Abstract
Background
Data on the prevalence and type of endocrine disorders in β-thalassemia intermedia (β-TI) patients are scarce. This multicenter study was designed to determine the prevalence of endocrine complications and the associated risk factors in a large group of β-TI patients.
Methods
In this cross-sectional multicenter study, 726 β-TI patients, aged 2.5–80 years, registered at 12 thalassemic centers, from nine countries, were enrolled during 2017. In a subgroup of 522 patients (mean age 30.8 ± 12.1; range: 2.5–80 years) from Qatar, Iran, Oman, Cyprus, and Jordan detailed data were available.
Results
Overall, the most prevalent complications were osteopenia/osteoporosis (22.3%), hypogonadism (10.1%), and primary hypothyroidism (5.3%). In the subgroup multivariate analysis, older age was a risk factor for osteoporosis (Odds ratio: 7.870, 95% CI: 4.729–13.099, P < 0.001), hypogonadism (Odds ratio: 6.310, 95% CI: 2.944–13.521, P < 0.001), and non-insulin-dependent diabetes mellitus (NIDDM; Odds ratio: 17.67, 95% CI: 2.217–140.968, P = 0.007). Splenectomy was a risk factor for osteoporosis (Odds ratio: 1.736, 95% CI: 1.012–2.977, P = 0.045). Hydroxyurea was identified as a “protective factor” for NIDDM (Odds ratio: 0.259, 95% CI: 0.074–0.902, P = 0.034).
Conclusions
To the best of our knowledge, this is the largest cohort of β-TI patients with endocrine disorders evaluated in extremely heterogenic thalassemic populations for age, clinical, hematological, and molecular composition. The study demonstrates that endocrine complications are less common in patients with β-TI compared with β-TM patients. However, regular monitoring with timely diagnosis and proper management is crucial to prevent endocrine complications in β-TI patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
β-thalassemias are a group of hereditary anemias caused by either reduced or complete absence of the production of β-globin chains of the hemoglobin (Hb) tetramer [1]. β-thalassemias are extremely heterogeneous in terms both of genotype and phenotype, depending on the nature of β-gene mutation and the extent of impairment in β-globin chain production. As a rule, heterozygous carriers of β-thalassemia (one affected allele) are asymptomatic and only altered laboratory values are observed. In contrast, the inheritance of two defective β-globin genes results in a wide phenotype spectrum, ranging from transfusion-dependent (thalassemia major [TM]) to mild or moderate anemia (thalassemia intermedia [TI]). β0 refers to the complete absence of the production of β-globin on the affected allele‚ β+ refers to alleles with some residual production of β-globin‚ and β++ to a very mild reduction in β-globin production. TI mutations in both parental genes lead to a moderate reduction in β-globin production. Patients have in general later clinical onset, milder anemia not requiring transfusions for survival during the first few years of life, and quality of life is not severely impaired, but the clinical course of the disease, if remaining untreated, is complicated by the multiple effects of chronic hemolytic anemia and the consequent tissue hypoxia, as well as by their compensatory reactions, including increased erythropoiesis with bone marrow expansion and increased intestinal iron absorption [2,3,4,5,6,7].
Conversely, chronic blood transfusions although are important for anemic patients inevitably lead to iron overload as humans cannot actively remove excess of iron [8,9,10,11].
Iron accumulation in β-thalassemia intermedia (β-TI) patients occurs more slowly than in β-TM but can pose a serious risk to the patients’ health because complications associated with β-TI may be as serious as those observed in β-TM. However, since patients with β-TI usually have a milder and more slowly progressing phenotype than β-TM patients have, there is a risk that regular monitoring and treatment may be delayed until complications become obvious. Excess iron is extremely toxic to all cells of the body and can cause serious and irreversible organic damage [2, 6, 7].
In patients with β-TM an increased risk for developing several complications, including diabetes mellitus, hypothyroidism, hypoparathyroidism, hypogonadism, and osteoporosis, has been reported, as the patients advance in age, mainly associated with iron overload [12,13,14,15,16,17].
In view of the limited data available in the literature on endocrinopathies in patients with β-TI, the aim of this multicenter study was to determine the prevalence of endocrine complications in β-TI patients, who were registered in 12 Thalassemia centers of nine countries (n = 726), and to evaluate the potential risk factors for endocrine complications in a subgroup of these patients.
Patients and methods
Study design
Our cross-sectional multicountry study included all β-TI patients followed in 2017 at 12 thalassemia centers of Iran, Italy, and Turkey (two centers for each country), and Greece, Oman, Qatar, Jordan, Cyprus, and United Kingdom (one center of each country). Patients’ medical history was obtained by review of medical records.
The protocol of the study was approved by Shiraz University of Medical Sciences (Approval code; 1396-01-32-15525). Written informed consent was obtained from the patients or their parents or legal guardian before participation in the study.
Patients’ selection
The patients were diagnosed as having β-TI by Hb electrophoresis, complete blood count, clinical history, and in some of them by molecular analysis.
The term β-TI was used to define a type of non-transfusion-dependent thalassemia, with mild genotype and clinical phenotype not requiring regular transfusions for survival. As a rule, the milder cases had no or occasional blood transfusions while the more severe may needed up to 7–8 transfusions annually [12, 18].
The prevalence of registered β-TI patients and endocrine complications of patients were obtained from each center by the data collection form. Moreover, an additional data gathering form was designed to collect more detailed data, including age, sex, Hb and serum ferritin levels, hydroxyurea consumption, and splenectomy. The questionnaire was sent to all participating centers but only six centers: Qatar, Iran (Shiraz, Tehran), Oman, Cyprus, and Jordan were able to fully complete the requested information
The definitions of the endocrine disorders reported by the participating centers are described in Table 1 [19,20,21,22,23,24,25].
Statistical analysis
Data were analyzed by SPSS Version 21. Descriptive data were presented as mean, standard deviation, percentage, and prevalence. In the subgroup of TI patients, followed in six centers including Qatar, Iran (Shiraz, Tehran), Oman, Cyprus, and Jordan, information on patient’s age, sex, Hb, serum ferritin levels, hydroxyurea therapy, and splenectomy were also available for performing an inferential analysis.
Univariate analysis was done using Chi-square test to determine the relationship of probable risk factors with each registered endocrine disorder. Variables with P value < 0.2 were entered into a multiple logistic regression model with enter method to determine independent factors influencing on the prevalence of endocrine disorders. A two-sided P value < 0.05 was considered statistically significant.
Results
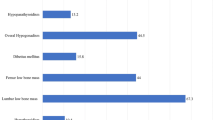
A total of 726 β-TI patients from 12 thalassemia centers from nine different countries were eligible for this study. The highest number of patients were from Iran [414 (57%)]. Overall disease prevalence in patients with β-TI is shown in Fig. 1.
The most common disease-related complications were osteopenia/osteoporosis (22.3%), hypogonadism (10.1%), and primary hypothyroidism (5.3%) (Fig. 1). The prevalence of endocrine disorders in patients with β-TI followed in 2017, in different countries, is presented in Table 2.
Two centers, Greece and Italy, reported the presence of an adrenal mass (3.3% and 1.85%, respectively). In first patient a diagnosis of nonfunctioning adenoma was made and in the second patient a myelolipoma (max diameter 3.5 cm) was diagnosed.
No clinical reports of adrenal insufficiency were registered by the participating centers.
In the subgroup of 522 β-TI patients more detailed data were available. The characteristics of these patients are shown in Table 3. The reported mean age of these patients was 30.8 ± 12.1 years (range: 2.5–80 years), with a male-to-female ratio of 1:1. The mean Hb and serum ferritin levels were 9.22 ± 1.21 (6.1–15.1) g/dl and 925 ± 1122 (13–8623) µg/l, respectively.
The highest Hb level reported in one patient was registered after blood transfusions and the lowest serum ferritin level (13 µg/l) relates to a woman with β-TI with iron deficiency, she had 11 pregnancies before the assessment of serum ferritin, requiring oral iron treatment.
The highest prevalence of endocrine disorders in this subgroup of patients was osteopenia/osteoporosis (26.2%), followed by hypogonadism (9.8%), and primary hypothyroidism (5.2%).
Osteopenia/osteoporosis showed significant positive association with age (P < 0.001), female gender (P = 0.047), lower Hb level (P = 0.003), and splenectomy (P < 0.001). Hypogonadism was significantly correlated with the older patients’ age (P < 0.001) and the sex (females) (P = 0.027). A significant association with the age (P = 0.001) was observed in patients with hypoparathyroidism.
The results of univariate analysis are shown in Table 4. Insulin-dependent diabetes mellitus (IDDM) showed a significant positive correlation with the age (P < 0.001), Hb level (P = 0.025), and splenectomy (P = 0.018). Non-insulin-dependent diabetes mellitus (NIDDM) showed a significant positive correlation with the age (P = 0.018) and hydroxyurea consumption (P = 0.031).
Finally, multiple logistic regression model was performed. In this model, age was arbitrarily subdivided into two groups: ≤35 and >35 years old. Older age (>35 years old) was associated with an increased risk of osteoporosis (Odds ratio: 7.870, 95% CI: 4.729–13.099, P < 0.001), hypogonadism (Odds ratio: 6.310, 95% CI: 2.944–13.521, P < 0.001), and NIDDM (Odds ratio: 17.67, 95% CI: 2.217–140.968, P = 0.007). An increased risk of osteopenia/osteoporosis was associated with splenectomy (Odds ratio: 1.736, 95% CI: 1.012–2.977, P = 0.045). On the contrary, hydroxyurea consumption resulted a “protective factor” for NIDDM (Odds ratio: 0.259, 95% CI: 0.074–0.902, P = 0.034) (Table 5).
It was not possible to perform a multivariate analysis for IDDM (n = 13) and hypoparathyroidism (n = 11) due to small number of patients present in our survey.
Discussion
Patients with β-TI experience with aging many clinical complications despite their independence from frequent transfusions. The main causes of endocrine disorders in β-TI are anemia, caused by ineffective erythropoiesis, relative bone marrow hyperactivity, medullary expansion, extramedullary hyperplasia, and iron overload [12].
Our results show that osteopenia/osteoporosis is the most common complication in the 3rd–5th decades of life, which is in agreement with the Optimal Care Study (OCS) reported by Taher et al. (~22%) [10]. The etiology of bone mineral loss is multifactorial. Medullary expansion due to anemia, patient age, duration of the disease, vitamin D deficiency, hypogonadism, and other endocrine-associated complications are significant contributory factors. Therefore, an annual screening for osteoporosis/osteopenia, after the second decade of life, is recommended. Although bisphosphonates remain the gold standard of osteoporosis [5]; the most beneficial effects for the treatment of low bone mass in thalassemia has yet to be established and needs to be considered in future prospective studies.
The second most common complication found was secondary hypogonadism, although the frequency in our study was lower compared with OCS study (10.1% vs.17.3%), followed by IDDM (6% vs. 1.7%) [10]. The prevalence of primary hypothyroidism was confirmed to be similar in both studies (~5%) [10]. The variable prevalence of endocrine complications reported in these two studies could be due to different severity of anemia, iron overload, patients’ age and gender.
In fact, in our subgroup of patients, older age was significantly associated with almost all complications including IDDM, NIDDM, osteopenia/osteoporosis, hypoparathyroidism, and hypogonadism.
In univariate analysis, IDDM was significantly associated with higher Hb levels and splenectomy. However, this association was not confirmed with a multivariate analysis, probably due to small sample size of patients with IDDM.
Female gender was significantly associated with osteopenia/osteoporosis and hypogonadism. Furthermore, hypogonadism was also significantly associated with splenectomy.
In brief, these findings reinforce the importance of regular follow-up of patients with β-TI for early detection and management of associated complications.
In general, when we compared β-TI patients with those with β-TM [13, 26, 27] a lower prevalence of endocrine complications was observed. We believe that, although the prevalence of endocrinopathies in β-TI tend to increase with age, it may be attributed to the lower extent, favorable genotype, slower rate, and hepatic predominance of iron loading in this group of patients. Nevertheless, a regular assessment of serum ferritin levels and T2* MRI of heart and liver, if available, are recommended for a prevention of endocrine complications secondary to iron overload. As reported in the literature, although serum ferritin is much less accurate and is weakly correlated with degree of siderosis, its monthly measurement could still have some value in predicting endocrine complications [28,29,30]. It has been also recommended that iron chelation therapy should be started if serum ferritin is above 500–800 µg/l or the liver iron concentration is above 5 mg/g dry weight [29, 30].
We recognize that one limit of our study is the heterogeneity of patients enrolled in the survey, the different severity of iron overload assessed by serum ferritin level (925 ± 1122 µg/l), and the lack of full information reported by some participating centers. As reported, in β-TM patients the prevalence of endocrine complications is quite variable in different countries [11.13–1722.26]. Various explanations have been reported in the current literature, but this aspect needs to be investigated furtherly in subjects with β-TI.
Nevertheless, our study report a wider view on the prevalence of endocrine and nonendocrine (osteopenia/osteoporosis) complications in a large cohort of patients with β-TI, and stimulates the attention for the development of further studies in order to assess the impact of anemia, frequency of transfusions, iron chelation therapy, splenectomy and hydroxyurea treatment on the outcome of these patients.
Finally, our study confirm that although endocrine complications are less common in patients with β-TI compared with data reported in the literature in β-TM patients. Nevertheless a regular monitoring with timely diagnosis and proper management is recommended to optimize the quality of life of these patients.
References
R. Galanello, R. Origa, Beta-thalassemia. Orphanet J. Rare Dis. 5(1), 11 (2010)
A. Taher, H. Isma’eel, M.D. Cappellini, Thalassemia intermedia: revisited. Blood Cells Molecules Dis. 37(1), 12–20 (2006)
K.M. Musallam, S. Rivella, E. Vichinsky, E.A. Rachmilewitz, Non-transfusion-dependent thalassemias. Haematologica 98(6), 833–844 (2013)
A. Taher, C. Hershko, M.D. Cappellini, Iron overload in thalassaemia intermedia: reassessment of iron chelation strategies. Br. J. Haematol. 147(5), 634–640 (2009)
M. Cappellini, A. Cohen, A. Eleftheriou, A. Piga, J. Porter, A. Taher, Guidelines for the Clinical Management of Thalassaemia. (Thalassaemia International Federation TIF, 2014)
M. Karimi, H. Darzi, M. Yavarian, Hematologic and clinical responses of thalassemia intermedia patients to hydroxyurea during 6 years of therapy in Iran. J. Pediatr. Hematol. Oncol. 27(7), 380–385 (2005)
P. Pootrakul, P. Sirankapracha, J. Sankote, U. Kachintorn, W. Maungsub, K. Sriphen, K. Thakernpol, K. Atisuk, S. Fucharoen, U. Chantraluksri, Clinical trial of deferiprone iron chelation therapy in β‐thalassaemia/haemoglobin E patients in Thailand. Br. J. Haematol. 122(2), 305–310 (2003)
R. Origa, R. Galanello, T. Ganz, N. Giagu, L. Maccioni, G. Faa, E. Nemeth, Liver iron concentrations and urinary hepcidin in β-thalassemia. Haematologica 92(5), 583–588 (2007)
H. Isma’eel, A.H. El Chafic, F. El Rassi, A. Inati, S. Koussa, R. Daher, W. Gharzuddin, S. Alam, A. Taher, Relation between iron-overload indices, cardiac echo-Doppler, and biochemical markers in thalassemia intermedia. Am. J. Cardiol. 102(3), 363–367 (2008)
A.T. Taher, K.M. Musallam, M. Karimi, A. El-Beshlawy, K. Belhoul, S. Daar, M.-S. Saned, A.-H. El-Chafic, M.R. Fasulo, M.D. Cappellini, Overview on practices in thalassemia intermedia management aiming for lowering complication rates across a region of endemicity: the OPTIMAL CARE study. Blood 115(10), 1886–1892 (2010)
A.U. Kurtoglu, E. Kurtoglu, A.K. Temizkan, Effect of iron overload on endocrinopathies in patients with beta-thalassaemia major and intermedia. Endokrynol. Pol. 63(4), 260–263 (2012)
M. Karimi, N. Cohan, V. De Sanctis, N.S. Mallat, A. Taher, Guidelines for diagnosis and management of Beta-thalassemia intermedia. Pediatr. Hematol. Oncol. 31(7), 583–596 (2014)
V. De Sanctis, C. Pintor, M. Gamberini, M. Ughi, A. Pinamonti, M. Aliquo, S. Anastasi, S. Andò, C. Brancati, M. Bruno, Multicentre study on prevalence of endocrine complications in thalassaemia major. Clin. Endocrinol. 42(6), 581–586 (1995)
S. Mohammadian, U. Bazrafshan, A. Sadeghi-Nejad, Endocrine gland abnormalities in thalassemia major: a brief review. J. Pediatr. Endocrinol. Metab. 16(7), 957–964 (2003)
P. De, R. Mistry, C. Wright, S. Pancham, W. Burbridge, K. Gangopadhayay, T. Pang, G. Das, A review of endocrine disorders in thalassaemia. Open J. Endocr. Metab. Dis. 4(02), 25 (2014)
V. De Sanctis, A.T. Soliman, H. Elsedfy, N. Skordis, C. Kattamis, M. Angastiniotis, M. Karimi, M.A.D.M. Yassin, A. El Awwa, I. Stoeva, Growth and endocrine disorders in thalassemia: the international network on endocrine complications in thalassemia (I-CET) position statement and guidelines. Indian J. Endocrinol. Metab. 17(1), 8 (2013)
N. Perera, N. Lau, S. Mathews, C. Waite, P. Ho, I. Caterson, Overview of endocrinopathies associated with β-thalassaemia major. Intern. Med. J. 40(10), 689–696 (2010)
A.T. Taher, K.M. Musallam, M.D. Cappellini, Thalassaemia intermedia: an update. Mediterr. J. Hematol. Infect. Dis. 1(1), e2009004 (2009)
Writing Group for the ISCD Position, Development Conference: indications and reporting for dual-energy x-ray absorptiometry. J. Clin. Densitom. 7(1), 37–44 (2004)
S.M. Petak, H. Nankin, R. Spark, R. Swerdloff, L. Rodriguez-Rigau, American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients-2002 update. Endocr. Pract. 8(6), 440–456 (2002)
D.M. Nathan, J.B. Buse, M.B. Davidson, E. Ferrannini, R.R. Holman, R. Sherwin, B. Zinman, Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 32(1), 193–203 (2009)
N.E. Cusano, N.M. Maalouf, P.Y. Wang, C. Zhang, S.C. Cremers, E.M. Haney, D.C. Bauer, E.S. Orwoll, J.P. Bilezikian, Normocalcemic hyperparathyroidism and hypoparathyroidism in two community-based nonreferral populations. J. Clin. Endocrinol. Metab. 98(7), 2734–2741 (2013)
B.B. Bercu, D. Shulman, A.W. Root, B.E. Spiliotis, Growth hormone (GH) provocative testing frequently does not reflect endogenous GH secretion. J. Clin. Endocrinol. Metab. 63(3), 709–716 (1986)
M.A. Yialamas, F.J. Hayes, Androgens and the ageing male and female. Best Pract. Res. Clin. Endocrinol. Metabol. 17(2), 223–236 (2003)
E.B. Fung, P.R. Harmatz, P.D. Lee, M. Milet, R. Bellevue, M.R. Jeng, K.A. Kalinyak, M. Hudes, S. Bhatia, E.P. Vichinsky, Increased prevalence of iron‐overload associated endocrinopathy in thalassaemia versus sickle‐cell disease. Br. J. Haematol. 135(4), 574–582 (2006)
C. Vullo, V. Sanctis, M. Katz, B. Wonke, A. Hoffbrand, B. Bagni, T. Torresani, G. Tolis, M. Masiero, A. Palma, Endocrine abnormalities in thalassemia. Ann. N. Y. Acad. Sci. 612(1), 293–310 (1990)
V. De Sanctis, A.T. Soliman, D. Canatan, H. Elsedfy, M. Karimi, S. Daar, H. Rimawi, S. Christou, N. Skordis, P. Tzoulis, An ICET—a survey on hypoparathyroidism in patients with thalassaemia major and intermedia: a preliminary report. Acta Bio Med. Atenei Parmensis 88(4), 435–444 (2017)
I. Kanbour, P, Chandra, A. Soliman, V. De Sanctis, A. Nashwan, S. Abusamaan, A. Moustafa, M.A. Yassin, Severe liver iron concentrations (LIC) in 24 patients with β-thalassemia major: correlations with serum ferritin, liver enzymes and endocrine complications. Mediterr. J. Hematol. Infect. Dis. 10(1), e2018062 (2018)
Z, Majd, S, Haghpanah, G.H. Ajami, S. Matin, H. Namazi, M. Bardestani, M. Karimi, Serum ferritin levels correlation with heart and liver MRI and LIC in patients with transfusion-dependent thalassemia. Iran. Red Crescent Med. J. 17(4), e24959 (2015)
A.T. Taher, J.B. Porter, V. Viprakasit, A. Kattamis, S. Chuncharunee, P. Sutcharitchan, N. Siritanaratkul, R. Galanello, Z. Karakas, T. Lawniczek, Deferasirox effectively reduces iron overload in non-transfusion-dependent thalassemia (NTDT) patients: 1-year extension results from the THALASSA study. Ann. Hematol. 92(11), 1485–1493 (2013)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The protocol of the study was approved by Shiraz University of Medical Sciences (Approval code: 1396-01-32-15525).
Informed consent
Written informed consent was obtained from the patients or their parents or legal guardian before participation in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karimi, M., Zarei, T., Haghpanah, S. et al. Evaluation of endocrine complications in beta-thalassemia intermedia (β-TI): a cross-sectional multicenter study. Endocrine 69, 220–227 (2020). https://doi.org/10.1007/s12020-019-02159-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-02159-6