A. Ramachandran, B. Zinman, A.F. Hernandez; E.S. Group, Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 377(13), 1228–1239 (2017). https://doi.org/10.1056/NEJMoa1612917
M. Husain, A.L. Birkenfeld, M. Donsmark, K. Dungan, F.G. Eliaschewitz, D.R. Franco, O.K. Jeppesen, I. Lingvay, O. Mosenzon, S.D. Pedersen, C.J. Tack, M. Thomsen, T. Vilsboll, M.L. Warren, S.C. Bain; P. Investigators, Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 381(9), 841–851 (2019). https://doi.org/10.1056/NEJMoa1901118
H.C. Gerstein, H.M. Colhoun, G.R. Dagenais, R. Diaz, M. Lakshmanan, P. Pais, J. Probstfield, J.S. Riesmeyer, M.C. Riddle, L. Ryden, D. Xavier, C.M. Atisso, L. Dyal, S. Hall, P. Rao-Melacini, G. Wong, A. Avezum, J. Basile, N. Chung, I. Conget, W.C. Cushman, E. Franek, N. Hancu, M. Hanefeld, S. Holt, P. Jansky, M. Keltai, F. Lanas, L.A. Leiter, P. Lopez-Jaramillo, E.G.Cardona Munoz, V. Pirags, N. Pogosova, P.J. Raubenheimer, J.E. Shaw, W.H. Sheu, T. Temelkova-Kurktschiev; R. Investigators, Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394(10193), 121–130 (2019). https://doi.org/10.1016/S0140-6736(19)31149-3
M. Elashoff, A.V. Matveyenko, B. Gier, R. Elashoff, P.C. Butler, Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 141(1), 150–156 (2011). https://doi.org/10.1053/j.gastro.2011.02.018
A.E. Butler, M. Campbell-Thompson, T. Gurlo, D.W. Dawson, M. Atkinson, P.C. Butler, Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 62(7), 2595–2604 (2013). https://doi.org/10.2337/db12-1686
S. Singh, H.Y. Chang, T.M. Richards, J.P. Weiner, J.M. Clark, J.B. Segal, Glucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern. Med. 173(7), 534–539 (2013). https://doi.org/10.1001/jamainternmed.2013.2720
B. Gier, A.V. Matveyenko, D. Kirakossian, D. Dawson, S.M. Dry, P.C. Butler, Chronic GLP-1 receptor activation by exendin-4 induces expansion of pancreatic duct glands in rats and accelerates formation of dysplastic lesions and chronic pancreatitis in the Kras(G12D) mouse model. Diabetes 61(5), 1250–1262 (2012). https://doi.org/10.2337/db11-1109
L.M. Knapen, J. van Dalem, Y.C. Keulemans, N.P. van Erp, M.T. Bazelier, M.L. De Bruin, H.G. Leufkens, S. Croes, C. Neef, F. de Vries, J.H. Driessen, Use of incretin agents and risk of pancreatic cancer: a population-based cohort study. Diabetes Obes. Metab. 18(3), 258–265 (2016). https://doi.org/10.1111/dom.12605
J.A. Romley, D.P. Goldman, M. Solomon, D. McFadden, A.L. Peters, Exenatide therapy and the risk of pancreatitis and pancreatic cancer in a privately insured population. Diabetes Technol. Ther. 14(10), 904–911 (2012). https://doi.org/10.1089/dia.2012.0075
L. Azoulay, K.B. Filion, R.W. Platt, M. Dahl, C.R. Dormuth, K.K. Clemens, M. Durand, D.N. Juurlink, L.E. Targownik, T.C. Turin, J.M. Paterson, P. Ernst; I. Canadian Network for Observational Drug Effect Studies, Incretin based drugs and the risk of pancreatic cancer: international multicentre cohort study. BMJ 352, i581 (2016). https://doi.org/10.1136/bmj.i581
E. Raschi, C. Piccinni, E. Poluzzi, G. Marchesini, F. De Ponti, The association of pancreatitis with antidiabetic drug use: gaining insight through the FDA pharmacovigilance database. Acta Diabetol. 50(4), 569–577 (2013). https://doi.org/10.1007/s00592-011-0340-7
I. Tkac, I. Raz, Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 diabetes. Diabetes Care 40(2), 284–286 (2017). https://doi.org/10.2337/dc15-1707
M. Monami, B. Nreu, A. Scatena, B. Cresci, F. Andreozzi, G. Sesti, E. Mannucci, Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): data from randomized controlled trials. Diabetes Obes. Metab. 19(9), 1233–1241 (2017). https://doi.org/10.1111/dom.12926
Y. Liu, Q. Tian, J. Yang, H. Wang, T. Hong, No pancreatic safety concern following glucagon-like peptide-1 receptor agonist therapies: a pooled analysis of cardiovascular outcome trials. Diabetes Metab. Res. Rev. 34(8), e3061 (2018). https://doi.org/10.1002/dmrr.3061
D. Moher, A. Liberati, J. Tetzlaff, D.G. Altman; P. Group, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8(5), 336–341 (2010). https://doi.org/10.1016/j.ijsu.2010.02.007
G.H. Guyatt, A.D. Oxman, G.E. Vist, R. Kunz, Y. Falck-Ytter, P. Alonso-Coello, H.J. Schunemann; G.W. Group, GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650), 924–926 (2008). https://doi.org/10.1136/bmj.39489.470347.AD
M.J. Bradburn, J.J. Deeks, J.A. Berlin, A. Russell Localio, Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat. Med. 26(1), 53–77 (2007). https://doi.org/10.1002/sim.2528
J.P. Higgins, S.G. Thompson, Quantifying heterogeneity in a meta-analysis. Stat. Med. 21(11), 1539–1558 (2002). https://doi.org/10.1002/sim.1186
H. Hui, A. Nourparvar, X. Zhao, R. Perfetti, Glucagon-like peptide-1 inhibits apoptosis of insulin-secreting cells via a cyclic 5’-adenosine monophosphate-dependent protein kinase A- and a phosphatidylinositol 3-kinase-dependent pathway. Endocrinology 144(4), 1444–1455 (2003). https://doi.org/10.1210/en.2002-220897
J. Zhang, Y. Tokui, K. Yamagata, J. Kozawa, K. Sayama, H. Iwahashi, K. Okita, M. Miuchi, H. Konya, T. Hamaguchi, M. Namba, I. Shimomura, J.I. Miyagawa, Continuous stimulation of human glucagon-like peptide-1 (7-36) amide in a mouse model (NOD) delays onset of autoimmune type 1 diabetes. Diabetologia 50(9), 1900–1909 (2007). https://doi.org/10.1007/s00125-007-0737-6
A.A. Gumbs, Obesity, pancreatitis, and pancreatic cancer. Obes. Surg. 18(9), 1183–1187 (2008). https://doi.org/10.1007/s11695-008-9599-3
M.A. Nauck, A critical analysis of the clinical use of incretin-based therapies: the benefits by far outweigh the potential risks. Diabetes Care 36(7), 2126–2132 (2013). https://doi.org/10.2337/dc12-2504
C. Cao, S. Yang, Z. Zhou, GLP-1 receptor agonists and risk of cancer in type 2 diabetes: an updated meta-analysis of randomized controlled trials. Endocrine (2019). https://doi.org/10.1007/s12020-019-02055-z
M. Monami, I. Dicembrini, C. Nardini, I. Fiordelli, E. Mannucci, Glucagon-like peptide-1 receptor agonists and pancreatitis: a meta-analysis of randomized clinical trials. Diabetes Res Clin. Pract. 103(2), 269–275 (2014). https://doi.org/10.1016/j.diabres.2014.01.010
S. Yachida, S. Jones, I. Bozic, T. Antal, R. Leary, B. Fu, M. Kamiyama, R.H. Hruban, J.R. Eshleman, M.A. Nowak, V.E. Velculescu, K.W. Kinzler, B. Vogelstein, C.A. Iacobuzio-Donahue, Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467(7319), 1114–1117 (2010). https://doi.org/10.1038/nature09515
Author contributions
All authors collectively designed the study. C.C. and S.Y. collected, extracted, and analyzed the data. C.C. wrote the manuscript, and Z.Z. reviewed and edited the manuscript. All authors critically revised the paper for important intellectual content and approved the final paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Cao, C., Yang, S. & Zhou, Z. GLP-1 receptor agonists and pancreatic safety concerns in type 2 diabetic patients: data from cardiovascular outcome trials. Endocrine 68, 518–525 (2020). https://doi.org/10.1007/s12020-020-02223-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02223-6