Abstract
Purpose
Emerging data supports an association between parathyroid hormone (PTH) and aldosterone. It has been speculated, that potential adverse cardiovascular effects of vitamin D insufficiency may partly be caused by the development of secondary hyperparathyroidism with increased activity of the renin-angiotensin-aldosterone system (RAAS).
We aimed to investigate the effect of normalizing vitamin D status and/or reducing PTH levels on RAAS activity and other markers of cardiovascular health.
Methods
In a double-blinded study during wintertime, we randomized 81 healthy postmenopausal women with secondary hyperparathyroidism (PTH > 6.9 pmol/l) and 25-hydroxy-vitamin D (25(OH)D) levels < 50 nmol/l to 12 weeks of treatment with vitamin D3 70 µg/day (2800 IU/day) or identical placebo.
Markers of cardiovascular health were defined as changes in the plasma RAAS, glycated hemoglobin, lipids, and lipoproteins, blood pressure, vascular stiffness, heart rate, and cardiac conductivity.
Results
Compared to placebo, vitamin D3 treatment significantly increased plasma levels of 25(OH)D and 1,25(OH)2D by 230% (95% CI: 189–272%) and 58% (190–271%), respectively. Vitamin D3 treatment reduced PTH by 17% (11–23%), but did not reduce RAAS activity. Compared to placebo, vitamin D3 treatment increased plasma levels of high-density lipoproteins (HDL) by 4.6% (0.12–9.12%), but did not affect other measured indices.
Conclusions
Vitamin D3 supplementation normalized vitamin D levels and reduced PTH. The supplement increased levels of HDL, but had no effects on RAAS activity or other indices of cardiovascular health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous observational studies report an association between low plasma levels of 25-hydroxyvitamin D (25(OH)D) and an increased risk of cardiovascular disease (CVD) [1, 2]. An effect of vitamin D on cardiovascular health is biologically plausible, as the vitamin D receptor and the 1-alpha hydroxylase (CYP27B1) are expressed in cardiovascular tissues. It has, therefore, been suggested, that 25(OH)D may be activated and exert biological function within the tissue.
Meta-analyses on findings from randomized clinical trials (RCTs) have shown a decreased overall mortality in elderly in response to vitamin D3 supplementation [3,4,5]. A decreased cardiovascular specific mortality is reported in observational studies, although not confirmed in pooled data from RCTs [2, 4, 5].
The potential adverse health effect of low 25(OH)D levels may partly be due to an increased activity of the renin-angiotensin-aldosterone system (RAAS) and the development of secondary hyperparathyroidism (SHPT).
RAAS activation increases blood pressure and exerts various deleterious effects on the cardiovascular system. Reduced levels of renin and aldosterone as well as a reduced blood pressure have been found in some [6,7,8], but not all RCTs [3, 9,10,11] investigating the effect of vitamin D.
High levels of PTH have been shown to be independently associated with an increased cardiovascular mortality in the general population [12]. Accumulating evidence supports an association between PTH and aldosterone [13,14,15]. A direct association between PTH and aldosterone is supported by the fact that PTH type 1 receptors have been shown in the adrenal cortex as well as mineralocorticoid receptors in the parathyroid gland [13, 16]. Similar to patients with hyperaldosteronism, patients with primary hyperparathyroidism are at increased risk of CVD and surgical cure by parathyroidectomy is associated with reduced concentrations of angiotensin 2, aldosterone, and a lower risk of CVD [17,18,19].
Most previous RCTs evaluating vitamin D supplementation on cardiovascular health included participants with a replete vitamin D status. Potential detrimental effect of high levels of PTH is poorly understood [3]. Accordingly, we hypothesized that vitamin D supplementation through a normalization of vitamin D levels and/or a PTH reduction improved markers of cardiovascular health, as assessed by changes the plasma RAAS (p-aldosterone is primary end point), glycated hemoglobin, lipids and lipoproteins, blood pressure, vascular stiffness, heart rate, and cardiac conductivity.
Methods
We designed our study as an investigator-initiated parallel group, single-center, randomized double-blinded placebo-controlled trial. The study was conducted in Aarhus, Denmark, at 56° N in winter season (November–April) during 2 consecutive years (2015/-16 and 2016/-17). In this period, cutaneous cholecalciferol synthesis is insignificant.
Recruitment of participants
By a letter outlined with a short description of the study, we invited postmenopausal women aged 60–80 to participate. Using the Danish Nationwide Civil Register, we contacted a randomly selected group of women from the general background population. Potential participants were excluded if they had a history of CVD (cerebral infarctions, heart attacks, or arterial insufficiency), liver- or renal disease (creatinine above upper limit of the reference range, known renal arterial stenosis or previous kidney transplantation). Potential participants were also excluded if they were on treatment with antihypertensives, diuretics, systemic glucocorticoids, non-steroidal anti-inflammatory drugs, or lithium or at any time had received treatment with anti-osteoporotic drugs. Furthermore, we excluded women who planned to travel to sunny destinations, or used sunbeds on a regular basis during the study.
The remaining potential participants were offered a biochemical screening sample. From the entire screened cohort, we only included women with 25(OH)D level below < 50 nmol/l, a PTH level above upper limit of the reference range ( > 6.9 pmol/l) as well as plasma levels of creatinine and calcium below upper reference range.
All participants had their medical history verified by an interview and a regular clinical examination was performed prior to randomization. Criteria for participation were re-assessed by interview at all clinical visits. No participants were withdrawn from the study.
Intervention
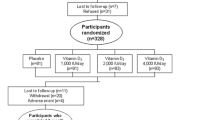
For 12 weeks, the participants underwent concealed 1:1 randomization to daily vitamin D3 supplementation (cholecalciferol 70 microgram) or similar placebo. The design was factorial for the two first weeks, as the participants were co-treated with an angiotensin 2-receptor blocker (valsartan 80 mg per day) or similar placebo in order to study whether treatment with valsartan lowered PTH levels (Fig. 1). The findings from these initial two-weeks of study have previously been reported, showing no effects of valsartan on PTH levels [20]. As we included women with vitamin insufficiency and wanted to assure a replete vitamin D status in participants randomized to vitamin D supplementation, we chose a daily dose of 70 microgram, as previous studies have suggested an increase in 25(OH)D levels of 0.7 to 1.1 nmol/l per 1 microgram/day of vitamin D supplementation [21, 22]. The hospital pharmacy randomized participants using a computer program (http://www.randomization.com) into blocks of 16, 16, 8, 8, 16, 8, and 8 participants. The block-sizes were unknown for the investigators.
Investigators and participants were blinded to the study drug allocations. We received treatment randomization codes from the pharmacy when all data were on-file.
Orkla Care A/S produced and supplied cholecalciferol and identical placebo tablets. The hospital pharmacy labeled all bottles.
For the two first weeks of trial, we instructed the women to take both tablets at bedtime with a glass of water, as food consumption decreases the absorption of valsartan with 40% [21]. Subsequent, vitamin D and identical placebo tablets were taken with breakfast in the morning, as food intake may potentiate the absorption of fat-soluble vitamins such as vitamin D [23]. There were no dietary instructions, and the participants were told to continue their normal daily living.
Study measurements
Clinical visits were performed at baseline and after 2 (±2 days), 6 (±1 week), and 12 (±2 weeks) weeks of treatment. All examinations and blood sampling were performed in the morning after an overnight fast. To minimize the intra-individual variability of the measurements, all participants were lying down resting for at least 15 min before examining blood pressure, arterial stiffness, and cardiac conductivity. The participants were lying down resting for 30 min before blood sampling.
We assessed the total dietary intake of calcium according to reported average dietary intake of milk, cheese, and other dairy products by a questionnaire [24].
Blood and urine samples
In accordance with standard operating procedures, blood samples for batch analyses were centrifuged at 4000 rpm at 5 °C for 10 min. Plasma for batch analysis and a 24-hour urine for measurement of cortisol was stored at −80 °C until assayed in summer 2017 after a maximum storage of 18 months. PTH was re-analyzed in January 2018.
Analytical methods, laboratory, reference intervals, measurement ranges, and coefficients of variation for all batch analysis are shown in the Supplementary Table.
The possibility of a regression toward the mean bias was quantified by looking at all PTH measurements performed in Central Denmark Region (a total population of 1.3 million) during year 2016.
Blood pressure, vascular stiffness, and cardiac conductivity
At all visits, we measured blood pressure with a dual-cuff monitor from WatchBP Office (Microlife AG, Widnau, Switzerland) allowing simultaneous measurement on both arms. The device reported systolic blood pressure (SBP) and diastolic blood pressure (DBP) as an average of three measurements in both arms. The blood pressure measurement on the non-dominant arm is reported unless we found a difference of ≥ 10 mm Hg between arms at baseline [25]. If so, the arm with the highest blood pressure is reported. We selected the appropriate cuff for the blood pressure recordings by measuring the circumference of the upper arm.
At week 0 and week 12, we measured 24-hour blood pressure in all participants using the Arteriograph24 equipment (TensioMed Inc., Hungary). Data on blood pressure was obtained every 20 min for 24 h. Participants with at least 14 daytime and seven night-time measurements of blood pressure were included in the analysis. Sleep pattern was monitored, and a mean during day- and night-time was calculated. A weighted 24 h mean of heart rate, SBP, and DBP are reported. Nocturnal dipping was calculated as percentage reduction in blood pressure while sleeping.
Blood pressure, central blood pressure estimates, pulse wave velocity (PWV), and pulse wave analysis were further measured by tonometry at week 0 and week 12 using the SphygmoCor XCEL equipment (AtCor Medical, Sydney, Australia). To assess central PWV, carotid pulse waves were measured by applanation tonometry. Simultaneously, an inflated cuff measured femoral pulse waves over the femoral artery. Central PWV was calculated as the direct distance between pulse measuring sites divided by the pulse transit time. Because the direct distance overestimates real PWV, the estimate was multiplied with 0.8 [26]. Only records meeting the quality requirements (quality value above or equal to 75% assessed by the program) were included in the analysis. Preferably, two measurements with a difference of less than 1 m/s was obtained, and the average of two measurements reported. Electrocardiograms (ECG) were collected using a calibrated standard 12-lead ECG machine. QC intervals were corrected by Bazett’s QTc = (QT/√RR) and Fridericias QTc = (QT∛RR) formula, respectively.
Statistical analyses
Sample size calculations were performed prior to initiating the trial. We assumed a mean plasma aldosterone at 91 (±59) pg/ml [27] and an reduction in aldosterone at 40%, similar to treatment with an angiotensin 2-receptor blocker [28]. In order to obtain 80% statistical power at the 5% level of significance, 80 women should be included.
The distribution of data was tested with QQ-plots and histograms. Normal distributed data were analyzed with parametric tests. Skewed variables were log-transformed and re-tested for normality before any parametric analysis.
Changes in response to treatment are reported as means or medians with 95% confidence intervals, or medians with 25 and 75% percentiles when appropriate. For categorical data, we used a χ2 test. Changes from baseline to end of study were analyzed using a test for two independent samples or Wilcoxon-Ranks tests. If we had end-points with more than two measurements, analysis of variance (ANOVA) with repeated measurements were used. To determine correlation coefficients between measurements of RAAS and the calcium metabolism, Pearson correlation analyses were conducted. A multivariate linear regression model investigated an association between renal calcium excretion and blood pressure. We evaluated all end-points using an intention to treat approach. We considered a two-tail p-value < 0.05 as statistical significant. SPSS version 24 was used for the statistical analysis.
Results
Participants
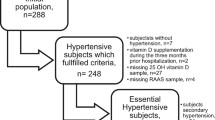
We invited 32,321 randomly selected women by letter, among whom 4113 women responded positively (Fig. 2). A blood test was performed in 1580 women. The test identified 109 women with SHPT in terms of high PTH- and low 25(OH)D levels, allowing for inclusion of the 81 women participating in the study. All included women completed the study in accordance with the study program.
Baseline characteristics of participants are shown in Table 1 and 2. Except from fewer current smokers (N = 1 vs. N = 8, p < 0.05) and more systolic non-dippers (N = 18 vs. N = 7, p < 0.01), in the vitamin D group, there was no differences between groups. None used anti-epileptic medication.
Vitamin D and PTH
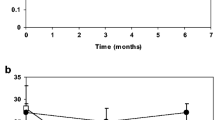
Levels of 25(OH)D and PTH are depicted in Table 2. Compared with placebo, vitamin D treatment increased levels of 25(OH)D and 1,25(OH)2D by a mean of 243% (95% CI: 203–289%) and 58% (44–72%), respectively (Table 2 and Fig. 3).
Plasma levels of 25-hydroxyvitamin D (25(OH)D, 1,25-dihydroxyvitamin D (1,25(OH)2D), parathyroid hormone (PTH) aldosterone, renin, and angiotensin 2 during follow-up. Data are presented as means with 95% confidence intervals. p-values for 25(OH)D, 1,25(OH)2D, PTH, aldosterone and renin are bases on analysis of variance (ANOVA) for repeated measurements (RM-ANOVA). Comparison between groups (independent t-test) are performed post-hoc (percentages change from baseline) if RM-ANOVA have indicated a significant between group difference. PTH is reported as the initial PTH Roche measurement. Angiotensin 2 were only measured in 41 women at week 0 and 12 and the p-value for angiotensin 2 is a comparison of changes between groups (independent t-test). The initial increase in renin levels is attributable to co-treatment with an angiotensin 2-receptor blocker during the first 2 weeks (see text). Vitamin D had no effect on renin at any time
Vitamin D treatment decreased PTH levels significantly by 11.0% (2.9–19.1%) after 2 weeks, 13.4% (6.9–19.8%) after 6 weeks and 16.8% (10.9–22.8%) after 12 weeks (Fig. 3).
Vitamin D and the RAAS
Compared with placebo, vitamin D treatment did not change plasma levels of aldosterone, renin, aldosterone/renin ratio, angiotensin 2, or arginine vasopressine at any time points of measurements (Table 2 and Fig. 3).
Vitamin D, renal function, and plasma electrolytes
Compared with placebo, vitamin D treatment did not affect plasma concentrations of calcium, phosphate, magnesium, or sodium (Table 2). Vitamin D treatment increased creatinine clearance by 8.5% (0.6–16.4%), and this was not changed by adjustment for body surface area (data not shown).
Vitamin D and 24-hour urine analyses
Compared to placebo, vitamin D treatment increased the renal calcium-, magnesium and phosphate excretion by 32% (16–49%), 20% (7–34%) and 16% (3–30%), respectively (Table 2). After 12 weeks of supplementation, significantly more women were hypercalcicuric in the vitamin D group, (N = 9), as compared with the placebo group (N = 2), p = 0.03.
Vitamin D treatment did not affect 24 h urinary cortisol or urine osmolality.
Vitamin D, lipids, lipoproteins, and glycated HbA1c
HDL cholesterol decreased in both groups during the winter, but compared with placebo, vitamin D treatment increased HDL cholesterol by 4.6% (0.12–9.1%, p = 0.04) (Table 2 and Fig. 4). One patient started treatment with simvastatin during the study. Excluding her from analyses did not change the results. Five participants had abnormal low HDL at baseline. Seven participants went from normal ( > 1.2 mmol/l) to low HDL ( ≤ 1.2 mmol/l) cholesterol status during the study, with most (N = 6) receiving placebo. One women (receiving vitamin D3) went from a low to a normal HDL level. The changes were borderline significant (p = 0.06).
There were no changes in total cholesterol, LDL cholesterol, triglycerides, or glycated HbA1c in response to treatment.
Vitamin D and cardiac conductivity
All participants had sinus rhythm with normal ECG intervals at all examinations. Vitamin D treatment did not change heart rate (as assessed by ECG, 24 h blood pressure measurements, and SphygmoCor XCEL, (Table 3) or the PR-, QRS-, and QTc-intervals, as assessed by ECG.
Vitamin D, blood pressure, and vascular stiffness
The number of successful blood pressure measurements in the 24-hour investigation was 53 (51–56) and did not differ between groups. There was no differences in different measures of diastolic and systolic blood pressure (Table 3) or estimates stratified in day- and night-time (data not shown).
Nor were there any changes in SBP or DBP after 2 and 6 weeks neither (data not shown). Ambulatory PVW and augmentation index (Aix) assessed by SphygmoCor XCEL did not change significantly in response to treatment (Table 3).
At baseline, 33% were systolic non-dippers and 19% diastolic non-dippers (defined as a < 10% reduction in night, as compared with awake blood pressure). Vitamin D supplementation did not affect dipper status. Aldosterone levels were lower in diastolic non-dippers (p < 0.05) at both baseline and end of study, with no other markers of the RAAS, lipids or arterial stiffness distinguishing dippers from non-dippers (data not shown).
Blood pressure and renal calcium excretion
The weighted 24 h blood pressure measurements and the renal calcium excretion correlated at baseline (DBP: r = 0.34, p = < 0.01, 0.04; SBP: r = 0.34, p < 0.01) and at end of study (DBP: r = 0.40, p < 0.001; SBP: r = 0.47, p = < 0.001). Mutual adjustments for age, weight, creatinine clearance, current smoking, and levels of 25(OH)D did not change the association for SBP and DBP at baseline or SBP at end of study, whereas the association between renal calcium excretion and DBP was no longer present at end of study (data not shown).
Women with 25(OH)D levels ≤ 30 nmol/l
Restricting analyses to the subgroup of women with plasma 25(OH)D levels ≤ 30 nmol/l at baseline (N = 11 in the placebo group and N = 20 in the vitamin D group) showed results similar to the overall findings. Between group differences following treatment in plasma levels of PTH, 25(OH)D, and 1,25(OH)2D were significant and slightly more pronounced compared to changes in the entire group of randomized women, with no significant effects between groups in any of the markers of cardiovascular health (data not shown).
Post-hoc analysis exploring the substantial PTH decrease
At baseline, PTH levels were 34% lower than at screening 12 (±6) days before.
To quantify a regression toward the mean effect, all PTH measurements in Central Denmark Region (a total population of 1.3 million) during 1 year (2016) were studied. A total of 2765 individuals had at least two available measurements of plasma PTH. Compared to the first measurement, plasma levels of PTH at the second measurement were 10.4% lower for the individuals with PTH > 6.9 pmol/l (N = 1075), and 81.0% higher for the individuals with PTH < 1.9 pmol/l (N = 40).
PTH reanalysis confirmed our initial findings and showed similar response to vitamin D treatment (Table 2).
Compliance and adverse events
Compliance rate assessed by pill count was > 99% at all visits. Overall, 71 adverse events were reported. The most common adverse event was infections in the upper airways (N = 27), with 14 (34%) in the placebo group and 13 (33%) in the vitamin D group (p = 0.89). For all adverse events reported, there were no significant differences between the treatment groups.
Discussion
Using a placebo-controlled, randomized design, we showed that a daily vitamin D3 supplement normalized vitamin D status and reduced PTH, but the supplement had no effects on RAAS activity or most measured cardiovascular markers. However, our study suggests an effect of vitamin D supplementation of plasma levels of HDL cholesterol, as HDL cholesterol decreased significantly more in the placebo group.
HDL cholesterol is known to exhibit a seasonal variation with peak levels in January [29, 30]. As most of our participants (78%) were included in January and February, this may explain the decrease in HDL cholesterol in both groups. The association between HDL and vitamin D3 is in accordance with several observational studies [31, 32], whereas findings from RCTs and Mendelian randomization studies are inconclusive [31, 33]. Although low levels of HDL cholesterol are associated with an increased risk of CVD, causality has so far not been established [34]. Changes in HDL levels in response to treatment was a secondary outcome, and we cannot exclude that our findings may be attributable to a statistical type one error. Larger RCTs to assess causality between vitamin D and HDL cholesterol are warranted.
In previous trials investigating the effect of vitamin D supplementation on RAAS activity, discrepant results have been reported. In a few trials, supplementation with vitamin D is shown to decrease plasma levels of renin, aldosterone, and blood pressure [6,7,8, 35]. In the most recently published study by McMullan et al. [9], supplementation with ergocalciferol (vitamin D2) did not cause significant effects on blood pressure or RAAS activity. No associations between 25(OH)D and the RAAS were found. It has been argued that vitamin D2 is less effective than vitamin D3 [36]. However, our data do not support such an explanation, as we trialed vitamin D3 and similar to McMullan et al. [9] found no effects on cardiovascular indices.
The potential effect of vitamin D on blood pressure has been extensively investigated. Most meta-analyses on RCTs have not documented beneficial effects of vitamin D supplementation on blood pressure [3]. A Mendelian randomization study including 142,255 individuals reported a 10% increase in genetically determined 25(OH)D concentration is associated with a 0.29 mm Hg decrease in DBP (95% CI –0.52 to –0.07), illustrating a minor clinical impact at best [37].
Increased arterial stiffness is an independent risk factor for the development of CVD, and intervention studies investigating vitamin D insufficiency and/or hyperparathyroidism reports reduced arterial stiffness in response to treatment with vitamin D or parathyroidectomy [38,39,40]. Our study does not support such effects which is in agreement with two recent published meta-analysis, showing no significant effects of vitamin D supplementation on PVW or AIx [41, 42].
Our study showed a slight, but significant increase in creatinine clearance in response to vitamin D supplementation. A priori, we did not expect effects of vitamin D supplementation on renal function. Further studies are needed to confirm whether this is a real effect of vitamin D supplementation or a by chance finding.
Vitamin D is known to increase the intestinal absorption of calcium, but most published studies have not shown increased renal excretion in response to vitamin D supplementation [10, 43, 44]. Surprisingly, our trial showed a 32% (95% CI: 16 to 49) increased renal calcium excretion in response to vitamin D supplementation. As an increase in urinary calcium may increase the risk of nephrocalcinosis and nephrolithiasis, it is of importance to assess such an possible adverse effect further in upcoming studies [45].
The renal calcium handling is of importance to calcium homeostasis, and calcium levels influence blood pressure regulation. In accordance with other studies, we found a weak correlation between renal calcium excretion and most 24 h blood pressure measurements [46,47,48].
Our primary aim was to investigate whether a vitamin D supplement and an assumed reduction in PTH reduced plasma levels of aldosterone. Interventional trials conducted ahead of ours, reported a positive interaction between PTH and aldosterone, but most of these studies intervened with a blood pressure reduction in terms of antihypertensive treatment or adrenalectomy (in patients with primary hyperaldosteronism) [13, 15, 49, 50]. Our study did not show an association between PTH and the RAAS. While we conducted this trial, the results from the only other RCT designed to explore potential interactions between PTH and aldosterone was published. The trial investigated effects of 8 weeks of aldosterone blockade with the selective mineralocorticoid receptor antagonist eplerenone in patients with primary hyperparathyroidism [51]. Eplerenone reduced blood pressure and tended to reduce urinary calcium excretion, but did not affect PTH levels.
We speculate whether an alternative explanation for studies reporting a positive association between PTH and aldosterone levels is a blood pressure mediated SHPT. In other words, lowering blood pressure reduces the renal calcium- and magnesium excretion thereby increasing plasma calcium and reducing PTH. Further studies should aim at investigating such mechanisms in details.
At screening, all studied participants had low 25(OH)D levels and PTH levels above the upper limit of normal. We assumed that subjects with high PTH levels are the one most likely to benefit from a vitamin D supplement. An increased overall- and CVD-specific mortality has been reported in people with levels of 25(OH)D < 30 and > 90 nmol/l [2]. Accordingly, it has been suggested that trials investigating vitamin D and CVD should focus on populations with initial 25(OH)D levels <30 nmol/l and that treatment concentrations above 100 nmol/l should be avoided [52]. We raised levels of vitamin D 25(OH)D to 90 nmol/l. Although our subgroup analysis on participants with very low 25(OHD levels ( ≤ 30 nmol/l) may be limited by a low number of subjects (N = 31), the subgroup analyses did not show any signs of a beneficial effect of vitamin D supplementation on cardiovascular markers. It would have been preferred to include women with very low levels of vitamin D only, but if so, the inclusion of participants would have been extremely difficult in our set-up.
Our study has several strengths as well as limitations. Importantly, the trial was designed according to the scientific gold standard as a randomized placebo-controlled trial designed to evaluate the effect of vitamin D treatment in women with low 25(OH)D levels and SHPT. Inclusion succeeded, and there was no drop out. Baseline characteristics were well balanced between study groups. Participants had a high compliance rate, and we avoided seasonal changes in 25(OH)D and PTH levels by conducting the study during winter season. We excluded conditions influencing the RAAS and/or the normal calcium homeostasis to minimize bias, leaving a group of healthy, vitamin D insufficient participants.
Surprisingly, we found a 34% (95% CI: 31–37%) lower PTH level at baseline compared to screening. An obvious explanation is a regression toward the mean bias, but as quantified, this may only partly explain the lower levels. In order to quantify a regression toward the mean effect, we performed an ancillary analysis, showing that the magnitude of such an effect is approximately 10%.
Other possible mechanisms are fasting and diurnal variation. PTH is known to show a mid-morning nadir, with a diurnal amplitude at 34% and fasting lowers PTH levels by around 10% [53,54,55]. As opposed to the screening measures collected at a random time during the day, all blood samples in the study were collected in the morning after an overnight fast. If PTH levels at baseline had been measured as non-fasting samples after the morning nadir, more participants would probably have had PTH levels above the upper limit of the reference interval, as all participants had at screening. As we are in a non-fasting state most of the day, this could be a more appropriate reflection of “true” PTH levels.
During the two weeks, the study was co-designed to investigate the PTH response to valsartan treatment. There was no interaction between treatment with the vitamin D3 supplement and valsartan [20] and although the factorial design is a limitation to measurements after two weeks, the half-life of valsartan is 6–9 h making an effect on reported 12 weeks measures unlikely [56].
It is also a limitation, that we did not estimate food intake during the study. The effect of randomization and comparable 24 h renal sodium excretions (the gold standard to assess sodium intake), however, argue against profound differences between groups. Finally, as most included women were normotensive, our results do not necessarily apply to hypertensive women.
In conclusion, a daily vitamin D3 supplementation normalized vitamin D status and reduced PTH levels without affecting RAAS activity. We found a weak positive association between blood pressure and renal calcium excretion, but our study does not support a direct association between 25(OH)D or PTH and the RAAS. Except from a small potential beneficial effect on HDL cholesterol, vitamin D3 had no effect on markers of cardiovascular health. Changes in HDL was a designed secondary end point, and larger studies primary designed to study the effect on cholesterol status are warranted.
References
M.R. Grübler, W. März, S. Pilz, T.B. Grammer, C. Trummer, C. Müllner, V. Schwetz, M. Pandis, N. Verheyen, A. Tomaschitz, A. Fiordelisi, D. Laudisio, E. Cipolletta, G. Iaccarino, Vitamin-D concentrations, cardiovascular risk and events - a review of epidemiological evidence. Rev. Endocr. Metab. Disord. 18, 259 (2017)
M. Gaksch, R. Jorde, G. Grimnes, R. Joakimsen, H. Schirmer, T. Wilsgaard, E.B. Mathiesen, I. Njølstad, M.L. Løchen, W. Maürz, M.E. Kleber, A. Tomaschitz, M. Gruübler, G. Eiriksdottir, E.F. Gudmundsson, T.B. Harris, M.F. Cotch, T. Aspelund, V. Gudnason, F. Rutters, J.W.J. Beulens, E. Van’t Riet, G. Nijpels, J.M. Dekker, D. Grove-Laugesen, L. Rejnmark, M.A. Busch, G.B.M. Mensink, C. Scheidt-Nave, M. Thamm, K.M.A. Swart, I.A. Brouwer, P. Lips, N.M. Van Schoor, C.T. Sempos, R.A. Durazo-Arvizu, Z. Škrabaókovaó, K.G. Dowling, K.D. Cashman, M. Kiely, S. Pilz, Vitamin D and mortality: Individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One. 12, 1 (2017)
L. Rejnmark, L.S. Bislev, K.D. Cashman, G. Eiríksdottir, M. Gaksch, M.R. Grübler, G. Grimnes, V. Gudnason, P. Lips, S. Pilz, N.M. Van Schoor, M. Kiely, R. Jorde, Non-skeletal health effects of vitamin D supplementation: A systematic review on findings from meta-analyses summarizing trial data. PLoS One 1, e010512, pp 39 (2017)
G. Bjelakovic, G. Ll, D. Nikolova, K. Whitfield, J. Wetterslev, S. Rg, M. Bjelakovic, G. Bjelakovic, L.L. Gluud, D. Nikolova, K. Whitfield, J. Wetterslev, R.G. Simonetti, Bje-, M.: Vitamin D supplementation for prevention of mortality in adults (Review). Cochrane database Syst. Rev. 1–205 (2014)
S. Afzal, P. Brondum-Jacobsen, S.E. Bojesen, B.G. Nordestgaard, Genetically low vitamin D concentrations and increased mortality: mendelian randomisation analysis in three large cohorts. Bmj. 349, g6330 (2014)
N.F. Schroten, W.P.T. Ruifrok, L. Kleijn, M.M. Dokter, H.H. Silljé, H.J. Lambers Heerspink, S.J.L. Bakker, I.P. Kema, W.H. Van Gilst, D.J. Van Veldhuisen, H.L. Hillege, R.A. De Boer, Short-term vitamin D3 supplementation lowers plasma renin activity in patients with stable chronic heart failure: An open-label, blinded end point, randomized prospective trial (VitD-CHF trial). Am. Heart J. 166, 357 (2013)
M.R. Grübler, M. Gaksch, K. Kienreich, N. Verheyen, J. Schmid, B.W.J. Ó Hartaigh, G. Richtig, H. Scharnagl, A. Meinitzer, B. Pieske, A. Fahrleitner-Pammer, W. März, A. Tomaschitz, S. Pilz, Effects of Vitamin D Supplementation on Plasma Aldosterone and Renin-A Randomized Placebo-Controlled Trial. J. Clin. Hypertens. 18, 608 (2016)
J.P. Forman, J.B. Scott, K. Ng, B.F. Drake, E. Suarez, D.L. Hayden, G.G. Bennett, P.D. Chandler, B.W. Hollis, K.M. Emmons, E.L. Giovannucci, C.S. Fuchs, A.T. Chan, Effect of vitamin d supplementation on blood pressure in blacks. Hypertension. 61, 779 (2013)
C.J. McMullan, L. Borgi, G.C. Curhan, N. Fisher, J.P. Forman, The effect of vitamin D on renin–angiotensin system activation and blood pressure. J. Hypertens. 35, 822 (2017)
S. Pilz, M. Gaksch, K. Kienreich, M. Grübler, N. Verheyen, A. Fahrleitner-Pammer, G. Treiber, C. Drechsler, B.ó Hartaigh, Barbara Obermayer-Pietsch, V. Schwetz, F. Aberer, J. Mader, H. Scharnagl, A. Meinitzer, E. Lerchbaum, J.M. Dekker, A. Zittermann, W. März, A. Tomaschitz, Effects of Vitamin D on Blood Pressure and Cardiovascular Risk Factors a Randomized controlled trial. Hypertension. 65, 0 (2015)
R. Scragg, A.W. Stewart, D. Waayer, C.M.M. Lawes, L. Toop, J. Sluyter, J. Murphy, K.-T. Khaw, C.A. Camargo, Effect of Monthly High-Dose Vitamin D Supplementation on Cardiovascular Disease in the Vitamin D Assessment Study. JAMA Cardiol. 2, 608 (2017)
A.J. Van Ballegooijen, I. Reinders, M. Visser, I.A. Brouwer, Parathyroid hormone and cardiovascular disease events: A systematic review and meta-analysis of prospective studies. Am. Heart J. 165, 655 (2013)
J.M. Brown, J.S. Williams, J.M. Luther, R. Garg, A.E. Garza, L.H. Pojoga, D.T. Ruan, G.H. Williams, G.K. Adler, A. Vaidya, Human interventions to characterize novel relationships between the renin-angiotensin-aldosterone system and parathyroid hormone. Hypertension. 63, 273 (2014)
Bislev, L.S., Sikjær, T., Rolighed, L., Rejnmark, L.: Relationship Between Aldosterone and Parathyroid Hormone, and the Effect of Angiotensin and Aldosterone Inhibition on Bone Health. Clin. Rev. Bone Miner. Metab. 194–205 (2015)
J. Brown, I.H. de Boer, C. Robinson-Cohen, D.S. Siscovick, B. Kestenbaum, M. Allison, A. Vaidya, I.H. De Boer, C. Robinson-Cohen, Aldosterone, Parathyroid hormone, and the Use of Renin-Angiotensin-Aldosterone System Inhibitors: The Multi-Ethnic Study of Atherosclerosis. J. Clin. Endocrinol. Metab. 1, 1–9 (2014)
C. Maniero, A. Fassina, V. Guzzardo, L. Lenzini, G. Amadori, M.R. Pelizzo, C. Gomez-Sanchez, G.P. Rossi, Primary hyperparathyroidism with concurrent primary aldosteronism. Hypertension. 58, 341 (2011)
R. Pacifici, H.M. Perry, W. Shieber, E. Biglieri, D.M. Droke, L.V. Avioli, Adrenal responses to subtotal parathyroidectomy for primary hyperparathyroidism. Calcif. Tissue Int. 41, 119 (1987)
B. Jespersen, E.B. Pedersen, P. Charles, H. Danielsen, H. Juhl, Elevated angiotensin II and vasopressin in primary hyperparathyroidism. Angiotensin II infusion studies before and after removal of the parathyroid adenoma. Acta Endocrinol. (Copenh). 120, 362 (1989)
P. Vestergaard, C.L. Mollerup, V.G. Frøkjaer, P. Christiansen, M. Blichert-Toft, L. Mosekilde, Cardiovascular events before and after surgery for primary hyperparathyroidism. World J. Surg. 27, 216 (2003)
Bislev, L.S., Rødbro, L., Bech, J., Pedersen, E., Rolighed, L., Sikjaer, T., Rejnmark, L.: Effects of treatment with an angiotensin 2 receptor blocker and/or vitamin D3 on parathyroid hormone and aldosterone: a randomized, placebo-controlled trial. Clin. Endocrinol. (Oxf). 1–11 (2018)
M. Burnier, H.R. Brunner, Angiotensin II receptor antagonists. Lancet 355, 637–645 (2000). https://doi.org/10.1016/S0140-6736(99)10365-9
R.P. Heaney, K.M. Davies, T.C. Chen, M.F. Holick, M. Janet Barger-Lux, Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am. J. Clin. Nutr. 77, 204 (2003).
G.B. Mulligan, A. Licata, Taking vitamin D with the largest meal improves absorption and results in higher serum levels of 25-hydroxyvitamin D. J. Bone Miner. Res. 25, 928 (2010)
A.P. Hermann, J. Thomsen, P. Vestergaard, L. Mosekilde, C.P. Assessment, A quick method compared to a 7 days food diary. Calcif Tissue Int 82, S82 (1999)
E. O’Brien, G. Parati, G. Stergiou, R. Asmar, L. Beilin, G. Bilo, D. Clement, A. De La Sierra, P. De Leeuw, E. Dolan, R. Fagard, J. Graves, G.A. Head, Y. Imai, K. Kario, E. Lurbe, J.M. Mallion, G. Mancia, T. Mengden, M. Myers, G. Ogedegbe, T. Ohkubo, S. Omboni, P. Palatini, J. Redon, L.M. Ruilope, A. Shennan, J.A. Staessen, G. Van Montfrans, P. Verdecchia, B. Waeber, J. Wang, A. Zanchetti, Y. Zhang, European society of hypertension position paper on ambulatory blood pressure monitoring. J. Hypertens. 31, 1731 (2013)
F. Mattace-Raso, A. Hofman, G.C. Verwoert, J.C.M. Wittemana, I. Wilkinson, J. Cockcroft, C. McEniery, YasminaS. Laurent, P. Boutouyrie, E. Bozec, T.W. Hansen, C. Torp-Pedersen, H. Ibsen, J. Jeppesen, S.J. Vermeersch, E. Rietzschel, M. de Buyzere, T.C. Gillebert, L. van Bortel, P. Segers, C. Vlachopoulos, C. Aznaouridis, C. Stefanadis, A. Benetos, C. Labat, P. Lacolley, C.D.A. Stehouwer, G. Nijpels, J.M. Dekker, I. Ferreira, J.W.R. Twisk, S. Czernichow, P. Galan, S. Hercberg, B. Pannier, A. Guérin, G. London, J. Kennedy Cruickshank, S.G. Anderson, A. Paini, E.A. Rosei, M.L. Muiesan, M. Salvetti, J. Filipovsky, J. Seidlerova, M. Dolejsova, Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘Establishing normal and reference values’. Eur. Heart J. 31, 2338 (2010)
S. Trenkel, C. Seifarth, H. Schobel, E.G. Hahn, J. Hensen, Ratio of serum aldosterone to plasma renin concentration in essential hypertension and primary aldosteronism. Exp. Clin. Endocrinol. Diabetes. 110, 80 (2002)
J.N. Cohn, I.S. Anand, R. Latini, S. Masson, Y.T. Chiang, R. Glazer, V.H.F.T. Investigators, Sustained reduction of aldosterone in response to the angiotensin receptor blocker valsartan in patients with chronic heart failure: results from the Valsartan Heart Failure Trial. Circulation. 108, 1306 (2003)
I.S. Ockene, D.E. Chiriboga, E.J. Stanek III, M.G. Harmatz, R. Nicolosi, G. Saperia, A.D. Well, P. Freedson, P.A. Merriam, G. Reed, Y. Ma, C.E. Matthews, J.R. Hebert, Seasonal Variation in Serum Cholesterol Levels. Arch. Intern. Med. 164, 863 (2004)
L. Rastam, P.J. Hannan, R.V. Luepker, M.B. Mittelmark, D.M. Murray, J.S. Slater, Seasonal variation in plasma cholesterol distributions: implications for screening and referral. Am. J. Prev. Med. 8, 360 (1992)
R. Jorde, G. Grimnes, Vitamin D and metabolic health with special reference to the effect of vitamin D on serum lipids. Prog. Lipid Res. 50, 303 (2011)
R. Jorde, G. Grimnes, Exploring the association between serum 25-hydroxyvitamin D and serum lipids—more than confounding? Eur. J. Clin. Nutr. 72(4), 526–533 (2018)
T. Skaaby, L.L.N. Husemoen, T. Martinussen, J.P. Thyssen, M. Melgaard, B.H. Thuesen, C. Pisinger, T. Jørgensen, J.D. Johansen, T. Menné, B. Carlsen, P.B. Szecsi, S. Stender, R.V. Fenger, M. Fenger, A. Linneberg, Vitamin D Status, Filaggrin Genotype, and Cardiovascular Risk Factors: A Mendelian Randomization Approach. PLoS One. 8, e57647 (2013)
M. Briel, I. Ferreira-Gonzalez, J.J. You, P.J. Karanicolas, E.A. Akl, P. Wu, B. Blechacz, D. Bassler, X. Wei, A. Sharman, I. Whitt, S. Alves da Silva, Z. Khalid, A.J. Nordmann, Q. Zhou, S.D. Walter, N. Vale, N. Bhatnagar, C. O’Regan, E.J. Mills, H.C. Bucher, V.M. Montori, G.H: Guyatt, Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. Bmj. 338, b92 (2009)
M. Pfeifer, B. Begerow, H.W. Minne, D. Nachtigall, C. Hansen, Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J. Clin. Endocrinol. Metab. 86, 1633 (2001)
L.A.G. Armas, B.W. Hollis, R.P. Heaney, Vitamin D2 is much less effective than vitamin D3 in humans. J. Clin. Endocrinol. Metab. 89, 5387 (2004)
K.S. Vimaleswaran, A. Cavadino, D.J. Berry, R. Jorde, A.K. Dieffenbach, C. Lu, A.C. Alves, H.J.L. Heerspink, E. Tikkanen, J. Eriksson, A. Wong, M. Mangino, Ka Jablonski, I.M. Nolte, D.K. Houston, T.S. Ahluwalia, Pf.J. van der Moasd, D. Pasko, L. Zgaga, E. Thiering, V. Vitart, R.M. Fraser, J.E. Huffman, Ra de Boer, B. Schöttker, K.-U. Saum, M.I. McCarthy, J. Dupuis, K.-H. Herzig, S. Sebert, A. Pouta, J. Laitinen, M.E. Kleber, G. Nadis, M. Lorentzon, K. Jameson, N. Arden, Ja Cooper, J. Acharya, R. Hardy, O. Raitakari, S. Ripatti, L.K. Billings, J. Lahti, C. Osmond, B.W. Penninx, L. Rejnmark, K.K. Lohman, L. Paternoster, R.P. Stolk, D.G. Hernandez, L. Byberg, E. Hagström, H. Melhus, E. Ingelsson, D. Mellström, O. Ljunggren, I. Tzoulaki, S. McLachlan, E. Theodoratou, C.M.T. Tiesler, A. Jula, P. Navarro, A.F. Wright, O. Polasek, J.F. Wilson, I. Rudan, V. Salomaa, J. Heinrich, H. Campbell, J.F. Price, M. Karlsson, L. Lind, K. Michaëlsson, S. Bandinelli, T.M. Frayling, Ca Hartman, T.Ia Sørensen, S.B. Kritchevsky, B.L. Langdahl, J.G. Eriksson, J.C. Florez, T.D. Spector, T. Lehtimäki, D. Kuh, S.E. Humphries, C. Cooper, C. Ohlsson, W. März, M.H. de Borst, M. Kumari, M. Kivimaki, T.J. Wang, C. Power, H. Brenner, G. Grimnes, P. van der Harst, H. Snieder, A.D. Hingorani, S. Pilz, J.C. Whittaker, M.-R. Järvelin, E. Hyppönen, Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. lancet. Diabetes Endocrinol. 2, 719 (2014)
C. Vlachopoulos, K. Aznaouridis, C. Stefanadis, Prediction of Cardiovascular Events and All-Cause Mortality With Arterial Stiffness. J. Am. Coll. Cardiol. 55, 1318 (2010)
A. Raed, J. Bhagatwala, H. Zhu, N.K. Pollock, S.J. Parikh, Y. Huang, R. Havens, I. Kotak, D.-H. Guo, Y. Dong, Dose responses of vitamin D 3 supplementation on arterial stiffness in overweight African Americans with vitamin D deficiency: A placebo controlled randomized trial. PLoS One 1, e0188424 (2017)
J. Rosa, I. Raska, D. Wichterle, O. Petrak, B. Strauch, Z. Somloova, T. Zelinka, R. Holaj, J. Widimsky, Pulse wave velocity in primary hyperparathyroidism and effect of surgical therapy. Hypertens. Res. 34, 296 (2011)
L.A. Beveridge, M.B. Chb, F. Khan, A.D. Struthers, J. Armitage, I. Barchetta, I. Bressendorff, M.G. Cavallo, R. Clarke, R. Dalan, G. Dreyer, A.D. Gepner, N.G. Forouhi, R.A. Harris, G.A. Hitman, T. Larsen, R. Khadgawat, P. Marckmann, F.H. Mose, S. Pilz, A. Scholze, M. Shargorodsky, S.I. Sokol, H. Stricker, C. Zoccali, M.D. Witham, Effect of Vitamin D Supplementation on Markers of Vascular Function: A Systematic Review and Individual Participant Meta- Analysis. J Am Heart Assoc 1, pii: e008273 (2018)
A.J. Rodríguez, D. Scott, V. Srikanth, P. Ebeling, Effect of Vitamin D supplementation on measures of arterial stiffness: A systematic review and meta-analysis of randomized controlled trials. Clin. Endocrinol. (Oxf) 84, 645 (2016)
E. Holmlund-Suila, H. Viljakainen, T. Hytinantti, C. Lamberg-Allardt, S. Andersson, O. Mäkitie, High-dose vitamin D intervention in infants - Effects on vitamin D status, calcium homeostasis, and bone strength. J. Clin. Endocrinol. Metab. 97, 4139 (2012)
B. Nygaard, N.E. Frandsen, L. Brandi, K. Rasmussen, O.V. Oestergaard, L. Oedum, H.C. Hoeck, D. Hansen, Effects of High Doses of Cholecalciferol in Normal Subjects: A Randomized Double-Blinded, Placebo-Controlled Trial. PLoS One. 9, e102965 (2014)
O.W. Moe, Kidney stones: Pathophysiology and medical management. Lancet. 367, 333 (2006)
H. Kesteloot, I. Tzoulaki, I.J. Brown, Q. Chan, A. Wijeyesekera, H. Ueshima, L. Zhao, A.R. Dyer, R.J. Unwin, J. Stamler, P. Elliott, Relation of urinary calcium and magnesium excretion to blood pressure. Am. J. Epidemiol. 174, 44 (2011)
D.A. McCarron, P.A. Pingree, R.J. Rubin, S.M. Gaucher, M. Molitch, S. Krutzik, Enhanced parathyroid function in essential hypertension: a homeostatic response to a urinary calcium leak. Hypertension. 2, 162 (1980)
P. Strazzullo, V. Nunziata, M. Cirillo, R. Giannattasio, L.A. Ferrara, P.L. Mattioli, M. Mancini, Abnormalities of calcium metabolism in essential hypertension. Clin. Sci. (Lond). 65, 137 (1983)
E. Rossi, C. Sani, F. Perazzoli, M.C. Casoli, A. Negro, Alterations of the calcium metabolism and of parathyroid function in primary aldosteronism and their revertal by spironolactone or by surgical removal of aldosterone-producing adenomas. Am. J. Hypertens. 7061, 884 (1995)
L. Ceccoli, V. Ronconi, L. Giovannini, M. Marcheggiani, F. Turchi, M. Boscaro, G. Giacchetti, Bone health and aldosterone excess. Osteoporos. Int. 24, 2801 (2013)
A. Tomaschitz, N. Verheyen, A. Meinitzer, B. Pieske, E. Belyavskiy, H. Brussee, J. Haas, W. Märzc, E. Pieske-Kraigherd, S. Verheyeni, L. Ofner-Ziegenfuss, B.O. Hartaigh, V. Schwetz, F. Aberer, M. Grübler, F. Lang, I. Alesutann, J. Voelkl, M. Gakschl, J.H. Horina, H.-P. Dimai, J. Rus-Machan, C. Stiegler, S. Pilz, Effect of eplerenone on parathyroid hormone levels in patients with primary hyperparathyroidism: results from the EPATH randomized, placebo-controlled trial. J Hypertens 34(7), 1347–56 (2016)
D.L. Zittermann, S. Pilz, Vitamin D Supplementation and Cardiovascular Risk. JAMA Cardiol. 2, 1280 (2017)
L. Rejnmark, A.L. Lauridsen, P. Vestergaard, L. Heickendorff, F. Andreasen, L. Mosekilde, Diurnal rhythm of plasma 1,25-dihydroxyvitamin D and vitamin D-binding protein in postmenopausal women: Relationship to plasma parathyroid hormone and calcium and phosphate metabolism. Eur. J. Endocrinol. 146, 635 (2002)
A. Schlemmer, C. Hassager, Acute fasting diminishes the circadian rhythm of biochemical markers of bone resorption. Eur. J. Endocrinol. 140, 332 (1999)
S.M. Stigler, Regression towards the mean, historically considered. Stat. Methods Med. Res. 6, 103 (1997)
R. Dina, M. Jafari, Angiotensin II-receptor antagonists: an overview. Am. J. Health. Syst. Pharm. 57, 1231 (2000)
Acknowledgements
We acknowledge the laboratory technicians at Aarhus University Hospital and Department of Medical Research Holstebro for invaluable help. Additionally, we acknowledge support from our Hospital Pharmacy, Danish Health Data Authority and Orkla Health A/S. Most importantly we thank all participants for their exceptional commitment and goodwill.
Funding
Aarhus University, The Research Foundation of Central Denmark Region, The Augustinus Foundation, The Foundation of Endocrinology Aarhus University Hospital, Toyota Foundation, A.P. Møller & wife Chastine MC-Kinney Møllers Foundation and P. A. Messerschmidt & wife foundation funded the study. Orkla Health donated vitamin D and vitamin D placebo tablets free of charge, but had no influence on analyses and interpretation of data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
We conducted the study in accordance to the Helsinki II declaration considering biomedical research regarding humans. The Danish Data Protection Agency (1-16-02-492-14), the Regional Committee on Biomedical Research Ethics (1-10-72-326-14), The Danish Health Authority (2014-003645-10) and Danish Health Data Authority (FSEID-00001274) approved the project. The local unit for Good Clinical Practice at Aarhus University Hospital monitored the study. Clinicaltrials.gov: #NCT02572960.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Bislev, L.S., Langagergaard Rødbro, L., Bech, J.N. et al. The effect of vitamin D3 supplementation on markers of cardiovascular health in hyperparathyroid, vitamin D insufficient women: a randomized placebo-controlled trial. Endocrine 62, 182–194 (2018). https://doi.org/10.1007/s12020-018-1659-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1659-4