Abstract
Purpose
To compare women diagnosed with gestational diabetes mellitus (GDM) according to the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) diagnostic criteria based on the number of OGTT diagnostic criteria, which OGTT parameters are altered and the glycemic deviation from proposed diagnostic cutoffs.
Methods
Cross-sectional, multicentric study of women diagnosed with GDM between 24–28 weeks of pregnancy according to the IADPSG criteria, in Portugal, between 2012–2014. Primary outcomes: large for gestational age (LGA) and maternal glucose metabolism status after delivery. Secondary outcome: small for gestational age (SGA).
Results
Three-thousand three-hundred fourteen patients were included; 67% had 1 OGTT altered value; 3.6% had LGA and 13% had SGA newborns; 7% had prediabetes/diabetes after delivery. Three diagnostic criteria in OGTT (OR 3.02; p < 0.001), a diagnostic value at 0 min (OR 2.09; p = 0.002) and 60 min (OR 1.70; p = 0.022) and glucose deviation at 0 min (OR 1.02; p = 0.014) were predictors of LGA. Having 2 (OR 1.94; p < 0.001) or 3 (OR 3.93; p < 0.001) diagnostic criteria in OGTT, a diagnostic value at 0 min (OR 1.76; p = 0.002), at 60 min (OR 1.57; p = 0.007) and at 120 min (OR 3.11; p < 0.001), the glucose deviation at 0 (OR 1.02; p = 0.017) and 120 min (OR 1.02; p < 0.001) were predictors of prediabetes/diabetes after delivery. Insufficient weight gain in pregnancy (OR 1.49; p < 0.001) and lower maternal BMI (OR 0.97; p = 0.024) were associated with SGA.
Conclusion
IADPSG diagnostic criteria include a heterogeneous group of women, for whom different management strategies should be adopted to obtain ideal pregnancy outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) has been a subject of controversy, regarding its definition, diagnostic criteria, treatment goals, and clinical implications.
According to the American Diabetes Association (ADA), GDM is defined as “diabetes that is first diagnosed in the second or third trimester of pregnancy that is not clearly either preexisting type 1 or type 2 diabetes” [1].
In 2008, the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study was the first large-scale multinational study to show that maternal hyperglycemia between 24–28 weeks was linearly and positively correlated with large for gestational age (LGA) infants, caesarian rate, cord-blood serum C-peptide level, and neonatal hypoglycemia. No glycemic threshold for a greater risk was identified for most outcomes [2].
Based on the findings of the HAPO study, the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) proposed, in 2011, a major change in the diagnostic criteria of GDM: a one-step diagnostic approach based on a 2 h 75-g oral glucose tolerance test (OGTT) between 24–28 weeks of gestation in all women not previously known to have diabetes or GDM [3]. Based on the average fasting, 1 h, and 2 h plasma glucose values obtained in the HAPO study population and on an odds ratio (OR) of 1.75 for the studied adverse outcomes, the IADPSG proposed new diagnostic criteria thresholds for the diagnosis of GDM. The diagnosis is made based on, at least, one altered parameter [3]. This was an attempt to uniformize diagnostic standards, as different diagnostic procedures and thresholds were used previously worldwide. However, these new guidelines imply that the individual glucose OGTT values are independent predictors of adverse outcomes and the that relative importance of each value is similar.
This change was not universally adopted and has been a topic of extensive debate, with some advocating that it led to a significant increased incidence of GDM, with consequent burden on the health care systems and medicalization of previously considered healthy pregnancies, with potential implications on women’s quality of life [1]. On the other hand, the pathophysiologic basis of impaired fasting glucose and impaired glucose tolerance in OGTT are different. While the former seems to be related to insulin resistance, the latter is due to decreased beta-cell function, either in early- and late-phases of insulin secretion [4].
In Portugal, the new IADPSG diagnostic criteria were nationally adopted in 2011.
However, there is lack of evidence from randomized controlled trials comparing the old vs. new diagnostic criteria for GDM and showing the benefits of treating modestly elevated glycemic values in the mother’s future risk of diabetes and the fetus metabolic benefits in its future life [1]. Moreover, there are no data showing the best treatment approach for these newly diagnosed women, namely the ideal intensity of treatment and monitoring during pregnancy [1]. In fact, the IADPSG diagnostic criteria classify equally a potentially heterogenous group of women, who are monitored and treated the same way, with the same treatment targets during pregnancy.
We hypothesized that women diagnosed according to these criteria in the second trimester of pregnancy using 2 h 75-g OGTT constitute an heterogenous group and may warrant different treatment strategies and targets.
In this study, our aim is to compare different subsets of women diagnosed with GDM according to the IADPSG 1-step approach, based on the number of OGTT diagnostic criteria, which OGTT parameter is/are altered and the glycemic deviation from proposed diagnostic cutoffs. Our primary outcomes are the occurrence of LGA newborn and the reclassification status of maternal glucose metabolism after birth as prediabetes or diabetes. Our secondary outcome is the occurrence of small for gestational age (SGA) newborn.
Methods
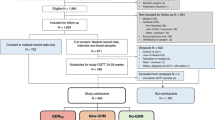
This is a cross-sectional, multicentric, nation-wide study of women diagnosed with GDM and followed at the medical centers of the Portuguese Group for the Study of Diabetes and Pregnancy, between 1st January 2012 and 31st December 2014. The centers included are representative of Portugal’s mainland. Each center sent their data, obtained from the patients’ medical records, to the coordinator of the study group. The merged data were blinded for both patient and hospital identification.
We included women diagnosed with GDM between 24–28 weeks of pregnancy according to the IADPSG criteria [3]. We excluded multiple pregnancies, women with pregestational diabetes, women diagnosed with GDM before or after 24–28 weeks, women who gave birth to newborns before 28 weeks, and cases with lack of information about any of the three parameters of 2 h 75-g OGTT.
We studied the following variables: maternal characteristics (age, diabetes mellitus in first degree relatives, previous GDM, previous macrosomia, pre-pregnancy body mass index (BMI)); factors related to the present pregnancy (OGTT parameters, weight gain, need for hypoglycaemic therapy); factors related to the newborn (sex, gestational age (GA) and birthweight); reclassification of maternal glucose metabolism 6 to 12 weeks after delivery, according to ADA criteria [1]. Women were treated with hypoglycemic drugs to achieve the following therapeutic goals: glucose before meals < 90 mg/dl; glucose 1 h after meals: < 120 mg/dl, according to national standards.
According to the pre-pregnancy BMI, we divided women in four groups: “Underweight” (BMI ≤ 18.4 Kg/m2), “Normal weight” (BMI 18.5–24.9 Kg/m2); “Overweight” (BMI 25.0–29.9 Kg/m2) and “Obese” (BMI ≥ 30.0 Kg/m2). We classified weight gain in pregnancy according to the Institute of Medicine’s recommendations as “insufficient”, “adequate” or “excessive” for BMI category [5].
We also classified newborns as “Small for Gestational Age (SGA)” if weight for gestational age below 10th percentile; “Adequate for Gestational Age (AGA)” if weight for gestational age between 10th–90th percentile and “Large for Gestational Age (LGA)” if weight for gestational age above 90th percentile, according to Fenton Growth Charts [6].
For statistical analysis, we used STATA IC 14® software. In the descriptive analysis, for quantitative variables, we used central tendency measures and dispersion measures and for qualitative variables we used absolute numbers and percentages. We looked for associations between outcomes and qualitative covariates using the Chi-square test and for differences in the distribution of quantitative variables using Anova and Kruskall–wallis tests.
For the studied outcomes, we created three multiple logistic regression models based on different ways of analyzing glucose values at 0, 60, and 120 min in OGTT: model 1 (number of diagnostic criteria); model 2 (which OGTT parameters were altered); model 3 (glycemic difference from the proposed diagnostic cutoffs). In logistic model building, we adjusted for potential confounders using a stepwise regression with a backward elimination approach. We constructed receiver operating characteristic (ROC) curves and calculated areas under the curves (AUCs) to compare the different models of logistic regression for each outcome.
A 2-sided p-value ≤ 0.05 was considered statistically significant. In logistic regression, for the two primary outcomes, we adopted a conservative approach and used a Bonferroni correction, considering a significant value if p < 0.025. A complete-case analysis was used to deal with missing data.
This research was conducted according to the Declaration of Helsinki.
Results
In the study period, 8911 women with GDM were followed at the medical centers participating in the study. After applying the inclusion and exclusion criteria, 3314 women were included in the study. The number of participants with missing data for each variable of interest was as follows: maternal age (n = 6), DM in first degree relatives (n = 116), previous GDM (n = 82), previous macrosomia (n = 94), pre-pregnancy BMI (n = 150), weight gain (n = 379), need for insulin (n = 36) or oral antidiabetic agents (n = 2311), fetal sex (n = 162), week of delivery (n = 108), birthweight (n = 101), reclassification of maternal glucose metabolism after delivery (n = 943). There were no missing values in all categories related to OGTT results.
The patients’ mean age was 33.4 ± 5.3 years. More than 50% were overweight or obese and 25% had an excessive weight gain during pregnancy. Most women were diagnosed with GDM based on only one diagnostic criteria in OGTT; 27% had a diagnostic value at 0 min and >50% had diagnostic values at 60 and 120 min. The glucose value deviation from the proposed diagnostic cutoffs varied greatly in this population. After GDM diagnosis, >40% of the women required insulin treatment to achieve the desired therapeutic goals. Only 113 newborns (3.5%) were LGA; however, a greater proportion (13.2%) were SGA. After delivery, 7% of the women were classified as having prediabetes/diabetes (Table 1).
Mothers of LGA infants had higher glucose values at 0 and 60 min in OGTT, a higher percentage of diagnosis based on three OGTT criteria, higher initial BMI, greater prevalence of previous GDM, previous macrosomia, need for insulin treatment and an increased prevalence of excessive weight gain during pregnancy than mothers of non LGA infants. Mothers of SGA infants had a significantly lower BMI and a greater frequency of insufficient weight gain in pregnancy; 38% of these women were treated with insulin. Most of these women had only one diagnostic value in OGTT (Table 2).
The diagnosis of prediabetes/diabetes after delivery was positively associated with maternal age, family history of diabetes, excessive weight gain in pregnancy, insulin treatment, the number of diagnostic criteria and higher glucose values in OGTT at 0, 60, and 120 min (Table 3).
Table 4 shows the OR for LGA and SGA and for prediabetes/diabetes diagnosis after pregnancy in the three different logistic regression models previously described. Having three diagnostic criteria in OGTT, a diagnostic value at 0 and 60 min and the absolute glucose difference at 0 min from proposed cutoff were significant predictors of LGA. Previous macrosomia, maternal BMI, and excessive weight gain in pregnancy were also associated with LGA (Table 4).
Having 2 or 3 GDM diagnostic criteria, a diagnostic value at 0, 60, or 120 min and the glucose difference from proposed cutoffs at 0 and 120 min were predictors of reclassification status after birth. A family history of diabetes was also associated with this outcome (Table 4).
Insufficient weight gain in pregnancy (OR 1.49; CI 95% 1.19–1.86, p < 0.001) and lower maternal BMI (OR 0.97; CI 95% 0.95–0.997, p = 0.024) were significant predictors of SGA in multiple logistic regression. Need for insulin treatment was not (OR 0.82; CI 95% 0.65–1.02, p = 0.08). When the analysis was adjusted for the OGTT values as described in Table 4, only a diagnostic value at 0 min and the glycemic difference at 0 min were significant predictors of SGA (Table 4).
Discussion
GDM affects a significant proportion of women, following the increasing global prevalence of overweight and obesity in women of reproductive age. Although it has been demonstrated that even slightly elevated glycemic levels are associated with adverse outcomes in pregnancy, the relationship between different OGTT characteristics, namely the number of diagnostic criteria and which values are elevated, as well as the appropriate management strategies for different patients remain controversial.
Aiming to contribute to further clarify this question, we compared women diagnosed with GDM between 24–28 weeks of pregnancy through 75-g OGTT according to the number of diagnostic criteria, which criteria were altered and the magnitude of the deviation from proposed cutoffs.
In this cohort, the diagnosis of GDM was mostly made based on a single altered OGTT value, namely after 60 or 120 min. Black et al. [7], in a study of 1691 women with untreated GDM diagnosed by IADPSG criteria, have reported that most women were also diagnosed based on one single altered OGTT value, but in that cohort the fasting value was the most frequently elevated.
In our study, in all three parameters, the median glycemic difference above the proposed cutoff was low and showed a narrow interquartile range. Therefore, only a minority of these women showed a marked hyperglycemic value. This may be relevant since the inter- and intra-assay variability is not considered when the diagnosis is made in a 1-step diagnostic approach. It can be questioned if, in women with only one slightly elevated glycemic value, repeating the diagnostic test would confirm the diagnosis.
Despite this, more than 40% of women received insulin treatment, which may have been due to the strict glycemic targets adopted at that time in Portugal (glucose before meals < 90 mg/dl; 1 h after meal: < 120 mg/dl).
We found a positive association between the number of diagnostic criteria in OGTT and LGA, but only the presence of three diagnostic criteria was predictor of this outcome. A Chinese study, including 2927 women with GDM according to the same criteria, found similar results, with the strongest association in patients with three abnormal values [8].
In our study, higher glucose levels at 0 and 60 min were associated with LGA, with a strongest association for the fasting value. In the HAPO study, none of the three OGTT values was superior in predicting fetal adiposity [9]. However, others have shown the association of fasting hyperglycemia and LGA. Black et al. reported that a diagnostic value at 0 and 60 min in OGTT was associated with increased risk for LGA, with greater risk for abnormal fasting glucose (either isolated or associated with impaired tolerance test (IGT)), compared to patients with only IGT [7]. Feng et al. [8] also found that fasting hyperglycemia had the strongest association with LGA (OR 1.7; CI 1.29–2.25), with increasing risk of LGA as fasting glucose levels increased. Others have reported similar findings [10, 11]. Maternal BMI and excessive weight gain in pregnancy were predictors of LGA in all logistic regression models, independently of glycemic values, as previously reported [12,13,14].
In this GDM population, we found a relatively low prevalence of LGA infants and a surprisingly higher percentage of SGA infants, namely in mothers with lower pre-pregnancy BMI and with insufficient weight gain in pregnancy. Although this may reflect the achievement of a therapeutic goal (low LGA prevalence), it could also be questioned if it reflects over diagnosis of GDM and/or overtreatment of these women, due to the strict glycemic targets adopted, at expenses of a high prevalence of insulin treatment and of insufficient weight gain. It should be noticed that either too much or too little weight gain in pregnancy can adversely impact the health of children, as very low weight gain in pregnancy is associated with SGA neonates which, in turn, is a risk factor for preterm delivery, hypertension, cardiovascular disease, and diabetes in future life [8, 15]. This may support the idea that women with GD diagnosed with IADPSG criteria may not all need the same intensive treatment approach. In fact, others have found that about 1/3 of women with GDM according to IADPSG criteria have rates of fetal macrosomia only slightly above women with no GDM [16].
It has long been recognized that women with GDM have an increased risk for type 2 diabetes mellitus. Maternal BMI, GDM severity, and low C-peptide levels have been associated with this outcome [15]. In our study, women with post-delivery diagnosis of prediabetes/diabetes had a higher age, greater prevalence of diabetes family history and of excessive weight gain in pregnancy than women with normal glucose status at reclassification. In multiple logistic regression, however, only diabetes family history remained a significant predictor of this outcome.
Although only a minority of patients were diagnosed as having prediabetes/diabetes after delivery, women with a greater glycemic dysfunction (diagnosed either based on the number of diagnostic criteria in OGTT, which diagnostic criteria were elevated and the magnitude of the glycemic deviation) had an increased risk for diabetes in the near future. The association with the number of abnormal values in OGTT has previously been shown in women diagnosed with GDM through the 100-g diagnostic OGTT [17]. However, it must be noted that, in a GDM women population according to these diagnostic criteria, more than 90% had normal OGTT after delivery. More than 40% of these women were treated with insulin and a similar proportion had insufficient weight gain during pregnancy. The fact that most women had only one slightly elevated parameter in OGTT and, therefore, a near-normal glycemic metabolism, could have contributed to the relatively low prevalence of prediabetes/diabetes after delivery.
In this study, we included a large number of patients, representing a significant percentage of the total number of Portuguese women with GDM in the study period. All patients were diagnosed with GDM using the IADPSG diagnostic criteria, were regularly followed by multidisciplinary teams and were treated with the same glycemic targets. Reclassification of maternal glucose metabolism after delivery was done using the same standard procedure in all women. However, this study has some limitations to the generalizability of its findings. As it was a multicentric retrospective study, OGTT determinations were not performed in the same laboratory and we cannot exclude the existence of interlaboratory variability in glucose determinations. We only had a small number of patients with three altered parameters and, thus, with greater glycemic dysfunction, which may also have underestimated differences between groups. As all patients received some form of medical intervention through pregnancy, the differences between groups regarding hyperglycemic dysfunction and its effect in the occurrence of LGA may be underestimated. Also, we cannot exclude differences among centers regarding insulin therapy and strategies for weight control during pregnancy, namely the number of medical visits, number of plasma glucose measurements, different thresholds for insulin treatment, and different nutrition plans. All patients participated in nutritional consultations at all medical centers. Nutrition plans were adjusted according to the clinical condition of the patients, namely the presence of GDM, the pre-pregnancy BMI and the trimester of pregnancy. However, there was no standard protocol shared by all centers. As this is a retrospective study, we cannot assess patients’ compliance.
In conclusion, we confirmed our hypothesis that women diagnosed with IADPSG criteria in the second trimester of pregnancy constitute a heterogeneous group regarding some neonatal and maternal outcomes. We found that the number of abnormal OGTT values identifies groups with different risks for LGA and prediabetes/diabetes after delivery. Fasting hyperglycemia and glycemia at 60 min seem to be the most important glycemic alterations associated with LGA, while all glucose values seem to be associated with the risk for glucose metabolism dysfunction after pregnancy. Our findings are supported by other recent studies [7, 8, 11]. We also found that, in women with lower BMI and lower fasting glycemic values, strict glycemic targets may lead to high insulin treatment rates and insufficient weight gain in pregnancy, with consequent SGA newborns. These findings suggest that different management strategies should be adopted for different subsets of patients with GDM to obtain ideal pregnancy outcomes, both for the mother and the child.
References
American Diabetes, A., 2. Classification and diagnosis of diabetes. Diabetes Care 40(Suppl 1), S11–S24 (2017). https://doi.org/10.2337/dc17-S005
H.S.C.R. Group, B.E. Metzger, L.P. Lowe, A.R. Dyer, E.R. Trimble, U. Chaovarindr, D.R. Coustan, D.R. Hadden, D.R. McCance, M. Hod, H.D. McIntyre, J.J. Oats, B. Persson, M.S. Rogers, D.A. Sacks, Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 358(19), 1991–2002 (2008). https://doi.org/10.1056/NEJMoa0707943
International Association of Diabetes and Pregnancy Study Groups Consensus Panel, B.E. Metzger, S.G. Gabbe, B. Persson, T.A. Buchanan, P.A. Catalano, P. Damm, A.R. Dyer, A. Leiva, M. Hod, J.L. Kitzmiler, L.P. Lowe, H.D. McIntyre, J.J. Oats, Y. Omori, M.I. Schmidt, International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33(3), 676–682 (2010). https://doi.org/10.2337/dc09-1848
M. Hanefeld, C. Koehler, K. Fuecker, E. Henkel, F. Schaper, T. Temelkova-Kurktschiev, Insulin secretion and insulin sensitivity pattern is different in isolated impaired glucose tolerance and impaired fasting glucose: the risk factor in Impaired Glucose Tolerance for Atherosclerosis and Diabetes study. Diabetes Care 26(3), 868–874 (2003)
K.M. Rasmussen, A.L. Yaktine (eds.) Weight Gain During Pregnancy: Reexamining the Guidelines. (The National Academies Collection: Reports funded by National Institutes of Health, Washington (DC), 2009)
T.R. Fenton, J.H. Kim, A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 13, 59 (2013). https://doi.org/10.1186/1471-2431-13-59
M.H. Black, D.A. Sacks, A.H. Xiang, J.M. Lawrence, Clinical outcomes of pregnancies complicated by mild gestational diabetes mellitus differ by combinations of abnormal oral glucose tolerance test values. Diabetes Care 33(12), 2524–2530 (2010). https://doi.org/10.2337/dc10-1445
H. Feng, W.W. Zhu, H.X. Yang, Y.M. Wei, C. Wang, R.N. Su, M. Hod, E. Hadar, Relationship between oral glucose tolerance test characteristics and adverse pregnancy outcomes among women with gestational diabetes mellitus. Chin. Med. J. (Engl.). 130(9), 1012–1018 (2017). https://doi.org/10.4103/0366-6999.204928
H.S.C.R. Group, Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes 58(2), 453–459 (2009). https://doi.org/10.2337/db08-1112
K. Brankica, V.N. Valentina, S.K. Slagjana, J.M. Sasha, Maternal 75-g OGTT glucose levels as predictive factors for large-for-gestational age newborns in women with gestational diabetes mellitus. Arch. Endocrinol. Metab. 60(1), 36–41 (2016). https://doi.org/10.1590/2359-3997000000126
E. Disse, J. Graeppi-Dulac, G. Joncour-Mills, O. Dupuis, C. Thivolet, Heterogeneity of pregnancy outcomes and risk of LGA neonates in Caucasian females according to IADPSG criteria for gestational diabetes mellitus. Diabetes Metab. 39(2), 132–138 (2013). https://doi.org/10.1016/j.diabet.2012.09.006
H.S.C.R. Group, Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG 117(5), 575–584 (2010). https://doi.org/10.1111/j.1471-0528.2009.02486.x
A.M. Stuebe, M.B. Landon, Y. Lai, C.Y. Spong, M.W. Carpenter, S.M. Ramin, B. Casey, R.J. Wapner, M.W. Varner, D.J. Rouse, A. Sciscione, P. Catalano, M. Harper, G. Saade, Y. Sorokin, A.M. Peaceman, J.E. Tolosa, Eunice Kennedy Shriver National Institute of Child and Human Development Maternal-FetalMedicine Units Network, Bethesda, MD, Maternal BMI, glucose tolerance, and adverse pregnancyoutcomes. Am. J. Obstet. Gynecol 207(1), 62e61–62e67 (2012). https://doi.org/10.1016/j.ajog.2012.04.035
P.M. Catalano, H.D. McIntyre, J.K. Cruickshank, D.R. McCance, A.R. Dyer, B.E. Metzger, L.P. Lowe, E.R. Trimble, D.R. Coustan, D.R. Hadden, B. Persson, M. Hod, J.J. Oats, H.S.C.R. Group, The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 35(4), 780–786 (2012). https://doi.org/10.2337/dc11-1790
S.J. Herring, E. Oken, Obesity and diabetes in mothers and their children: can we stop the intergenerational cycle? Curr. Diab. Rep. 11(1), 20–27 (2011). https://doi.org/10.1007/s11892-010-0156-9
O. Kalter-Leibovici, L.S. Freedman, L. Olmer, N. Liebermann, A. Heymann, O. Tal, L. Lerner-Geva, N. Melamed, M. Hod, Screening and diagnosis of gestational diabetes mellitus: critical appraisal of the new International Association of Diabetes in Pregnancy Study Group recommendations on a national level. Diabetes Care 35(9), 1894–1896 (2012). https://doi.org/10.2337/dc12-0041
F. Pallardo, L. Herranz, T. Garcia-Ingelmo, C. Grande, P. Martin-Vaquero, M. Janez, A. Gonzalez, Early postpartum metabolic assessment in women with prior gestational diabetes. Diabetes Care 22(7), 1053–1058 (1999)
Acknowledgements
Multidisciplinary teams belonging to The Portuguese Pregnancy and Diabetes Study Group of the Portuguese Society of Diabetology from the following portuguese medical centers: Centro Hospitalar Barreiro Montijo; Centro Hospitalar de Entre o Douro e Vouga; Centro Hospitalar de Lisboa Norte; Centro Hospitalar de Lisboa Ocidental; Centro Hospitalar de Vila Nova de Gaia/Espinho; Centro Hospitalar do Algarve; Centro Hospitalar do Alto Ave; Centro Hospitalar do Baixo Vouga; Centro Hospitalar do Médio Ave; Centro Hospitalar do Oeste; Centro Hospitalar do Porto; Centro Hospitalar do Tâmega e Sousa; Centro Hospitalar e Universitário de Coimbra; Centro Hospitalar São João; Hospital de Braga; Hospital Garcia de Orta; Unidade Local de Saúde de Matosinhos; Unidade Local de Saúde do Alto Minho.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Santos, M.J., Fernandes, V. & The Portuguese Pregnancy and Diabetes Study Group. Gestational diabetes mellitus: different management strategies should be adopted for different subsets of patients diagnosed by oral glucose tolerance test. Endocrine 62, 602–610 (2018). https://doi.org/10.1007/s12020-018-1704-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1704-3