Abstract
Purpose
Hypothyroidism is a common side effect of Sunitinib (SUN) treatment in metastatic renal cell carcinoma (mRCC) patients. We aimed to evaluate thyroid profile during the alternative 2/1 SUN treatment schedule and to assess the predictive value of hypothyroidism in terms of survival.
Methods
We performed a prospective observational study enrolling 42 consecutive mRCC patients starting first-line alternative SUN dosing 2/1 schedule. Thyroid function was assessed at baseline and during the first three SUN cycles (1 cycle = 6 weeks = 2 ON/1 OFF + 2 ON/1 OFF), and then after 6 and 12 months. Thyroid ultrasound was performed at baseline and after 3, 6, and 12 months.
Results
Subclinical hypothyroidism developed in 24% of patients during the first cycle; in other 24% in the second cycle and in 14% in the third cycle. The highest TSH values were reached during the second cycle, ON phase (6.58 ± 5.74 μI U/l). We observed a reduction in thyroid size, in echogenicity and in parenchymal perfusion in all patients. Progression-free survival (PFS) tended to be longer in patients with TSH ≥ 5 μI U/ml during the second cycle (p = 0.069). TSH level was an independent risk factor for PFS in men (p = 0.009) but not in women (p = 0.285).
Conclusions
This is the first study investigating functional and morphological effects on thyroid during the alternative 2/1 SUN schedule in mRCC patients. We detected an early onset of subclinical hypothyroidism, observing the association between TSH ≥ 5 μI U/ml and: (i) longer PFS in men; (ii) progressive decrease of thyroid size in absence of significant changes in autoimmune thyroid profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sunitinib (SUN) is an oral, small-molecule tyrosine kinases inhibitor (TKI) that is structurally similar to adenosine triphosphate. SUN inhibits cellular signaling by targeting multiple receptor tyrosine kinases, including all receptors for the three isoforms of VEGF, PDGF, FMS-like tyrosine kinase-3, and stem cell factor receptor. SUN also binds RET, CD114, and CD135 receptors [1]. The simultaneous inhibition of these targets reduces tumor vascularization and triggers cancer cell apoptosis, thus resulting in tumor shrinkage. It has been approved by the Food and Drug Administration for imatinib-resistant gastrointestinal stromal tumor, advanced pancreatic neuroendocrine tumor and as a standard first-line treatment for patients with metastatic renal cell carcinoma (mRCC) [2,3,4]. This recommendation has particular weight if it is estimated that mRCC accounts for 20–30% of all kidney cancer at the initial diagnosis and that 20–40% of localized renal cell carcinoma will develop distant metastases after radical surgery [5]. Preclinical and clinical studies suggested that standard dosing of SUN is the “4/2 schedule” with a dose of 50 mg/day taken orally (4 weeks ON and 2 weeks OFF). This schedule has a superior survival benefit [3, 6], but high exposure of SUN was associated with severe adverse events (sAE), impacting compliance and medication taken [7, 8]. To balance efficacy and sAE better, alternative SUN schedules have been evaluated to improve patients tolerance and survival outcomes, showing noninferior clinical benefits of a 2/1 dosing schedule [9,10,11]. Nevertheless, the most common adverse events associated with SUN are hypothyroidism, fatigue, diarrhea, nausea, anorexia, hypertension, a yellow skin discolouration, hand–foot skin reaction, stomatitis, altered taste, constipation and may reduce quality of life, requiring monitoring and treatment [12]. Biochemical and clinical hypothyroidism is commonly reported in patients with mRCC receiving SUN [13, 14], with variable prevalence: from 50–85% in retrospective trials [14, 15] to 36–46% in prospective and observational studies [16,17,18,19,20,21,22,23]. This discrepancy is probably due to differences in the study design, sample size, definition of hypothyroidism and previous use of cytokine therapy [23].
The controversial association between hypothyroidism and survival in patients with mRCC [16, 19,20,21,22, 24] and the clinical usefulness of TSH as a potential tumor biomarker in SUN-treated mRCC patients are not completely explored. The aim of the present study is to prospectively observe thyroid profile and autoimmunity during SUN administration in mRCC patients.
Subjects and methods
Participants
The local review board approved the protocol, and all patients provided written informed consent. All the patients with pathologically confirmed mRCC were enrolled from patients starting first-line SUN treatment in the Medical Oncology Department—“Sapienza” University of Rome between March 2013 and June 2015. Patients previously treated with IL-2 and/or IFN-α, those with severe hypertension under treatment with three or more drugs at the time of enrolment, those under steroids therapy and those with previous thyroid dysfunctions were excluded.
Setting and methods
SUN was given at a dose of 50 mg daily for 2 weeks ON followed by 1 week OFF (1 cycle = 6 weeks = 2 ON/1 OFF + 2 ON/1 OFF). Enrolled subjects underwent thyroid hormonal investigations and thyroid ultrasound. Blood samples were assessed at baseline and at the end of 2nd week (end of first phase ON—first SUN cycle), at the end of 3rd week (end of first phase OFF—first SUN cycle), at the end of 8th week (end of first phase ON—second SUN cycle), at the end of 9th week (end of first phase OFF—second SUN cycle) (Fig. 1). TSH (reference range: 0.35–4.9 μI U/l), FT3 (1.7–3.7 pg/ml), FT4 (0.7–1.4 ng/dL), TGAb (<115/ml), and TPOAb (<35 IU/mL) were measured by chemiluminescent microparticle immunoassay technology (Architect System, Abbott). Subclinical hypothyroidism was defined as a serum TSH of more than 5 μI U/l and <10 μI U/l and normal FT3 and FT4 levels [25, 26]. According to the literature Levothyroxine (LT4) therapy (25–75 mcg) was started in all patients who developed subclinical hypothyroidism or who showed a persistent serum TSH ≥ 5 μI U/l at the end of the second cycle (OFF period, at the end of 9th week) [27, 28]. Thyroid Ultrasounds (US) were performed using an IU22 unit (Philips, Bothell, Wash), with compound spatial and pulse inversion imaging and a 7–15-MHz wide-band linear probe, by the same sonographer with more than 10 years of experience. Images were transferred to our local picture archiving system (eFilm; Merge, Milwaukee, Wis). US were performed before starting SUN treatment and after 3, 6, and 12 months, and included measurement of right and left lobes diameters, evaluation of thyroid echotexture and echogenicity, evaluation of blood flow on color Doppler and description of any nodules [29, 30]. Objective response to SUN treatment was evaluated by the Response Evaluation Criteria in Solid Tumors version 1.1.
Statistical methods
Statistical analysis was performed using SPSS 20.0 [SPSS, Chicago, Ill]. Normality of the continuous variables was tested with the Shapiro–Wilk test. Normally continuous variables were presented as mean ± SD, while those not normally distributed were presented as median (25th–75th percentile). A one-way repeated measures ANOVA was conducted to determine whether there was a statistically significant difference in TSH concentration and thyroid lobe diameters over the course of SUN treatment. Progression-free survival (PFS) and overall survival (OS) was counted from the start of treatment with SUN until disease progression or death from any cause, or was censored at the end of the study. Both PFS and OS were estimated with Kaplan–Meier method and compared with log-rank test. Prognostic factors were tested using the standard ordinary least squares regression. Statistical significance was considered at p < 0.05.
Results
Forty-two consecutive patients (26 men, 16 women) were included in the study. Mean age was 61 ± 8 years for men and 60 ± 16 years for women. All patients presented an advanced tumor stage with the following distribution of distant metastases: 48% lung, 36% bone, 9% liver, 7% brain, 5% adrenal, 5% contralateral kidney, 2% pancreas. At the time of enrolment all patients were euthyroid and 20/42 (48%) had thyroid nodules. No patient had positive autoantibodies. The median duration of SUN treatment was 34 weeks (range 3–49 weeks).
Median basal and follow-up data of thyroid function of all 42 patients are reported in Table 1. Subclinical hypothyroidism was developed in 10/42 patients (24%) in the first SUN cycle (ON phase, end of 2nd week); in other ten patients after the second SUN cycle (ON phase, end of 8th week) (total = 20/42 patients, 48%); During the third SUN cycle (ON phase, end of 14th week) 6 of the 20 patients who developed subclinical hypothyroidism in the first two cycles still showed TSH levels above 5 μI U/l. Overall, 20/42 patients developed subclinical hypothyroidism during SUN treatment. Peak TSH was reached during the second cycle (ON phase, end of 8th week) (Fig. 2). This was confirmed for the entire population as well as when sex and age classes were considered separately. No patient reached overt hypothyroidism. Figure 3 shows FT4 values during the study, which remained within the normal range in each data point. Two patients showed a pattern of subclinical hyperthyroidism during the first SUN cycle (ON phase, end of 2nd week) followed by subclinical hypothyroidism in the second SUN cycle (ON phase, end of 8th week). A one-way repeated measures ANOVA was conducted to determine whether there was a statistically significant difference in TSH concentration over the course of SUN cycles, before patients started hormone replacement therapy. Epsilon (ε) was 0.608, as calculated according to Greenhouse and Geisser [31], and was used to correct the one-way repeated measures ANOVA. TSH concentration was significantly different at the different time points during the SUN administration, F(1.216, 18.243) = 6.310, p = 0.017, with TSH concentration varying from 2.43 ± 1.80 μI U/l preintervention to 3.81 ± 2.89 μI U/l during the first SUN cycle (ON phase, end of 2nd week) and 6.58 ± 5.74 μI U/l during the second SUN cycle (ON phase, end of 8th week). Thyroid hormone replacement therapy was started in patients with TSH ≥ 5 μI U/l and with clinical symptoms as follow: eight patients after the first cycle; of these, five needed an increase in LT4 dosing at the end of the second cycle; eight different patients began LT4 replacement therapy after the second cycle; one patient began LT4 replacement therapy after the third cycle; of the 16 patients who started therapy during the first two cycles, three needed an increase in LT4 dosing at the end of the third cycle. Overall, 17/42 patients started LT4 therapy during SUN treatment. The therapeutic intervention changed significantly TSH values in the third cycle (ON phase, end of 14th week) (4.8 ± 5.1 μI U/l, p = 0.016) that remained stable until the end of the study (12 months). No patient developed a statistically significant increase in antibody titre (TGAb, TPOAb) during SUN treatment.
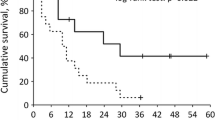
PFS was 33 months (95% CI 21.5–43.9 months) for women and 20 months (95% CI 12.4–27.1 months) for men. OS was 18.6 months (95% CI 2.3–34.9 months) for women and 18.9 months (95% CI 10.2–27.5 months) for men (Fig. 4). The Kaplan–Meier method was used to compare PFS and OS curves between euthyroid and patients with subclinical hypothyroidism. Patients with TSH ≥ 5 μI U/l tended to have a longer PFS than those with TSH < 5 μI U/l (p = 0.069). No difference in OS was seen between the two groups (Fig. 5). Standard regression analysis demonstrated that TSH level was an independent risk factor for PFS in men (p = 0.009) but not in women (p = 0.285) (Table 2).
At baseline, all patients showed thyroid size within normal ranges and normal thyroid echogenicity; 20/42 (48%) had thyroid nodules (12 men and 8 women).
SUN administration elicited significant changes in antero-posterior diameters of both lobes over time [F(3, 12) = 4.121, p < 0.032, partial η2 = 0.507 for the right lobe and F(3, 12) = 13.180, p < 0.001, partial η2 = 0.767 for the left lobe], with right lobe diameters decreasing from 1.6 ± 0.18 cm pre intervention to 1.46 ± 0.25 cm at 3 months and 1.36 ± 0.23 cm at 12 months (post intervention) and left lobe diameters decreasing from 1.46 ± 0.16 cm pre intervention to 1.34 ± 0.16 cm at 3 months, and 1.1 ± 0.17 cm at 12 months (post intervention).
Marked reductions in echogenicity and in parenchymal perfusion were observed in all patients from the early months of SUN therapy. No change was observed in the size of existing nodules. Only in one patient after three months of SUN treatment an US detection of a 0.5 cm thyroid nodule (TIRADS 2) [32] was found.
Discussion
The present study showed two innovative aspects related to thyroid profile in mRCC treated with SUN: [1] the assessment of thyroid function, morphology, and autoimmune profile during the alternative SUN 2/1 schedule in mRCC patients and [2] the trend of SUN-related hypothyroidism that followed the ON and the OFF phases of SUN administration, associated with a progressive decrease in thyroid size.
SUN-related hypothyroidism is usually subclinical (TSH elevation alone with normal FT4 levels) or overt (TSH elevation and low FT4) but rarely severe [18], arising from 12 to 50 weeks after treatment beginning [33]. Our sample of patients showed a subclinical hypothyroidism that is revealed at the 8th week. This study demonstrated for the first time such early detection of SUN-related hypothyroidism. This is an important aspect for oncological patients, because an early identification of this side effect leads to an early correction and monitoring, improving the symptoms related to hypothyroidism and the quality of life of mRCC patients. Thyroid parameters were measured during the alternative dosing schedule of SUN 50 mg once daily for 2 consecutive weeks followed by 1 week off (2/1 schedule). The OFF period is recommended to allow patients to recover from treatment-related adverse events, which frequently appear after the first 2 weeks of treatment and tend to worsen in the following days [34]. The early detection of SUN-related hypothyroidism in this alternative schedule dosing is of particular oncological relevance for the management of patients, given the data about the advantages of this new modality of administration of the SUN. Indeed, single-center studies demonstrated improved tolerability and clinical outcomes with a 2/1 schedule [35, 36]. Recently, Bracarda et al. showed in the RAINBOW study that the use of the 2/1 schedule in patients who did not tolerate the standard schedule resulted in an improved safety profile and a median PFS of 30.2 months [37]. Furthermore, a randomized multicenter phase 2 study comparing the 2/1 and 4/2 schedules found less toxicity and higher failure-free survival at 6 months with the 2/1 schedule, without compromising the efficacy in terms of overall response rate (ORR) and time to progression. This study used the alternative 2/1 schedule and investigated various parameters to confirm SUN-induced hypothyroidism. Hypothyroidism developed in 24% of patients during the first SUN cycle, in 48% during the second and in 14% during the third cycle. In line with what has been demonstrated in this study, peak TSH was found at the end of the ON phase of the second SUN cycle (at the end of 8th week) independently of gender, age and LT-4 replacement therapy status on enrolment [9]. Data from the literature have not yet clarified the mechanism of SUN-associated hypothyroidism and the course of the disease [21, 23]. Several underlying mechanisms might be involved: reduced synthesis of thyroid hormones through inhibition of thyroid peroxidase (TPO) activity and progressive depletion of the thyroid reserve [15]; inhibition of iodine thyroidal uptake [23]; glandular atrophy induced by TKIs through the inhibition of vascularization (direct action on VEGFR and/or PDGFR) [38]; possible autoimmune damage that causes lymphocytic thyroiditis in patients receiving SUN [23].
Recent reviews on the effect of TKIs on thyroid function concluded that thyroid autoimmunity cannot be considered as a trigger of SUN-induced hypothyroidism [18]. Conversely, Pani et al. [28, 39] found strong evidence that thyroid autoimmunity contributed to SUN-induced thyroid dysfunction.
In our study, autoantibodies titer was measure but no significant changes were detected in the autoimmune profile of the enrolled patients. Of the forty-two, three patients (7%) showed a small increase in AbTPO values during treatment that did not impact the statistical analysis. On the other hand, we demonstrated throught ultrasound assessment a progressive reduction in thyroid size, mainly after three months of SUN treatment, regardless of the ON/OFF phase, in agreement with the majority of the literature [28, 40, 41]. This was combined with marked hypoechogenicity and reduced parenchymal perfusion, probably as a result of SUN’s antiangiogenic effect. These data suggest that a vascular-mediated effect instead of autoimmune mechanism could be more responsible of the induction of a thyroid dysfunction. No significant changes were observed in the size of existing nodules and the onset of a thyroid nodule during SUN treatment was detected just in one patient.
SUN-induced hypothyroidism has also been proposed as a possible predictive factor for the outcome of the cancer treatment, although the conclusions are still unclear [16, 19,20,21,22, 24]. A preliminary study of SUN treatment for mRCC suggested that hypothyroid patients had a significantly longer PFS than euthyroid patients [23]. Schmidinger prospectively studied the correlation between the onset of hypothyroidism and the rate of disease remission, discovering a higher ORR in hypothyroid than in euthyroid patients (ORR: 28.3% vs. 3.3%; p < 0.001) [21]. In contrast, in a prospective observational multicentre study Sabatier found that thyroid dysfunction did not increase survival in SUN-treated patients with mRCC; after 6 months of SUN, 53% of patients developed hypothyroidism, with a median PFS of 18.9 months compared with 15.9 for the euthyroid group [20]. Given the high variability of the TSH values in women and the smaller caseload, we cannot exclude that in a larger group TSH level might also be a predictive factor for PFS in women. TSH was not predictive of OS in both gender, but the early correction of subclinical hypothyroidism with LT4 could mask this result.
Our study has some limitations. First, the small sample size due to the inclusion criteria that provides mRCC patients treated as a first line with the alternative 2/1 SUN schedule. Second, the a priori TSH cut-off of 5 μI U/l that was selected to early identify subclinical hypothyroid patients could be considered as dependent on our specific cohort. However, even if endocrinologists use the upper limit of their own TSH assays reference range to identify subclinical hypothyroidism, the cutoff of 5 μI U/l is widely acknowledged to identify this condition irrespective of its origin [42, 43]. Third, the early beginning of thyroid hormone replacement therapy masked the potential development of overt hypothyroidism and consequently prevented the PFS measure related to this adverse event as described in literature [44].
Conclusion
This is the first study investigating the thyroid functional and morphological effects of the alternative 2/1 SUN schedule in patients with mRCC. We detect an early onset of subclinical hypothyroidism associated with a progressive decrease of thyroid size in absence of significant changes in autoimmune thyroid profile. Moreover, the onset of subclinical hypothyroidism was associated with a longer PFS in men. In this view, our results underlined a gender difference in the role exerted by TSH level as an independent risk factor for PFS.
In line with a precision medicine point of view, we could suggest that an early LT4-mediated improvement of TSH levels could positively impact on symptoms of hypothyroidism and improve quality of life in SUN-treated mRCC patients.
Further analysis and larger series are needed to confirm our finding in order to improve the management of mRCC patients that may benefit of the alternative SUN dosing 2/1 schedule.
Abbreviations
- RCC:
-
renal cell carcinoma
- mRCC:
-
metastatic renal cell carcinoma
- IL-2:
-
interleukin-2
- IFN-α:
-
interferon-α
- VHL:
-
von Hippel Lindau
- VEGF:
-
vascular endothelial growth factor
- PDGF:
-
platelet-derived growth factor
- VEGFR:
-
vascular endothelial growth factor receptor
- PDGFR:
-
platelet-derived growth factor receptor
- TKIs:
-
tyrosine kinase inhibitors
- SUN:
-
Sunitinib
- ATP:
-
adenosine triphosphate
- PFS:
-
progression free survival
- ORR:
-
overall response rate
- OS:
-
overall survival
- FFS:
-
free survival rate
- TTP:
-
time to progression
- CTC:
-
common toxicity criteria
- TSH:
-
thyroid stimulating hormone
- FT4:
-
free thyroxine
- T3:
-
triiodothyronine
- FT3:
-
free triiodothyronine
- TPO:
-
thyroid peroxidase
- TGAb:
-
anti-thyroglobulin antibody
- TPOAb:
-
anti-thyroid peroxidase antibody
- FSH:
-
follicle stimulating hormone
- D3:
-
type III deiodinase
- RECIST:
-
response evaluation criteria in solid tumors
- OLS:
-
ordinary least squares
- LT:
-
levothyroxine
References
D.B. Mendel, A.D. Laird, X. Xin, S.G. Louie, J.G. Christensen, G. Li, R.E. Schreck, T.J. Abrams, T.J. Ngai, L.B. Lee, L.J. Murray, J. Carver, E. Chan, K.G. Moss, J.O. Haznedar, J. Sukbuntherng, R.A. Blake, L. Sun, C. Tang, T. Miller, S. Shirazian, G. McMahon, J.M. Cherrington, In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 9, 327–337 (2003)
G.D. Demetri, P. Reichardt, Y.K. Kang, J.Y. Blay, P. Rutkowski, H. Gelderblom, P. Hohenberger, M. Leahy, M. von Mehren, H. Joensuu, G. Badalamenti, M. Blackstein, A. Le Cesne, P. Schoffski, R.G. Maki, S. Bauer, B.B. Nguyen, J. Xu, T. Nishida, J. Chung, C. Kappeler, I. Kuss, D. Laurent, P.G. Casali; investigators Gs, Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381, 295–302 (2013)
R.J. Motzer, T.E. Hutson, P. Tomczak, M.D. Michaelson, R.M. Bukowski, S. Oudard, S. Negrier, C. Szczylik, R. Pili, G.A. Bjarnason, X. Garcia-del-Muro, J.A. Sosman, E. Solska, G. Wilding, J.A. Thompson, S.T. Kim, I. Chen, X. Huang, R.A. Figlin, Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 27, 3584–3590 (2009)
E. Raymond, L. Dahan, J.L. Raoul, Y.J. Bang, I. Borbath, C. Lombard-Bohas, J. Valle, P. Metrakos, D. Smith, A. Vinik, J.S. Chen, D. Horsch, P. Hammel, B. Wiedenmann, E. Van Cutsem, S. Patyna, D.R. Lu, C. Blanckmeister, R. Chao, P. Ruszniewski, Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. New Engl. J. Med. 364, 501–513 (2011)
N.K. Janzen, H.L. Kim, R.A. Figlin, A.S. Belldegrun, Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urologic Clin. N Am. 30, 843–852 (2003)
B.E. Houk, C.L. Bello, B. Poland, L.S. Rosen, G.D. Demetri, R.J. Motzer, Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother. Pharmacol. 66, 357–371 (2010)
M.E. Gore, C. Szczylik, C. Porta, S. Bracarda, G.A. Bjarnason, S. Oudard, S.H. Lee, J. Haanen, D. Castellano, E. Vrdoljak, P. Schoffski, P. Mainwaring, R.E. Hawkins, L. Crino, T.M. Kim, G. Carteni, W.E. Eberhardt, K. Zhang, K. Fly, E. Matczak, M.J. Lechuga, S. Hariharan, R. Bukowski, Final results from the large sunitinib global expanded-access trial in metastatic renal cell carcinoma. Br. J. Cancer 113, 12–19 (2015)
R.J. Motzer, T.E. Hutson, D. Cella, J. Reeves, R. Hawkins, J. Guo, P. Nathan, M. Staehler, P. de Souza, J.R. Merchan, E. Boleti, K. Fife, J. Jin, R. Jones, H. Uemura, U. De Giorgi, U. Harmenberg, J. Wang, C.N. Sternberg, K. Deen, L. McCann, M.D. Hackshaw, R. Crescenzo, L.N. Pandite, T.K. Choueiri, Pazopanib versus sunitinib in metastatic renal-cell carcinoma. New Engl. J. Med. 369, 722–731 (2013)
J.L. Lee, M.K. Kim, I. Park, J.H. Ahn, D.H. Lee, H.M. Ryoo, C. Song, B. Hong, J.H. Hong, H. Ahn, Randomized phase II trial of Sunitinib four weeks on and two weeks off versus two weeks on and one week off in metastatic clear-cell type REnal cell carcinoma: RESTORE trial. Ann. Oncol. 26, 2300–2305 (2015)
H. Miyake, K. Harada, A. Miyazaki, M. Fujisawa, Improved health-related quality of life of patients with metastatic renal cell carcinoma treated with a 2 weeks on and 1 week off schedule of sunitinib. Med. Oncol. 32, 78 (2015)
X. Zhang, G. Sun, J. Zhao, K. Shu, P. Zhao, J. Liu, Y. Yang, Q. Tang, J. Chen, P. Shen, J. Wang, H. Zeng, Improved long-term clinical outcomes and safety profile of sunitinib dosing schedule with 4/2 switched to 2/1 in patients with metastatic renal cell carcinoma. J. Cancer 9, 3303–3310 (2018)
G. Di Lorenzo, C. Porta, J. Bellmunt, C. Sternberg, Z. Kirkali, M. Staehler, S. Joniau, F. Montorsi, C. Buonerba, Toxicities of targeted therapy and their management in kidney cancer. Eur. Urol. 59, 526–540 (2011)
H. Miyake, T. Kurahashi, K. Yamanaka, Y. Kondo, M. Muramaki, A. Takenaka, T.A. Inoue, M. Fujisawa, Abnormalities of thyroid function in Japanese patients with metastatic renal cell carcinoma treated with sorafenib: a prospective evaluation. Urologic Oncol. 28, 515–519 (2010)
B.I. Rini, I. Tamaskar, P. Shaheen, R. Salas, J. Garcia, L. Wood, S. Reddy, R. Dreicer, R.M. Bukowski, Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J. Natl. Cancer Inst. 99, 81–83 (2007)
E. Wong, L.S. Rosen, M. Mulay, A. Vanvugt, M. Dinolfo, C. Tomoda, M. Sugawara, J.M. Hershman, Sunitinib induces hypothyroidism in advanced cancer patients and may inhibit thyroid peroxidase activity. Thyroid 17, 351–355 (2007)
V. Baldazzi, R. Tassi, A. Lapini, C. Santomaggio, M. Carini, R. Mazzanti, The impact of sunitinib-induced hypothyroidism on progression-free survival of metastatic renal cancer patients: a prospective single-center study. Urologic Oncol. 30, 704–710 (2012)
J. Desai, L. Yassa, E. Marqusee, S. George, M.C. Frates, M.H. Chen, J.A. Morgan, S.S. Dychter, P.R. Larsen, G.D. Demetri, E.K. Alexander, Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann. Intern. Med. 145, 660–664 (2006)
D. Mannavola, P. Coco, G. Vannucchi, R. Bertuelli, M. Carletto, P.G. Casali, P. Beck-Peccoz, L. Fugazzola, A novel tyrosine-kinase selective inhibitor, sunitinib, induces transient hypothyroidism by blocking iodine uptake. J. Clin. Endocrinol. Metab. 92, 3531–3534 (2007)
L.M. Riesenbeck, S. Bierer, I. Hoffmeister, T. Kopke, P. Papavassilis, L. Hertle, B. Thielen, E. Herrmann, Hypothyroidism correlates with a better prognosis in metastatic renal cancer patients treated with sorafenib or sunitinib. World J. Urol. 29, 807–813 (2011)
R. Sabatier, J.C. Eymard, J. Walz, J.L. Deville, H. Narbonne, J.M. Boher, N. Salem, M. Marcy, S. Brunelle, P. Viens, F. Bladou, G. Gravis, Could thyroid dysfunction influence outcome in sunitinib-treated metastatic renal cell carcinoma? Annals of oncology: official journal of the European Society for. Med. Oncol. 23, 714–721 (2012)
M. Schmidinger, U.M. Vogl, M. Bojic, W. Lamm, H. Heinzl, A. Haitel, M. Clodi, G. Kramer, C.C. Zielinski, Hypothyroidism in patients with renal cell carcinoma: blessing or curse? Cancer 117, 534–544 (2011)
N. Shinohara, M. Takahashi, T. Kamishima, H. Ikushima, N. Otsuka, A. Ishizu, C. Shimizu, H. Kanayama, K. Nonomura, The incidence and mechanism of sunitinib-induced thyroid atrophy in patients with metastatic renal cell carcinoma. Br. J. Cancer 104, 241–247 (2011)
P. Wolter, C. Stefan, B. Decallonne, H. Dumez, M. Bex, P. Carmeliet, P. Schoffski, The clinical implications of sunitinib-induced hypothyroidism: a prospective evaluation. Br. J. Cancer 99, 448–454 (2008)
O. Bozkurt, H. Karaca, I. Hacibekiroglu, M.A. Kaplan, Y. Duzkopru, M. Uysal, V. Berk, M. Inanc, A.O. Duran, E. Ozaslan, M. Ucar, M. Ozkan, Is sunitinib-induced hypothyroidism a predictive clinical marker for better response in metastatic renal cell carcinoma patients? J. Chemother. 28, 230–234 (2016)
V. Fatourechi, Subclinical hypothyroidism: an update for primary care physicians. Mayo Clin. Proc. 84, 65–71 (2009)
M.I. Surks, E. Ortiz, G.H. Daniels, C.T. Sawin, N.F. Col, R.H. Cobin, J.A. Franklyn, J.M. Hershman, K.D. Burman, M.A. Denke, C. Gorman, R.S. Cooper, N.J. Weissman, Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. J. Am. Med. Assoc. 291, 228–238 (2004)
D.S. Cooper, B. Biondi, Subclinical thyroid disease. Lancet 379, 1142–1154 (2012)
F. Pani, F. Atzori, G. Baghino, F. Boi, L. Tanca, M.T. Ionta, S. Mariotti, Thyroid dysfunction in patients with metastatic carcinoma treated with Sunitinib: is thyroid autoimmunity involved? Thyroid 25, 1255–1261 (2015)
J. Brunn, U. Block, G. Ruf, I. Bos, W.P. Kunze, P.C. Scriba, [Volumetric analysis of thyroid lobes by real-time ultrasound (author’s transl)]. Dtsch. Med. Wochenschr. 106, 1338–1340 (1981)
A.M. Isidori, V. Cantisani, E. Giannetta, D. Diacinti, E. David, V. Forte, D. Elia, C. De Vito, E. Sbardella, D. Gianfrilli, F. Monteleone, J. Pepe, S. Minisola, G. Ascenti, V. D’Andrea, C. Catalano, F. D’Ambrosio, Multiparametric ultrasonography and ultrasound elastography in the differentiation of parathyroid lesions from ectopic thyroid lesions or lymphadenopathies. Endocrine 57, 335–343 (2017)
S.W. Greenhouse, S. Geisser, On methods in the analysis of profile data. Psychometrika 24, 95–112 (1959)
E.G. Grant, F.N. Tessler, J.K. Hoang, J.E. Langer, M.D. Beland, L.L. Berland, J.J. Cronan, T.S. Desser, M.C. Frates, U.M. Hamper, W.D. Middleton, C.C. Reading, L.M. Scoutt, A.T. Stavros, S.A. Teefey, Thyroid ultrasound reporting lexicon: white paper of the ACR Thyroid Imaging, Reporting and Data System (TIRADS) committee. J. Am. Coll. Radiol. 12, 1272–1279 (2015)
M.L. Vetter, S. Kaul, N. Iqbal, Tyrosine kinase inhibitors and the thyroid as both an unintended and an intended target. Endocr. Pract. 14, 618–624 (2008)
S. Faivre, C. Delbaldo, K. Vera, C. Robert, S. Lozahic, N. Lassau, C. Bello, S. Deprimo, N. Brega, G. Massimini, J.P. Armand, P. Scigalla, E. Raymond, Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J. Clin. Oncol. 24, 25–35 (2006)
T. Kondo, T. Takagi, H. Kobayashi, J. Iizuka, T. Nozaki, Y. Hashimoto, E. Ikezawa, K. Yoshida, K. Omae, K. Tanabe, Superior tolerability of altered dosing schedule of sunitinib with 2-weeks-on and 1-week-off in patients with metastatic renal cell carcinoma–comparison to standard dosing schedule of 4-weeks-on and 2-weeks-off. Jpn. J. Clin. Oncol. 44, 270–277 (2014)
Y.G. Najjar, K. Mittal, P. Elson, L. Wood, J.A. Garcia, R. Dreicer, B.I. Rini, A 2 weeks on and 1 week off schedule of sunitinib is associated with decreased toxicity in metastatic renal cell carcinoma. Eur. J. Cancer 50, 1084–1089 (2014)
S. Bracarda, R. Iacovelli, L. Boni, M. Rizzo, L. Derosa, M. Rossi, L. Galli, G. Procopio, M. Sisani, F. Longo, M. Santoni, F. Morelli, G. Di Lorenzo, A. Altavilla, C. Porta, A. Camerini, B. Escudier, G. Rainbow, Sunitinib administered on 2/1 schedule in patients with metastatic renal cell carcinoma: the RAINBOW analysis. Ann. Oncol. 26, 2107–2113 (2015)
F. Baffert, T. Le, B. Sennino, G. Thurston, C.J. Kuo, D. Hu-Lowe, D.M. McDonald, Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am. J. Physiol. Heart Circulatory Physiol. 290, H547–H559 (2006)
F. Pani, F. Atzori, G. Baghino, F. Boi, M.T. Ionta, L. Tanca, M. Scartozzi, S. Mariotti, Hypothyroidism and thyroid autoimmunity as a prognostic biomarker of better response in metastatic cancer long-term survivors treated with Sunitinib. Thyroid 26, 1336–1337 (2016)
N. Makita, M. Miyakawa, T. Fujita, T. Iiri, Sunitinib induces hypothyroidism with a markedly reduced vascularity. Thyroid 20, 323–326 (2010)
A. Rogiers, P. Wolter, K. Op de Beeck, M. Thijs, B. Decallonne, P. Schoffski, Shrinkage of thyroid volume in sunitinib-treated patients with renal-cell carcinoma: a potential marker of irreversible thyroid dysfunction? Thyroid 20, 317–322 (2010)
Z. Javed, T. Sathyapalan, Levothyroxine treatment of mild subclinical hypothyroidism: a review of potential risks and benefits. Therapeutic Adv. Endocrinol. Metab. 7, 12–23 (2016)
S.H. Pearce, G. Brabant, L.H. Duntas, F. Monzani, R.P. Peeters, S. Razvi, J.L. Wemeau, 2013 ETA guideline: management of subclinical hypothyroidism. Eur. Thyroid J. 2, 215–228 (2013)
M.G. Lechner, C.M. Vyas, O.R. Hamnvik, E.K. Alexander, P.R. Larsen, T.K. Choueiri, T.E. Angell, Hypothyroidism during tyrosine kinase inhibitor therapy is associated with longer survival in patients with advanced nonthyroidal cancers. Thyroid 28, 445–453 (2018)
Authors’ contributions
L.R., C.P., E.S., and E.G. designed and coordinated the study. F.L. and G.D.B. enrolled and followed patients. L.R., E.S., D.G., R.L., and M.T. followed and managed endocrine aspects of patients and performed US images. C.P., E.G., and A.F. performed quality control checks and separately analyzed thyroid US images. C.P. performed statistical analysis of the data. A.L. verified the analytic method. L.R., E.S., E.G., C.P., and A.F. contributed to the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the iwenstitutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rizza, L., Sbardella, E., Gianfrilli, D. et al. Thyroid profile during the alternative Sunitinib dosing 2/1 schedule in metastatic renal cell carcinoma. Endocrine 67, 597–604 (2020). https://doi.org/10.1007/s12020-019-02088-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-02088-4