Abstract
Background and purpose
Conformal, fractionated radiation therapy (XRT) is variably used as a treatment alternative for active acromegaly patients, usually, after failed pituitary surgery. Our objective was to evaluate the long-term efficacy and safety of XRT using strict criteria of biochemical control.
Setting, design, patients, and methods
Retrospective cohort study of 94 patients (73 women, mean age at radiation 53.16 ± 12.9 years) attending a specialized multidisciplinary clinic between 1998 and 2014 with a mean duration of follow-up of 12.9 ± 7.3 years.
Results
A basal growth hormone < 1 ng/mL and an IGF-1 < 1.2 × the upper limit of normal was achieved by 41% and 50.8%, respectively, at 5 years of follow-up, and by 44% and 66%, respectively, 10 years after XRT. Median tumor volume decreased significantly from 904 mm3 at baseline to 424 mm3 upon last follow-up (p = 0.01). The prevalence of central hypogonadism, central hypocortisolism, and central hypothyroidism increased from 18%, 35%, and 35% at baseline, to 38%, 53%, and 64%, respectively, after 10 years of follow-up. One patient was diagnosed with a meningioma and another one developed optic neuritis. No cerebrovascular events were recorded, and all patients are currently alive.
Conclusion
XRT is an effective and reasonably safe means of controlling acromegalic activity. Its main disadvantages are the time required to achieve biochemical control and the development of anterior pituitary hormone deficiencies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acromegaly is a chronic systemic disorder resulting from the excessive secretion of growth hormone (GH) by a pituitary adenoma [1]. Transsphenoidal surgery (TSS), performed by experienced hands, is successful in 70–80% of microadenomas and intrasellar, confined macroadenomas, however, this is the case for only 40% of macroadenomas with extrasellar extension and in < 10% of invasive macroadenomas [2]. Thus, in order to achieve control of GH secretion, ~ 40% of patients with acromegaly require further therapy, be it pharmacological or radiotherapeutical. Pharmacological therapy with first generation somatostatin analogs (SA) (octreotide LAR and lanreotide autogel) alone or in combination with the dopamine agonist (DA) cabergoline is currently considered as the secondary treatment of choice in acromegaly, whereas radiotherapy has been regarded as a third-line option [2, 3]. Although SA are effective in 30–40% of patients, their main disadvantages are the need to continue treatment for life and their elevated cost [4, 5].
External beam radiotherapy is being used less frequently in developed countries because of the long time required to obtain an objective response and the risk of side effects, including the induction of hypopituitarism and the potential damage to the optic apparatus [3, 6]. In contrast, in developing countries with limited resources to cover the expense of life-long pharmacological treatment, radiotherapy is still a cost-effective alternative [7, 8]. A frequently used strategy is the use of SA while awaiting the response to radiotherapy [2]. The present report constitutes an update of our experience with the use of external beam, conformal, fractionated radiotherapy (XRT) in a large number of patients with acromegaly followed for up to15 years at a specialized multidisciplinary clinic.
Patients and methods
We analyzed the outcome of all patients attending the Acromegaly Clinic of Hospital de Especialidades, Centro Médico Nacional S.XXI who received XRT between 1998 and 2014. This clinic gathers clinical, biochemical, imaging, therapeutic, and histopathological data in over 620 patients who are followed and treated according to a pre-established protocol. The study was approved by our local ethics and scientific committees. All patients sign an informed consent upon enrollment in the clinic.
Definitions
Active acromegaly was diagnosed based on the typical symptoms and signs of the disease, as well as on a glucose-suppressed GH > 1 ng/mL and an elevated, age-adjusted IGF-1 level. We defined biochemical control according to the following criteria: basal GH (bGH) < 2.5 ng/mL, bGH < 1 ng/mL and bGH <1 ng/mL plus age-adjusted IGF-1 < 1.2 times the upper limit of normal (× ULN).
Central hypocortisolism was defined by a morning serum cortisol < 3 μg/dL; central hypothyroidism by a serum-free T4 level < 0.5 ng/dL along with a low or inappropriately normal thyroid-stimulating hormone (TSH); the diagnosis of central hypogonadism was established when the serum estradiol was < 10 pg/mL in women and the serum total testosterone < 300 ng/dL in men in the presence of low or inappropriately normal LH and FSH. Panhypopituitarism was diagnosed when two or more pituitary hormone deficiencies were documented.
Treatment protocol
Of the 94 patients included in this analysis, 79 (84%) had been subjected to pituitary surgery (74 transsphenoidal, 5 transcranial), 7 (7.4%) had been started on primary pharmacological treatment with SA and/or DA and 8 (8.5%) received XRT as their primary treatment. They were all biochemically active and the majority had evidence of a tumor remnant on MRI. Octreotide LAR (20–40 mg every 4 weeks) or lanreotide autogel (90–120 mg every 4 weeks) therapy was initiated 3–6 months after failed surgery and upon biochemical documentation of disease activity. Some patients were also receiving cabergoline, either as monotherapy or in combination with either of the two SA. Pharmacological therapy was discontinued at least 3 months prior to radiation therapy.
Conformal XRT was administered using a lineal accelerator. Two equipments were used during the study period, the “TrueBeam” radiotherapy system (Varian Medical Systems, Palo Alto, CA) and the Versa HD Radiotherapy System (Elekta, Stockholm, Sweden). Throughout the years, there have been several software modifications that allow a more-precise treatment. The median radiation dose was 52 Gy (range 52–57), delivered over 3–5 weeks, in 2–2.5 Gy fractions administered 4–7 days per week. The individual radiation protocol was decided by the radiation oncologist, based on the availability of appointments and the specific circumstances of each patient. For instance, in patients living out of town, an attempt is usually made to complete treatment in as short a period of time as possible (i.e., in 3 weeks). Thus, some patients were treated daily for 3 weeks, each day receiving 2.5 Gy (2.5 × 21 = 52.5 Gy), whereas others were treated 4 days per week for 5 weeks, each day receiving 2.5 Gy (2.5α × 20 = 50 Gy), and still others were treated 5 days per week, for 5 weeks, each day receiving 2 Gy (2 × 25 = 50). Optic apparatus radiation dose was kept below 50.4 Gy using a multi-leave collimator.
As part of a pre-established treatment algorithm, 1 month after finishing XRT, patients are re-started on pharmacological treatment, consisting of octreotide LAR (20 mg monthly) or lanreotide autogel (120 mg monthly), alone (64%) or in combination with cabergoline (2.5 mg weekly) (24%); 12% with minimal disease activity were left without pharmacological treatment. Biochemical evaluations took place immediately prior to XRT and at year intervals thereafter withholding pharmacological treatment for at least one month. Magnetic resonance imaging (MRI) of the sella turcica was done at least every 2 years after XRT; tumor volume was calculated using the modified De Chiro Nelson formula [9].
Hormonal measurements
From 1998 to 2008, GH was measured by means of the Immulite, 2-site chemiluminescent assay (Diagnostic Products Corporation, Los Angeles, CA), with a sensitivity of 0.01 ng/mL and intra- and inter-assay coefficient of variation (CV)s of 6%. As of 2009, GH was determined using the Diasorin-Liaison assay (Salugia, Italy), which has a detection limit of 0.009 ng/mL and intra-and inter-assay CVs of 2.5% and 5.8%, respectively. The International Reference Preparation (IRP) used in these GH assays is the WHO second 95/574.
IGF-1 was separated from its binding proteins by acid-ethanol extraction prior to immunoassay. From 2000 to 2008, we used the Diagnostic Systems Laboratory 2-site chemiluminescent assay (DSL, Webster, TX) with intra- and inter-assay CVs of 2.6 and 4.4%, respectively. Since 2009, IGF-1 has been determined by the Diasorin-Liaison chemiluminescent assay (Salugia, Italy). The IRP in these IGF-1 assays is WHO second 02/254. We established our own normative IGF-1 data analyzing serum samples from 400 healthy individuals. Other hormones (LH, FSH, testosterone, estradiol, TSH, free T4, adrenocorticotropic hormone, and cortisol) were measured by different commercially available immunoassays.
Statistical analysis
Data distribution was established using the Shapiro–Wilks test. Normally distributed variables are presented as means ± standard deviation (SD) and non-normally distributed variables are expressed as medians with inter-quartile ranges (IQR). The X2 test was used to analyze categorical variables, whereas for continuous variables we used Kruskal–Wallis and analysis of variance–Bonferoni tests. A Cox proportional hazards model was carried out to ascertain, which baseline characteristics (age, gender, pre-XRT bGH, and IGF-1, as well as tumor volume) were significantly associated with the probability of reaching the composite goal (bGH < 1 ng/mL and an IGF-1 level < 1.2 × ULN) upon last follow-up. A p value < 0.05 was considered as statistically significant. Statistical package included STATA v.8 and SPSS v.16.
Results
We evaluated the long-term outcome of 94 patients (21 men, 73 women) with acromegaly who received XRT, the majority of them, after pituitary surgery (Table 1). Mean age at the time of irradiation was 53.16 ± 12.9 years. The median interval between surgery and XRT was 24 months (IQR 12–24), and the mean duration of follow-up, after radiation was 13 ± 7.3 years (Table 1). Ninety-four patients had been followed for 1 year; 72 (76.6%) for 3 years; 62 (66%) for 5 years; 44 (46.8%) for 10 years; and 15 (16%) for 15 years (Table 1).
bGH levels fell from 8.4 ng/ml at the beginning of XRT, to 3.7 ng/mL (54% decrease, p = 0.01) at 1 year of follow-up, 2.2 ng/mL (74% decrease, p = 0.01) at 3 years, 1.5 ng/mL (82% decrease, p = 0.01) at 5 years, 1.1 ng/mL (86% decrease, p = 0.01) at 10 years and 0.68 ng/mL (92% decrease, p = 0.001) at 15 years. Median glucose-suppressed GH levels were 4.7 ng/ml before XRT and they fell to 2.7 ng/mL (42.5% decrease, p = 0.01), 2.4 ng/mL (49% decrease, p = 0.001), 1.2 ng/mL (74% decrease, p = 0.001), 0.73 ng/mL (84% decrease, p = 0.001), and 0.6 ng/mL (86% decrease, p = 0.001), at 1, 3, 5, 10, and 15 years of follow-up, respectively. IGF-1 levels declined from 1.94 × ULN prior to XRT, to 1.71 × ULN (11.8% decrease, p = 0.01) after 1 year follow-up, 1.37 × ULN (29.3% decrease, p = 0.001) after 3 years, 1.2 × ULN (38% decrease, p = 0.001) after 5 years, 1.03 × ULN (47% decrease, p = 0.001) after 10 years and 0.94 × ULN (51.5% decrease, p = 0.001) after 15 years.
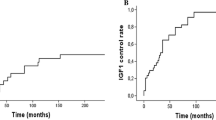
Table 2 and Fig. 1 depict the hormonal outcome after XRT. A bGH < 2.5 ng/mL was achieved by 31, 56, 67, 91, and 93%, 1, 3, 5, 10, and 15 years after XRT, respectively, whereas 11%, 31%, 41%, 44%, and 66% achieved a bGH < 1 ng/mL at the same time points of follow-up. An IGF-1 < 1.2 × ULN was reached by 19.7%, 42%, 50.8%, 61%, and 66% at 1, 3, 5, 10, and 15 years and 12%, 37%, 45%, 51%, and 72% achieved both, a basal GH < 1 ng/mL and an IGF-1 level < 1.2 × ULN at the same time points of follow-up. The proportion of patients requiring pharmacological treatment with SA or cabergoline to maintain biochemical control decreased progressively from 84% at 1 year follow-up, to 62% at 3 years, 55% at 5 years, 48% at 10 years, and 28% at 15 years.
Patients with higher bGH levels experienced significantly greater percent decrements only at 1 year follow-up (GH >30 ng/mL 84.6%, GH 10–29.9 ng/mL 67%, GH < 10 ng/mL 48%, p = 0.001). A Cox proportional hazards model including variables such as age, gender and pre-XRT bGH and IGF-1 levels, revealed that only the pre-XRT IGF-1 was significantly associated with the probability of achieving a bGH < 1 ng/mL (HR 1.2, 95% Confidence Interval 0.996–1.48, p = 0.05) and an IGF-1 < 1.2 × ULN (HR 1.31, 95% confidence interval 1.11–1.55, p = 0.001) after 10 years of follow-up. Median tumor volume decreased from 904 mm3 (IQR 130–3708) immediately before XRT to 424 mm3 (IQR 52–497) upon last follow-up (p = 0.01).
At the time of irradiation, 18% of the patients had central hypogonadism, 35% central hypocortisolism, 35% central hypothyroidism, and 23% had two or more anterior pituitary hormone deficiencies (Table 1). The prevalence of pituitary hormone deficiencies increased progressively so after 10 years of follow-up, 38% of patients had central hypogonadism, 53% central hypocortisolism, 64% central hypothyroidism, and 57% had two or more anterior pituitary hormone deficiencies (Table 2). One patient was diagnosed with a meningioma 10 years after radiation and there was one case of documented optic neuritis. No cerebrovascular events were recorded, and all patients were alive at the time of preparation of this manuscript.
Discussion
The present study is perhaps one of the largest and longest single-center studies evaluating the efficacy and safety of XRT in acromegaly patients with persistent disease activity. Although XRT has been used as an adjunctive treatment of acromegaly since the 1970s [10, 11], there are no prospective or controlled trials evaluating this therapeutic alternative. Most if not all studies evaluating the efficacy and safety of XRT published in the English medical literature have been single-center, retrospective studies including < 80 patients. The only multicenter study addressing this issue is that of Jenkins et al. [12], who evaluated data from 14 centers in the United Kingdom, comprising over 400 patients. Besides the present report from our group, there are only two large, single-center studies one from Brazil including 91 patients [13] and another one from France including 128 patients [14].
Table 3 summarizes the main studies assessing the outcome of acromegaly patients subjected to XRT [12,13,14,15,16,17,18,19,20,21,22,23]. Remission rates after XRT have been as low as 5–7% [15, 16] and as high as 90–100% [17, 23], with a mean of 47 ± 23.6% (median 44). Such a heterogeneous response rate to XRT is likely the result of several factors including the variability of radiation protocols, the different biochemical remission criteria used in each study as well as the length of follow-up. Further complicating the interpretation of data is the fact that published studies use different GH and IGF-1 assays, from in-house polyclonal RIAs to commercially available ultrasensitive immunoassays, throughout follow-up periods that can be as long as 15–20 years. The majority of studies published in the late 1990s and early 2000s defined biochemical control as the achievement of a bGH < 2.5 ng/mL, as this used to be the cutoff value considered as “safe” in terms of mortality rate reduction [14,15,16, 19, 20]. The proportion of patients achieving bGH levels < 2.5 ng/mL varies between 50 and 60% and 70 and 80% after 5 and 10 years of follow-up, respectively [12,13,14,15,16,17,18,19,20,21,22,23]. More-recently published studies using a bGH < 1 ng/mL as the criterion for biochemical remission, report rates that vary between 40 and 50% and 60 and 70% 5 and 10 years after radiation, respectively [20, 22, 23]. The normalization of IGF-1 levels has also been used in several studies to evaluate the efficacy of radiation therapy in acromegaly. As with GH, the proportion of patients achieving a normal IGF-1 increases along with the duration of follow-up, with 20–40% of patients reaching this goal after 2–5 years of follow-up and 60–80% after > 10 years [12,13,14,15, 17,18,19,20,21,22,23]. Our study shows that XRT is capable of significantly reducing GH and IGF-1 levels. Ten years after radiation 91% of patients achieved a bGH of < 2.5 ng/mL, 44% achieved a bGH < 1 ng/mL, and 45% reached the composite biochemical goal of a basal bGH < 1 ng/mL and an IGF-1 < 1.2 × ULN. We have previously reported similar outcome figures in a smaller study including 40 patients of whom 57% and 43% achieved a bGH < 1 ng/mL and an IGF-1 < 1.2 × ULN, respectively, after 10 years of follow-up [22].
Early remission rates in our study (1–3 years post XRT) are somewhat higher than in series published before 2010 [12, 14, 16,17,18,19,20,21], but quite similar to those found in more recently published studies, like the one by Patt et al. [23]. In most series evaluating the outcome of XRT in acromegaly, including our own, target GH is reached before IGF-1 normalization, when using a GH cutoff of 2.5 ng/mL [12,13,14,15,16,17,18,19,20,21,22,23]. However, if we use a more stringent GH cutoff value of 1 ng/mL, concordance between GH and IGF-1 increases. Although some series report that patients who reach adequate GH levels but have persistently elevated IGF-1 have a higher risk of persistent hypertension and higher HbA1c [24], other studies do not find these biochemically discordant patients to be at higher cardiovascular and/or metabolic risk [25].
Despite the proven efficacy of XRT in acromegaly, reaching GH and IGF-1 targets can take several years, therefore patients need to be treated pharmacologically while the effect of radiation takes place. After 1 year of follow-up, only 12% of our patients had reached the composite biochemical goal of bGH < 1 ng/mL and an IGF-1 < 1.2 × ULN, and 84% of them required pharmacological treatment with SA and/or cabergoline. The therapeutic effect of XRT appears to take off after 3 years of follow-up, increasing steadily thereafter, along with a clear reduction in the proportion of patients requiring pharmacological therapy (Fig. 1) [3].
The disadvantages of XRT include the development of pituitary hormone deficiencies as well as the potential risk of cerebrovascular events, neurocognitive impairment, optic neuritis or secondary central nervous system tumors [3]. As shown in the present study and in other studies published before, even though radiation-induced hypopituitarism is unavoidable, it must be pointed out that a substantial number of patients already have one or more pituitary hormone deficiencies immediately prior to treatment [14, 17, 22, 23].
Radiation therapy has been associated with an increased acromegaly mortality risk mainly owing to cerebrovascular events [26,27,28]. Interestingly, however, in a large mortality study performed at our center, that included over 400 patients followed for more than 10 years, we did not find a single cerebrovascular event in those subjected to XRT and having received XRT was not associated at all with mortality [29]. The results of the present study, and those of a smaller study published 8 years ago confirm that radiation therapy is not significantly associated with the risk of cerebrovascular events. Furthermore, among 10 studies published after the year 2000 evaluating the efficacy and safety of XRT in acromegaly and comprising almost 1000 patients, only one case of an atherothrombotic stroke was documented [12,13,14, 17,18,19,20,21,22,23].
Neurocognitive impairment putatively resulting from radiation-induced brain necrosis has been rarely reported. Among the 10 studies alluded to before, only five cases of neurocognitive impairment were reported and none of these cases was documented with neither formal neuropsychological testing nor with proper brain imaging techniques [13, 14, 20]. As we did not perform formal neurocognitive testing, we cannot totally exclude the presence of subtle neuropsychological abnormalities. Neurogenic visual loss, another dreaded complication of XRT, occurred in only one of the 94 patients in our study and has a reported incidence of 1–3% in other series [12,13,14, 18, 22]. It has been estimated that radiation-induced optic neuropathy is rare when the total radiation dose is kept below 50–54 Gy [3, 30]. The development of a secondary central nervous system tumor is a rare complication of pituitary radiation with an incidence of 1–2% [9, 22]. Most of these tumors have been meningiomas developing 10 or more years after radiation [19, 22].
We conclude that conformal, fractionated radiotherapy is an efficient means of controlling GH and IGF-1 levels, as well as tumor mass in acromegaly. The effect of XRT starts to become apparent 2–3 years after radiation and is usually associated with significant hypopituitarism upon long-term follow-up. With the currently-in-use radiation techniques, side effects such as optic neuritis, cognitive impairment due to cerebral necrosis and cerebrovascular events are infrequent, although longer follow ups could be required in order to ascertain the life-time risk of these complications.
References
S. Melmed, Acromegaly pathogenesis and treatment. J. Clin. Invest. 119, 3189–3202 (2009)
E. Espinosa, C. Ramirez, M. Mercado, The multimodal treatment of acromegaly: current status and future perspectives. DEndocr. Immune. Disord. Drug. Targets 14, 169–181 (2014)
G. Minniti, C. Scaringi, R.M. Enrici, Radiation techniques for acromegaly. Radiat. Oncol. 6, 167 (2011)
M. Mercado, E. Espinosa, C. Ramirez, Current status and future directions of pharmacological therapy for acromegaly. Minerva Endocrinol. 41, 351–265 (2016)
A.L. Espinosa-de-los-Monteros, B. Gonzalez, G. Vargas, E. Sosa, M. Mercado, Octreotide LAR treatment of acromegaly in “real life”: long term outcome at a tertiary care center. Pituitary 18, 290–296 (2015)
S. Melmed, M.D. Bronstein, P. Chanson, A. Klibanski, F.F. Casanueva, J.A.H. Wass et al. A consensus on acromegaly therapeutic outcomes. Nat. Rev. Endocrinol. 14, 552–561 (2018)
M.D. Bronstein, O.D. Bruno, A. Abreu, R. Mangupli, M. Mercado, A practical approach to acromegaly management in Latin America. Pituitary 17, s30–s35 (2014)
L.A. Portocarrero-Ortiz, A. Vergara-López, M. Vidrio-Velazquez, A.M. Uribe-Días, A. García-Domínguez, A. Reza-Albarrán, D. Cuevas-Ramos, V. Melgar, J. Talavera, A.J. Rivera-Hernández, C.V. Valencia-Méndez, M. Mercado; (The Mexican Acromegaly Registry Group), The Mexican Acromegaly Registry: clinical-biochemical characteristics at diagnosis and therapeutic outcomes. J. Clin. Endocrinol. Metab. 101, 3997–4004 (2016)
T. Ertekin, N. Hacer, A.T. Turgut, K. Aycan, O. Ozceli, M. Turgut, Comparison of three methods for the estimation of pituitary volume using magnetic resonance imaging: a stereological study. Pituitary 14, 31–38 (2011)
J. Roth, P. Gorden, K. Brace, Efficacy of conventional pituitary irradiation in acromegaly. N. Engl. J. Med. 282, 1385–1391 (1970)
R. Eastman, P. Gorden, J. Roth, Conventional supervoltage irradiation is an effective treatment for acromegaly. J. Clin. Endocrinol. Metab. 48, 931–940 (1979)
P.J. Jenkins, P. Bates, N. Carson, P.M. Stewart, J.A.H. Wass, Conventional pituitary irradiation is effective in lowering serum growth hormone and insulin-like growth factor-I in patients with acromegaly. J. Clin. Endocrinol. Metab. 91, 1239–1245 (2006)
R.S. Jallad, N.R. Musolino, L.R. Salgado, M.D. Bronstein, Treatment of acromegaly: Is there still a place for radiotherapy. Pituitary 10, 53–59 (2007)
G. Barrande, M. Pittino-Lungo, J. Coste, D. Ponvert, X. Bertagna, J.P. Luton, J. Bertherat, Hormonal and metabolic effects of radiotherapy in acromegaly: Long term results in 128 patients followed in a single centre. J. Clin. Endocrinol. Metab. 85, 3779–3785 (2000)
A.L. Barkan, I. Halasz, K.J. Dornfield, C.A. Jaffe, R.D. Friberg, W.F. Chandler, H.M. Sandler, Pituitary irradiation is ineffective in normalization of insulin-like growth factor-I in patients with acromegaly. J. Clin. Endocrinol. Metab. 82, 3187–3191 (1997)
N.C. Thalassinos, S. Tsagarakis, G. Ioannides, I. Tzavara, C. Papavasilau, Megavoltage pituitary irradiation lowers but seldom leads to “safe” GH levels in acromegaly: a long-term follow up study. Eur. J. Endocrinol. 138, 160–163 (1998)
N.R. Biermasz, H. van Dulken, F. Roelfsema, Long-term follow up results of postoperative radiotherapy in 36 patients with acromegaly. J. Clin. Endocrinol. Metab. 85, 2476–2482 (2000)
J.S. Powell, S.L. Wardlaw, K.D. Post, P.D. Freda, Outcome of radiotherapy for acromegaly using normalization of insulin-like growth factor-I to define cure. J. Clin. Endocrinol. Metab. 85, 2068–2071 (2000)
P. Epaminonda, S. Poretti, V. Capiello, P. Beck-Pecoz, G. Faglia, M. Arosio, Efficacy of radiotherapy in normalizing serum IGF-1, acid-labile subunit (ALS) and IGFBP3 levels in acromegaly. Clin. Endocrinol. 55, 183–189 (2001)
G. Minniti, M.L. Jaffrain-Rea, M. Osti, V. Esposito, A. Santoro, F. Salda, P. Gargiulo, G. Tamburano, R.M. Enrici, The long-term efficacy of conventional radiotherapy in patients with GH-secreting pituitary adenomas. Clin. Endocrinol. 62, 210–216 (2005)
K. Mullan, C. Sanabria, W.P. Abram, E.M. McConnell, H.C. Courtney, S.J. Hunter et al. Long-term effect of external pituitary irradiation on IGF-1 levels in patients with acromegaly free of adjunctive treatment. Eur. J. Endocrinol. 161, 547–551 (2009)
B. Gonzalez, G. Vargas, A.L. Espinosa-de-los-Monteros, E. Sosa, M. Mercado, Efficacy and safety of radiotherapy in acromegaly. Arch. Med. Res. 42, 48–52 (2011)
H. Patt, R. Jalali, C. Yerawar, S. Khore, T. Gupta, A. Gael et al. High precision conformal fractionated radiotherapy is effective in achieving remission in patients with acromegaly after failed transsphenoidal surgery. Endocr. Pract. 22, 162–172 (2016)
O. Alexopoulou, M. Bex, R. Abs, E. T’Sjoen, B. Velkeniers, D. Maiter, Divergence between growth hormone and insulin-like growth factor-1 concentrations in the follow up of acromegaly. J. Clin. Endocrinol. Metab. 93, 1324–1330 (2008)
A.L. Espinosa-de-los-Monteros, E. Sosa, S. Cheng, R. Ochoa, C. Sandoval, G. Guinto, V. Mendoza, I. Hernández, M. Molina, Mercado:biochemical evaluation of disease activity after pituitary surgery in acromegaly: a critical analysis of patients who spontaneously change disease status. Clin. Endocrinol. 64, 245–249 (2006)
J. Ayuk, R.N. Clayton, G. Holder, M.C. Sheppard, P.M. Stewart, A.S. Bates, Growth hormone and pituitary radiotherapy but not serum insulin-like growth factor-I concentrations predict excess mortality in patients with acromegaly. J. Clin. Endocrinol. Metab. 89, 1613–1617 (2004)
M. Arosio, G. Reimondo, E. Malchiodi, P. Berchialla, A. Borraccino, L. De Marinis et al. Predictors of morbidity and mortality in acromegaly: an Italian survey. Eur. J. Endocrinol. 167, 189–198 (2012)
F. Bogazzi, A.M. Colao, G. Rossi, M. Lombardi, C. Urbani, C. Sardella et al. Comparison of the effects of primary somatostatin analogue therapy and pituitary adenomectomy on survival in patients with acromegaly: a retrospective cohort study. Eur. J. Endocrinol. 169, 367–376 (2013)
M. Mercado, B. Gonzalez, G. Vargas, C. Ramirez, A.L. Espinosa-de-los-Monteros, E. Sosa et al. Successful mortality reduction and control of comorbidities in patients with acromegaly followed at a highly specialized multidisciplinary clinic. J. Clin. Endocrinol. Metab. 99, 4438–4446 (2014)
J.S. Loeffler, H.A. Shih, Radiation therapy in the management of pituitary adenomas. J. Clin. Endocrinol. Metab. 96, 1992–2003 (2011)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Study was approved by our local Ethics and Scientific Committees.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gonzales-Virla, B., Vargas-Ortega, G., Martínez-Vázquez, KB. et al. Efficacy and safety of fractionated conformal radiation therapy in acromegaly: a long-term follow-up study. Endocrine 65, 386–392 (2019). https://doi.org/10.1007/s12020-019-01955-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-01955-4