Abstract
Purpose
To find the sonographic features of follicular variant of papillary thyroid carcinoma (FV-PTC) and evaluate the diagnostic performance of 2017 ACR TI-RADS in FV-PTC.
Methods
From June 2017 to June 2019, 104 FV-PTC patients (106 nodules) and 337 classic papillary thyroid carcinoma (C-PTC) patients (343 nodules) with both sonograms and pathologic results were included. Sonographic features of FV-PTC and C-PTC were evaluated and compared, and 2017 ACR TI-RADS scores and levels were calculated.
Results
Pathologically, the incidence of Hashimoto’s thyroiditis and extrathyroidal extension was higher in the FV-PTC group. Most of both the FV-PTC and C-PTC groups were diagnosed as PTC or suspicious PTC by fine-needle aspiration biopsy (FNAB). FV-PTC tended to be isoechoic, while most nodules of C-PTC were hypoechoic or very hypoechoic. Compared with C-PTC, FV-PTC had lower percentages of a taller-than-wide shape (11.3% vs. 46.6%) and lobulated or irregular margin (33.0% vs. 61.8%), and a higher percentage of extrathyroidal extension (20.8% vs. 8.2%). FV-PTC featured macrocalcifications, whereas punctate echogenic foci were more frequently seen in the C-PTC group. Other characteristic US appearances of FV-PTC included uneven hypoechoic halo and peripheral vascularity. The mean TI-RADS score of FV-PTC cases was lower in the FV-PTC group, 11.3%, 44.3%, and 42.5% of which were categorized as TI-RADS 3, 4, and 5, respectively.
Conclusions
FV-PTC features isoechoic appearance, macrocalcifications, uneven hypoechoic halo, and peripheral vascularity on US, with lower incidences of microcalcifications and taller-than-wide shape, and tends to have lower TI-RADS scores and levels. For the nodules categorized as TI-RADS 3 or 4, the FNAB criteria should be broaden when these nodules have FV-PTC US features.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The papillary thyroid carcinoma (PTC) family of tumor is histologically defined by the presence of clear, overlapping nuclei with grooves and pseudoinclusions [1]. The variants of PTC include classic, follicular, tall cell, columnar cell, diffuse sclerosing, encapsulated, and oncocytic carcinomas [2]. Follicular variant of papillary thyroid carcinoma (FV-PTC) is the second most common subtype after the classic variant [3], accounting for about 18 to 30% of all PTC [4]. This tumor is pathologically defined as a neoplasm with the nuclear features of PTC and a predominantly follicular growth pattern [5], featuring follicles lined by cells that have PTC nuclear features. FV-PTC has presented challenges to pathologists and clinicians because it resembles papillary carcinoma in biologic behavior and all morphologic features with the exception that papillae are not present [6].
Ultrasound (US) examination of thyroid nodules is well known for its high performance in discriminating between benign and malignant lesions. As a clinically distinct entity, unlike classic papillary thyroid carcinoma (C-PTC), FV-PTC has been reported to have more benign US features than C-PTC and is often missed by radiologists [7, 8]. The US features of FV-PTC are considerably different in many ways compared with classic PTC [9]. However, the US findings showed wide variability. The inconsistent features of FV-PTC identified by small univariate analyses included to be purely solid and isoechoic, regular smooth margins, microcalcifications or mixed calcifications, a taller-than-wide shape, and halos [8, 10, 11].
US is a useful tool of risk stratification of thyroid nodules and thus used as guidance for fine-needle aspiration biopsy (FNAB) and further treatment. In 2017, ACR Thyroid Imaging Reporting and Data System (ACR TI-RADS) provided an up-to-date suggestion to guide the differential diagnosis and decisions regarding the need for FNAB of thyroid nodules [12]. In the ACR TI-RADS, thyroid nodules are classified into TR1–TR5 according to their US scores, which are based on five sonographic feature categories. Recommendations for biopsy or US follow-up are based on the nodule’s ACR TI-RADS level and its maximum diameter [13]. Studies have shown that ACR TI-RADS had high diagnostic accuracy and outperformed in the ability to avoid unnecessary thyroid nodule FNAB [14, 15], and thus ACR TI-RADS has been widely used now. However, given that the suspected rate of malignancy on US examination is lower in FV-PTC [16], the usefulness of ACR TI-RADS in the malignancy prediction of FV-PTC is still unclear. Therefore, our study aimed to find the US features of FV-PTC and evaluate the diagnostic performance of 2017 ACR TI-RADS in FV-PTC through analyzing and comparing the US images of histologically diagnosed FV-PTC and C-PTC, and comparing the TI-RADS scores and categories between the two types.
Materials and methods
This study was approved by the institutional review board, who waived the requirement for informed consent because of the retrospective nature of this study.
Patients
This retrospective analysis was based on patient data collected from the inpatients who underwent thyroidectomy in Peking University Third Hospital from June 2017 to June 2019 and had the histopathologic diagnosis of FV-PTC or C-PTC. The main thyroidectomy criteria included: (1) the nodule had a confirmed or suspicious diagnosis of thyroid malignancy by FNA; (2) the nodule was highly suspicious for malignancy on US and larger than 1 cm, if there was no pathological diagnosis. The patients were identified by searching for FV-PTC and C-PTC in an electronic medical record system. In this process, 111 patients with FV-PTC and 362 patients with C-PTC were identified. The corresponding sonograms were searched for in the picture archiving and communication system (PACS), and 104 FV-PTC patients (106 nodules) and 337 C-PTC patients (343 nodules) with both sonograms and pathologic results were identified in the end.
US examinations and image analysis
Each US image in PACS was reviewed independently by two reviewers with 8 and 15 years of experience in thyroid US, respectively, to reach a consensus on the US features. In cases of discrepancy, a third investigator with 25 years of experience in thyroid imaging reviewed the images independently and made a final decision. The images of FV-PTC and C-PTC were mixed together and arranged in chronological order. All reviewers were blinded to the initial US interpretation, clinical data, pathological results, and the results of any other examinations when analyzing the images. If the patient has more than one record of US examination, the examination closest in time to the patient’s surgery was reviewed. All US examinations were performed by one of ten radiologists with more than 3 years of experience in thyroid US, using Philips IU22, HITACHI VISION Avius, or GE Logiq 9 devices equipped with a 5–12 MHz or 8–15 MHz linear-array transducer. The US examinations included transverse and longitudinal gray-scale images of the thyroid and nodules, as well as the color Doppler images of the nodules.
All thyroid nodules were evaluated on the basis of the 2017 ACR TI-RADS. The nodule’s ACR TI-RADS level ranging from TR1 to TR5 is determined by the point total. The sonographic features in the ACR TI-RADS are categorized as benign (TR1, 0 point), not suspicious (TR2, 2 points), mildly suspicious (TR3, 3 points), moderately suspicious (TR4, 4–6 points), or highly suspicious for malignancy (TR5, 7 points or more). The categories and their points are as follows: (1) Composition: cystic or almost completely cystic, 0 points; spo.pngorm, 0 points; mixed cystic and solid, 1 point; solid or almost completely solid, 2 points. (2) Echogenicity: anechoic, 0 points; hyperechoic or isoechoic, 1 point; hypoechoic, 2 points; very hypoechoic, 3 points. (3) Shape: wider-than-tall, 0 points; taller-than-wide, 3 points. (4) Margin: smooth, 0 points; ill-defined, 0 points; lobulated or irregular, 2 points; extrathyroidal extension, 3 points. (5) Echogenic foci: none or large comet-tail artifacts, 0 points; macrocalcifications, 1 point; peripheral (rim) calcifications, 2 points; punctate echogenic foci (<1 mm), 3 points. Features in the first four categories each have a single score derived from mutually exclusive choices, whereas more than one feature may be present in the echogenic foci category [13, 17]. When assessing a nodule, the reader summed the points from each of the five categories. Other US features, including maximum diameters on US, uneven hypoechoic halo, and color Doppler signals of the nodules were also evaluated.
Medical record review
The electronic medical record of each patient with FV-PTC or C-PTC was reviewed to collect the demographic information, diagnosis of FNAB, and pathologic results after surgery, including the type of PTC, existence of lymph node metastasis and extrathyroidal extension, and whether there was a Hashimoto’s thyroiditis background. In our study, according to the 2016 new criteria, noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) was not included in FV-PTC [18].
Statistical analysis
Statistical analyses were performed using SPSS software (Version 16.0, IBM, Armonk, NY, USA). The Kolmogorov–Smirnov test was used to measure the normality of quantitative data. Normally distributed data were expressed as the mean ± standard deviation (SD), whereas nonnormally distributed data were expressed as the median ± quantile. The normally distributed data were compared between the FV-PTC and C-PTC groups using an independent sample t-test, and for the nonnormally distributed data, differences were analyzed using a Mann–Whitney U test. Qualitative data were presented as frequencies, the differences of which between the two groups were compared using the χ2-test. A p value < 0.05 was considered to indicate statistical significance.
Results
Clinicopathological characteristics
The FV-PTC group comprised 104 patients (22 men, 82 women, age range: 29–68 years, mean: 43.7 ± 13.4 years), two of whom had two nodules. The C-PTC group consisted of 93 men and 244 women with an average age of 41.3 ± 12.0 years (range: 31–72 years), and six of them had two nodules. According to the pathologic results, Hashimoto’s thyroiditis background was more likely to present in the FV-PTC group than in the C-PTC group (47.1% vs. 31.2%, p = 0.003), and the incidence of extrathyroidal extension was higher in the FV-PTC group (19.2% vs. 7.4%, p = 0.001). There were no statistically significant difference in age, sex, and lymph node metastasis between the two groups (Table 1).
Comparison of FNAB diagnosis between FV-PTC and C-PTC
Of the nodules of FV-PTC or C-PTC, there were 35 FV-PTC nodules and 132 C-PTC nodules which had FNAB diagnosis, respectively. Most of both the FV-PTC and C-PTC groups were diagnosed as PTC or suspicious PTC (82.3% vs. 93.2%, p = 0.089) by FNAB, without statistically significant difference (Table 2).
Comparison of US features between FV-PTC and C-PTC
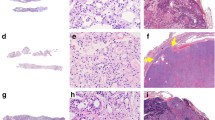
One hundred and six nodules of FV-PTC and three hundred and forty-three nodules of C-PTC were analyzed. Table 3 summarized the US features of FV-PTC and C-PTC. The maximum diameters of FV-PTC were larger than those of C-PTC (1.88 cm vs. 1.46 cm, p = 0.033). FV-PTC tended to be isoechoic, while most nodules of C-PTC were hypoechoic or very hypoechoic (Fig. 1a–c). Compared with C-PTC, FV-PTC had lower percentages of a taller-than-wide shape (11.3% vs. 46.6%, p < 0.001) and lobulated or irregular margin (33.0% vs. 61.8%, p < 0.001), which were frequently observed malignant US features, and a higher percentage of extrathyroidal extension (20.8% vs. 8.2%, p = 0.001). FV-PTC featured macrocalcifications, which were present in 50.0% of the FV-PTC group, whereas punctate echogenic foci were more frequently seen in the C-PTC group (40.2% vs. 28.3%, p = 0.029) (Fig. 1a–c). Other characteristic appearances of FV-PTC, including uneven hypoechoic halo and peripheral vascularity (>50% Doppler signals were in the periphery), were also observed (Fig. 1b–d). There were statistically significant differences in the parameters mentioned above between the two groups.
US images of FV-PTC. All these nodules were solid and isoechoic, without a taller-than-wide shape. White arrows showed macrocalcifications in the nodules (a, b). White arrowheads showed uneven hypoechoic halo around the nodules (b, c). CDFI showed peripheral vascularity (d). According to the 2017 ACR TI-RADS, the nodules in a, b, and c got the point totals of 4, 4, and 3, respectively, and were classified as TI-RADS category 4, 4, and 3, respectively
Comparison of ACR TI-RADS scores and categories
Table 4 showed ACR TI-RADS scores and categories of the FV-PTC and C-PTC groups. The mean ACR TI-RADS score of FV-PTC was lower than that of C-PTC (4 vs. 8, p < 0.001). 11.3%, 44.3%, and 42.5% of the FV-PTC group were categorized as TI-RADS 3, 4, and 5, respectively, while up to 85.2% of the C-PTC group were categorized as that suspicion group (Figs 1a–c and 2).
Comparison of ACR TI-RADS categories between FV-PTC and C-PTC. a FV-PTC. This nodule was solid, isoechoic, and smoothly marginated, without a taller-than-wide shape and echogenic foci. It was awarded 3 points and classified as TI-RADS 3. b C-PTC. This nodule was solid and very hypoechoic, with a taller-than-wide shape, irregular margin, and numerous punctate echogenic foci. It was awarded 13 points and classified as TI-RADS 5
Discussion
Clinically, FV-PTC was detected in patients around 40 years of age, with a male-to-female ratio of 1:6 [7]. In this retrospective analysis, age and sex were not significantly different between the FV-PTC and C-PTC groups, which was consistent with the previous studies [9, 11, 16]. A female predominance was also observed in the FV-PTC group, as shown in other studies. Our study found that Hashimoto’s thyroiditis background was more likely to present in the FV-PTC group, indicating a more significant association of FV-PTC with Hashimoto’s thyroiditis than that of C-PTC, which was not mentioned in the previous study.
In some previous studies, aggressive features, such as thyroid capsule invasion, extrathyroidal extension, lymph node metastasis, and disease recurrence, are considerably less common in FV-PTC than in C-PTC [9, 19]. However, our study showed a higher incidence of histologically confirmed extrathyroidal extension in the FV-PTC group, seemingly suggesting a poorer prognosis of FV-PTC. There is a possible explanation for this finding. The pathologic classification of FV-PTC has changed significantly since 2016 when noninvasive encapsulated FV-PTC was recategorized. FV-PTCs are traditionally divided into two main subtypes: infiltrative and encapsulated. According to the new classification, tumors that do not invade the tumor capsule and demonstrate vascular invasion (previously grouped as FV-PTC) are now specifically grouped separately as noninvasive follicular thyroid NIFTP [18]. Most of the recent large series comparing prognosis between C-PTC and FV-PTC were from multi-institutional databases without pathology review to evaluate whether patients included had NIFTP by the new criteria, and thus, their results could be skewed to show falsely favorable outcomes in FV-PTC patients [20].
In our study, the US feature analysis showed that the FV-PTC group had a larger average nodule size on US than C-PTC, as shown in other studies [4, 9]. The marked malignant US signs, including very hypoechoic appearance, taller-than-wide shape, lobulated or irregular margin, and punctate echogenic foci, were less frequently seen in the FV-PTC group, meaning that FV-PTC had more benign US features than C-PTC. This study also showed that FV-PTC featured macrocalcifications rather than microcalcifications, which were a marked malignant feature of C-PTC. The following theory may help explain this phenomenon: Microcalcifications have been thought to represent psammoma bodies [21], which exist in papillae. However, pathologically, FV-PTC does not have papillae [22], leading to the apparently lower incidence of punctate echogenic foci present in FV-PTC than in C-PTC. With respect to macrocalcifications, it is hypothesized that these calcifications may be secondary to tissue necrosis, hemorrhage, or both, which are more common in follicular carcinomas [23]. Because of the follicular growth pattern, we hypothesized that FV-PTC might resemble some features of follicular carcinomas and thus have a higher incidence of macrocalcifications.
In addition to the ACR TI-RADS sonographic features, we also evaluated other features of FV-PTC and found that uneven hypoechoic halo and peripheral vascularity were characteristic appearances of FV-PTC on US. These findings were in line with previous studies [7, 9]. A halo is thought to represent a capsule surrounding a nodule in resected specimens. The uneven hypoechoic halo may result from a progressing desmoplastic reaction or fibrosis of the capsule after the presence of microscopic capsular invasion [24].
Although FNAB is the mainstay and a convenient method in the evaluation of thyroid nodules, a previous study showed that the cytological diagnosis of FV-PTC was challenging due to overlapping features with both benign and malignant follicular-derived lesions [25]. However, our study showed that most of both the FV-PTC and C-PTC groups were diagnosed as PTC or suspicious PTC without statistically significant difference, meaning that FNAB is also useful in the diagnosis of FV-PTC, even though a specific diagnosis of FV-PTC was not made. FNAB is not recommended in follicular neoplasms, because it is extremely difficult to differentiate between follicular thyroid carcinoma and follicular thyroid adenoma before surgery due to the similarity of morphologies of carcinoma cells and adenoma cells obtained by FNAB. The final diagnosis of follicular neoplasms should be determined by postoperative pathological examination by the evaluation of the specific characteristics such as capsular infiltration and vascular invasion [26]. Given that FV-PTC has some sonographic features of follicular neoplasms such as isoechoic appearance and hypoechoic halo, we hypothesized that some of FV-PTCs did not undergo FNAB because of the suspicious diagnosis of follicular neoplasms. Our study showed that when the FV-PTC characteristics mentioned above were observed, FNAB was worth performing in the thyroid nodule.
In our study, FV-PTC got lower ACR TI-RADS scores than C-PTC. Most of the C-PTC group (85.1%) were categorized as the highly suspicious group (TI-RADS 5), indicating that ACR TI-RADS has high performance in predicting the malignancy risk of C-PTC. However, only 42.5% of the FV-PTC group were categorized as TI-RADS 5, and more than half of this group were categorized as TI-RADS 3 or 4, suggesting that more FV-PTC may fall in a nonbiopsy group than C-PTC with the same size. In the ACR TI-RADS, five groups of US features, including composition, echogenicity, shape, margin, and echogenic foci, were evaluated. Because the malignant US features, including very hypoechoic appearance, taller-than-wide shape, lobulated or irregular margin, and punctate echogenic foci, which were awarded more points according to ACR TI-RADS, were less common in FV-PTC, and smooth or ill-defined margin appeared in almost 50% of the FV-PTC nodules, FV-PTC had lower TI-RADS scores and risk levels. Thus, we hypothesized that ACR TI-RADS might underestimate the malignancy risk of FV-PTC, resulting in fewer FNABs and surgeries performed to FV-PTC. When the FV-PTC’s US characteristics, such as isoechoic appearance, macrocalcifications, uneven hypoechoic halo, and peripheral vascularity, are observed, the nodules may be FV-PTC and should undergo FNAB and be closely followed up or positively treated. For the nodules categorized as TI-RADS 3 or 4, the FNAB criteria should be broaden when these nodules have FV-PTC US features to ensure that more FV-PTC can be diagnosed and treated.
Some limitations deserve mention with respect to our study. First, because this was a retrospective study and only a small percentage of the enrolled patients had FNAB results, the sample sizes of the patients with FNAB results in both the FV-PTC and C-PTC groups were small, and thus our study was influenced by sampling bias. Further trials with larger patient cohorts are necessary to evaluate the usefulness of FNAB based on ACR TI-RADS in the diagnosis of FV-PTC. Second, our sample of patients was generated by a search of the electronic medical record system, and thus there might be a selection bias. We only selected patients who had undergone surgery, which was usually performed in patients with highly suspicious thyroid nodules on US, and different results might be found in the whole population.
Conclusions
Compared with C-PTC, FV-PTC has higher incidences of Hashimoto’s thyroiditis background and extrathyroidal extension. FV-PTC features isoechoic appearance, macrocalcifications, uneven hypoechoic halo, and peripheral vascularity on US, with relatively low incidences of microcalcifications and taller-than-wide shape, and tends to have lower TI-RADS scores and levels. A nodule with the features mentioned above deserves close attention, and FNAB is worth performing. For the nodules categorized as TI-RADS 3 or 4, the FNAB criteria should be broaden when these nodules have FV-PTC US features.
References
S.Y. Sohn, J.J. Lee, J.H. Lee, Molecular profile and clinicopathologic features of follicular variant papillary thyroid carcinoma. Pathol. Oncol. Res. (2019). https://doi.org/10.1007/s12253-019-00639-8
S.Y. Hahn, J.H. Shin, H.K. Lim, S.L. Jung, Follicular variant of papillary thyroid carcinoma: comparison of ultrasound-guided core needle biopsy and ultrasound-guided fine needle aspiration in a multicentre study. Clin. Endocrinol. 86, 113–119 (2017)
G.H. Daniels, What if many follicular variant papillary thyroid carcinomas are not malignant? A review of follicular variant papillary thyroid carcinoma and a proposal for a new classification. Endocr. Pract. 17, 768–787 (2011)
X. Shi, R. Liu, F. Basolo, R. Giannini, X. Shen, D. Teng, H. Guan, Z. Shan, W. Teng, T.J. Musholt, K. Al-Kuraya, L. Fugazzola, C. Colombo, E. Kebebew, B. Jarzab, A. Czarniecka, B. Bendlova, V. Sykorova, M. Sobrinho-Simões, P. Soares, Y.K. Shong, T.Y. Kim, S. Cheng, S.L. Asa, D. Viola, R. Elisei, L. Yip, C. Mian, F. Vianello, Y. Wang, S. Zhao, G. Oler, J.M. Cerutti, E. Puxeddu, S. Qu, Q. Wei, H. Xu, C.J. O’Neill, M.S. Sywak, R. Clifton-Bligh, A.K. Lam, G. Riesco-Eizaguirre, P. Santisteban, H. Yu, G. Tallini, E.H. Holt, V. Vasko, M. Xing, Differential clinicopathological risk and prognosis of major papillary thyroid cancer variants. J. Clin. Endocrinol. Metab. 101, 264–274 (2016)
J. Rosaí, G. Zampi, M.L. Carcangiu, Papillary carcinoma of the thyroid. A discussion of its several morphologic expressions, with particular emphasis on the follicular variant. Am. J. Surg. Pathol. 7, 809–817 (1983)
A.E. Walts, J.M. Mirocha, S. Bose, Follicular variant of papillary thyroid carcinoma (FVPTC): histological features, BRAF V600E mutation, and lymph node status. J. Cancer Res. Clin. Oncol. 141, 1749–1756 (2015)
C. Anuradha, M.T. Manipadam, H.S. Asha, N. Dukhabandhu, D. Abraham, M.J. Paul, Can new ultrasound signs help in identifying follicular variant of papillary carcinoma of thyroid?—A pilot study. Ultrasound Int. Open 2, E47–E53 (2016)
D. Ozdemir, R. Ersoy, N. Cuhaci, D. Arpaci, E.P. Ersoy, B. Korukluoglu, G. Guler, B. Cakir, Classical and follicular variant papillary thyroid carcinoma: comparison of clinical, ultrasonographical, cytological, and histopathological features in 444 patients. Endocr. Pathol. 22, 58–65 (2011)
S.C. Ng, S.F. Kuo, C.C. Hua, B.Y. Huang, K.C. Chiang, Y.Y. Chu, C. Hsueh, J.D. Lin, Differentiation of the follicular variant of papillary thyroid carcinoma from classic papillary thyroid carcinoma: an ultrasound analysis and complement to fine-needle aspiration cytology. J. Ultrasound Med. 37, 667–674 (2018)
D.S. Kim, J.H. Kim, D.G. Na, S.H. Park, E. Kim, K.H. Chang, C.H. Sohn, Y.H. Choi, Sonographic features of follicular variant papillary thyroid carcinomas in comparison with conventional papillary thyroid carcinomas. J. Ultrasound Med. 28, 1685–1692 (2009)
J.H. Yoon, H.J. Kwon, E.K. Kim, H.J. Moon, J.Y. Kwak, The follicular variant of papillary thyroid carcinoma: characteristics of preoperative ultrasonography and cytology. Ultrasonography 35, 47–54 (2016)
L. Gao, X. Xi, Y. Jiang, X. Yang, Y. Wang, S. Zhu, X. Lai, X. Zhang, R. Zhao, B. Zhang, Comparison among TIRADS (ACR TI-RADS and KWAK-TI-RADS) and 2015 ATA Guidelines in the diagnostic efficiency of thyroid nodules. Endocrine 64, 90–96 (2019)
F.N. Tessler, W.D. Middleton, E.G. Grant, Thyroid imaging reporting and data system (TI-RADS): a user’s guide. Radiology 287, 29–36 (2018)
E.J. Ha, D.G. Na, W.J. Moon, Y.H. Lee, N. Choi, Diagnostic performance of ultrasound-based risk-stratification systems for thyroid nodules: comparison of the 2015 American Thyroid Association Guidelines with the 2016 Korean Thyroid Association/Korean Society of Thyroid Radiology and 2017 American College of Radiology Guidelines. Thyroid 28, 1532–1537 (2018)
G. Grani, L. Lamartina, V. Ascoli, D. Bosco, M. Biffoni, L. Giacomelli, M. Maranghi, R. Falcone, V. Ramundo, V. Cantisani, S. Filetti, C. Durante, Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “Right” TIRADS. J. Clin. Endocrinol. Metab. 104, 95–102 (2019)
E.J. Jeon, Y.J. Jeong, S.H. Park, C.H. Cho, H.S. Shon, E.D. Jung, Ultrasonographic characteristics of the follicular variant papillary thyroid cancer according to the tumor size. J. Korean Med. Sci. 31, 397–402 (2016)
F.N. Tessler, W.D. Middleton, E.G. Grant, J.K. Hoang, L.L. Berland, S.A. Teefey, J.J. Cronan, M.D. Beland, T.S. Desser, M.C. Frates, L.W. Hammers, U.M. Hamper, J.E. Langer, C.C. Reading, L.M. Scoutt, A.T. Stavros, A.C.R. Thyroid Imaging, Reporting and data system (TI-RADS): white paper of the ACR TI-RADS committee. J. Am. Coll. Radio. 14, 587–595 (2017)
Y.E. Nikiforov, R.R. Seethala, G. Tallini, Z.W. Baloch, F. Basolo, L.D. Thompson, J.A. Barletta, B.M. Wenig, G.A. Al, K. Kakudo, T.J. Giordano, V.A. Alves, E. Khanafshar, S.L. Asa, A.K. El-Naggar, W.E. Gooding, S.P. Hodak, R.V. Lloyd, G. Maytal, O. Mete, M.N. Nikiforova, V. Nosé, M. Papotti, D.N. Poller, P.M. Sadow, A.S. Tischler, R.M. Tuttle, K.B. Wall, V.A. LiVolsi, G.W. Randolph, R.A. Ghossein, Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2, 1023–1029 (2016)
Cancer Genome Atlas Research Network, Integrated genomic characterization of papillary thyroid carcinoma. Cell 159, 676–690 (2014)
L.E. Henke, J.D. Pfeifer, T.J. Baranski, T. DeWees, P.W. Grigsby, Long-term outcomes of follicular variant vs classic papillary thyroid carcinoma. Endocr. Connect 7, 1226–1235 (2018)
H.S. Seo, D.H. Lee, S.H. Park, H.S. Min, D.G. Na, Thyroid follicular neoplasms: can sonography distinguish between adenomas and carcinomas. J. Clin. Ultrasound 37, 493–500 (2009)
K.T. Chem, J. Rosai, Follicular variant of thyroid papillary carcinoma: a clinicopathologic study of six cases. Am. J. Surg. Pathol. 1, 123–130 (1977)
J.H. Shin, B.K. Han, E.Y. Ko, Y.L. Oh, J.H. Kim, Differentiation of widely invasive and minimally invasive follicular thyroid carcinoma with sonography. Eur. J. Radiol. 74, 453–457 (2010)
J.Z. Zhang, B. Hu, Sonographic features of thyroid follicular carcinoma in comparison with thyroid follicular adenoma. J. Ultrasound Med. 33, 221–227 (2014)
Z.W. Baloch, P.K. Gupta, G.H. Yu, M.J. Sack, V.A. LiVolsi, Follicular variant of papillary carcinoma. Cytologic and histologic correlation. Am. J. Clin. Pathol. 111, 216–222 (1999)
L. Zhao, X.Y. Zhu, R. Jiang, M. Xu, N. Wang, G.G. Chen, Z.M. Liu, Role of GPER1, EGFR and CXCR1 in differentiating between malignant follicular thyroid carcinoma and benign follicular thyroid adenoma. Int J. Clin. Exp. Pathol. 8, 11236–11247 (2015)
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (No. 2016 YFA0201400).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, F., Chen, W. Sonographic features of follicular variant of papillary thyroid carcinoma (FV-PTC) and diagnostic performance of the 2017 ACR TI-RADS in FV-PTC. Endocrine 67, 379–386 (2020). https://doi.org/10.1007/s12020-019-02184-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-02184-5