Abstract
Purpose
We aimed to investigate associations among serum levels of LCN2, bone resorption marker carboxy-terminal cross-linking telopeptide of type-1 collagen (CTx), bone formation marker osteocalcin (OCN), and bone mineral densities (BMDs) in ambulatory healthy women.
Methods
This cross-sectional study analyzed 1012 previously enrolled outpatient Han Chinese women. BMDs of the lumbar spine and femoral neck were measured using dual energy X-ray absorptiometry. Serum levels of LCN2, CTx, OCN, and creatinine (Scr) were measured.
Results
Circulating LCN2 was inversely correlated with BMDs at the lumbar spine and femoral neck (Spearman’s r = −0.08, P = 0.010 and r = −0.14, P < 0.001; respectively). A significant positive correlation between LCN2 and CTx (r = 0.11, P < 0.001), OCN (r = 0.06, P = 0.047), age (r = 0.21, P < 0.001), and Scr (r = 0.24, P < 0.001) was also observed. After adjusting for age and Scr, the correlation among LCN2, BMDs and OCN disappeared, but LCN2 was still positively associated with CTx (r = 0.08, P = 0.010). The circulating concentration of LCN2 showed no significant difference between subjects with and without osteoporotic fractures (43.63 (35.29, 53.66) vs. 42.25 (34.43, 51.46) ng/ml, respectively, P = 0.111). Serum CTx concentrations rose with serum LCN2 increasing from the lowest to the highest quartile (P for trend = 0.005), even after adjusting for age and Scr (P for trend = 0.040). In multivariate regression analysis, LCN2 was one of the main determinants for changes in serum CTx (standard β = 0.061, P = 0.005).
Conclusions
In ambulatory healthy women, the relationships among serum LCN2 level, BMDs, and OCN were confounded by age and Scr. Although LCN2 was positively related with CTx, the correlation was very weak and may not be physiologically relevant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone mass is maintained by the dynamic balance of bone-forming osteoblasts and bone-resorbing osteoclasts [1]. Osteoporosis, the most common skeletal disease characterized by low bone mass, compromised bone quality, and risk of fractures, results from an imbalance in bone remodeling [2]. Due to the high morbidity of osteoporotic fractures, much research has been performed to identify factors or molecules that participate in bone remodeling [3,4,5].
Lipocalin 2 (LCN2), also known as 24p3 or neutrophil gelatinase-associated lipocalin (NGAL), is a 25-kDa secretory protein that binds and transports small hydrophobic substances [6, 7]. LCN2 was initially recognized as having an antibacterial effect by binding and sequestering bacterial siderophores [8]. Subsequent studies demonstrated that Lcn2 was expressed on adipocytes [9], whereas more recently Lcn2 was shown to be mainly expressed by osteoblasts under basal conditions with at least ten-fold higher expression levels than adipocytes [10]. Lcn2 expression is induced in different cell types and tissues under pathological conditions [11,12,13,14]. Thus, LCN2 has been implicated in diverse pathophysiological conditions, such as obesity and insulin resistance [9, 11], renal disease [12], cardiovascular disease [15], inflammation [8, 16], and brain dysfunction [14].
Lcn2 has generally been considered to be a mechanosensitive gene in bone homeostasis. It was first identified as the most upregulated gene in mouse primary osteoblasts under simulated microgravity conditions [17]. Subsequent studies demonstrated that mice receiving experimentally induced mechanical unloading, a condition facilitating bone mass decline, exhibited higher bone Lcn2 expression [18, 19]. In addition, a significant progressive increase in serum LCN2 levels was observed in healthy subjects undergoing 15 days of bed rest, a condition favouring bone loss [18]. Further studies found that osteoblast differentiation was retarded with the overexpression of Lcn2 in primary osteoblasts (OBs-LCN2), whereas osteoclast activity was enhanced when bone marrow mononuclear cells were co-cultured with OBs-LCN2-conditioned medium (OBs-LCN2-CM) [18].
In contrast to these findings, studies by Lu et al. found that the osteoblast-lineage differentiation was enhanced when bone marrow stromal cells were co-cultured with LCN2 [20]. Kim et al. reported suppressed osteoclast proliferation and differentiation when osteoclast precursors were pre-incubated with LCN2 [21]. Nevertheless, Lcn2 knockout (Lcn2−/−) mice and mice with a specific deletion of Lcn2 in osteoblasts showed a normal bone phenotype with no apparent differences in osteoclast development [10, 22]. Considering these conflicting findings in vitro and in mice studies, we wished to know whether LCN2 has any relationship with bone metabolism in humans. Therefore, we performed a cross-sectional study to investigate associations among serum LCN2, bone turnover markers, bone mineral density (BMD), and osteoporotic fractures in outpatient women.
Materials and methods
Subjects
The current study was performed in Ruijin hospital, Shanghai Jiao-tong University School of Medicine. Through posters, 1012 pre-menopausal and post-menopausal healthy outpatient women aged 20–88 years were enrolled from November 2007 to December 2008. All participants were Han Chinese women living in Shanghai, China. Individuals with disorders and conditions that may potentially cause abnormalities in bone metabolism—such as chronic disorders involving heart, lungs, liver, and/or kidneys, diabetes mellitus, history of any cancers, rheumatoid arthritis, chronic diarrhea, inflammatory bowel disease, etc.—were excluded. Women taking drugs known to influence bone metabolism, including but not limited to glucocorticoids, selective estrogen receptor modulators, hormone replacement therapy, bisphosphonates, teriparatide, selective serotonin reuptake inhibitors, levothyroxine, etc., were also excluded.
Baseline data including age and menopausal status were obtained by questionnaires completed by all participants. Osteoporotic fracture was self-reported and defined as fractures occurring in the forearm, hip, and/or spine with trauma equivalent to a fall from a standing height or lower. The age, site, number, and time of fractures were queried, but no medical records or X-ray films were required to confirm the diagnosis of fracture. Body height was assessed without shoes using a ruler fixed on a wall. Body weight was measured using digital scales with light clothes. Both body height and weight were measured by the same physician. Body mass index (BMI) was calculated in kg/m2. BMDs (g/cm2) at the lumbar spine and hip of all participants were measured using dual-energy X-ray absorptiometry (GE-Lunar Prodigy; GE Healthcare, Madison, WI).
The study protocol was approved by the Institutional Review Board of the Ruijin Hospital in Shanghai and complied with the World Medical Association Declaration of Helsinki’s Ethical Principles for Medical Research Involving Human Subjects. Informed consent was obtained from each participant after full explanation of the purpose and nature of all procedures used.
Measurements of serum LCN2, CTx, OCN, and Scr
Blood samples of all subjects were obtained after an overnight fast. The sera were multiple aliquoted after centrifugation and stored at −70° C until use. The concentration of serum LCN2 was measured after 20-fold dilution using a Human Lipocalin-2/NGAL Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, USA) with intra-assay and inter-assay CVs of less than 5% and 8%, respectively. The bone resorption marker carboxy-terminal cross-linking telopeptide of type-1 collagen (CTx) (Elecsys ß-Crosslaps/serum, Cobas 601, Roche Diagnostics, Basel, Switzerland) and bone formation marker osteocalcin (OCN) (Elecsys N-MID Osteocalcin, Cobas 601, Roche Diagnostics, Basel, Switzerland) were assessed using commercial kits according to the manufacturer’s instructions. Scr was measured using the automatic biochemical analyzer (Unicel DxC800, Beckman Coulter, US). All serum parameters were measured in batches.
Statistics
Of the 1012 participants, 1010 were ultimately used for analysis due to the lack of serum samples for two subjects. Kolmogorov–Smirnov tests were employed to assess a normal distribution of the variables. Normally distributed data were shown as the mean ± SD, whereas skewed distributed variables including age, height, weight, Scr, OCN, CTx, LCN2 were expressed as the median (interquartile range). Spearman correlation coefficients were calculated to describe the correlations between LCN2 and various variables. Partial correlation analysis was used to adjust for age and Scr. The difference between two groups was compared by the Mann–Whitney U test. ANOVA was performed to compare differences among quartiles of LCN2. To control for age and Scr, covariance analysis was employed. To define the relationship between LCN2 and CTx more precisely, multivariate stepwise regression analysis was conducted using CTx as the dependent variable and age, BMI, OCN, BMDs at the lumbar spine and the femoral neck, LCN2, and menopausal status as independent variables. A P-value of less than 0.05 was considered significant. All statistical analyses were performed using SPSS 20.0 (SPSS, Inc., Chicago, Illinois, USA).
Results
Baseline characteristics of the participants
The demographics of the subjects are outlined in Table 1. Briefly, postmenopausal women were older and shorter with a higher BMI and Scr level compared with premenopausal women. Their BMDs were markedly reduced both at the lumbar spine and the femoral neck. More rapid bone turnover was observed as indicated by significantly elevated serum levels of OCN and CTx (Table 1). Osteoporotic fractures were more frequent in postmenopausal women than premenopausal women (16.7 vs. 8.5%, respectively) (Table 1). The median concentration of serum LCN2 was 42.50 (34.55, 51.81) ng/ml, with postmenopausal women having higher LCN2 levels than premenopausal women (42.85 (34.55, 52.20) vs. 39.59 (34.48, 49.37) ng/ml, respectively, P = 0.031) (Table 1).
Correlation analysis between serum LCN2 and BMDs and bone turnover biomarkers
According to Spearman correlation analysis, LCN2 showed an inverse correlation with BMDs both at the lumbar spine and the femoral neck (r = -0.08, P = 0.010; r = -0.14, P < 0.001). A significant positive correlation between LCN2 and the bone resorption marker CTx (r = 0.11, P < 0.001), the bone formation marker OCN (r = 0.06, P = 0.047), age (r = 0.21, P < 0.001), and Scr (r = 0.24, P < 0.001) was also observed (Table 2).
To control for the potential influence of age and renal function on the relationships between serum LCN2 levels and CTx, OCN and BMDs, we reanalyzed the correlations among LCN2, BMDs, and bone turnover markers after first adjusting for age. Serum LCN2 retained its positive correlation with CTx (r = 0.07, P = 0.036); however, the correlations among LCN2, OCN, and BMDs disappeared. After further adjusting for Scr, LCN2 was still positively related to CTx (r = 0.08, P = 0.010) (Table 2).
Comparison of serum LCN2 levels between subjects with and without osteoporotic fractures
All participants were divided into two groups according to their osteoporotic fracture history. Comparison was performed by the Mann–Whitney U test. No significant difference between subjects with (n = 161) and without (n = 849) osteoporotic fractures was found for serum LCN2 concentrations (43.63 (35.29, 53.66) vs. 42.25 (34.43, 51.46) ng/ml, P = 0.111).
Comparison of serum CTx concentration among LCN2 quartiles
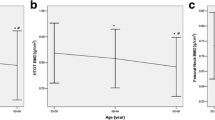
To further understand the relationship between LCN2 and CTx, participants were divided into four groups according to the quartiles of their serum LCN2 concentration. The results showed a sustained rise in serum CTx concentration with an increase in serum LCN2 from the lowest (first) to the highest (fourth) quartile (P for trend = 0.005) (Fig. 1a). When age and Scr were included as covariants, this trend of increase remained, with the fourth quartile of LCN2 showing the highest CTx levels (P for trend = 0.040) (Fig. 1b).
The influence of LCN2 on variations in serum CTx
In addition to its positive correlation with LCN2, CTx was positively correlated to age (r = 0.17, P < 0.001) and OCN (r = 0.73, P < 0.001) and negatively correlated to BMI (r = −0.06, P = 0.042) and BMDs at the lumbar spine (r = −0.29, P < 0.001) and femoral neck (r = −0.27, P < 0.001). There was also an increase in CTx in postmenopausal women (0.44 (0.32, 0.57) vs. 0.26 (0.19, 0.35) ng/ml, P < 0.001). To determine the factors responsible for variations in serum CTx, we further conducted multivariate stepwise regression analysis, which showed OCN (standard β = 0.688, P < 0.001), menopausal status (standard β = 0.072, P = 0.002), LCN2 (standard β = 0.061, P = 0.005) and BMD at the lumbar spine (standard β = −0.065, P = 0.005) as predictors of CTx, accounting for 54.2% variations in serum CTx (P < 0.001). LCN2 only contributed to 0.4% of changes in serum CTx (Table 3).
Discussion
The major findings of our study are that LCN2 is negatively associated with BMDs and positively related to OCN, but both correlations disappear after adjusting for age and Scr. LCN2 shows no association with osteoporotic fractures. Although LCN2 positively correlates with the bone resorption marker CTx, the magnitude of the correlation is very low and may not be physiologically relevant.
LCN2 has been shown to mediate the osteoclastogenic effect of microgravity-treated endothelial cell-conditioned medium (Micro-EC-CM) on total bone marrow cells [19]. Enhanced osteoclastogenesis was also observed when mouse bone marrow mononuclear cells were co-cultured with OBs-LCN2 or OBs-LCN2-CM [18]. Moreover, mice with mechanical unloading exhibited enhanced osteoclast surface per bone surface (Oc.S/BS) and higher levels of both serum and bone-expressed LCN2 [18]. Calvaria injected with Micro-EC-CM displayed increased TRAP staining, an indicator of osteoclast activity [19]. Furthermore, in humans, increased circulating LCN2 concentrations together with elevated urine CTx levels were revealed in subjects receiving head-down tilt bed rest, a state imitating microgravity-induced bone and muscle loss [18, 23]. In this study, when the correlation between LCN2 and bone resorption marker CTx was evaluated in ambulatory women under physiological condition, these two markers were only weakly associated. Since the contribution of LCN2 to the changes of CTx was almost negligible (0.4%), the real correlation between them could not be established.
The weak or the null correlation between LCN2 and bone turnover markers found in our study may further explain the lack of correlation between LCN2 and BMDs, since bone remodeling is one of the most important determinants for BMDs. Recent mice studies added further credence to the absense of relationship between LCN2 and BMDs. It was shown that in the physiological condition, mice with either deletion of Lcn2 specifically in osteoblasts or globally showed normal bone phenotypes, such as BMD, bone volume fraction and trabecular number [10, 22]. These findings were also echoed in another two human studies [24, 25], showing that there was no association between serum LCN2 and BMDs.
Other than bone turnover markers and BMDs, the relationship between LCN2 and the fracture-related outcome was also investigated in humans. In a prospective study involving 1009 older women, even though baseline above the median LCN2 (76.6 ng/ml) predicted increased risk of any osteoporotic fracture-related hospitalization and hip fracture-related hospitalization in a 14.5-year follow-up, baseline LCN2 levels were not different between participants with or without prevalent fractures [24]. Similarly, in the current study, we also failed to find differences in serum LCN2 concentrations between subjects with and without osteoporotic fractures.
All these findings, including ours, suggest that at least under the physiological condition, serum LCN2 concentration had little association with bone metabolism. However, when interpreting these data, several other factors, such as BMI, kidney function, and age, should be considered. It was shown that obese individuals usually have higher serum LCN2 concentrations [26,27,28], and the increased LCN2 levels in overweight and obese subjects were positively associated with BMI [9, 27, 28]. In contrast to findings in obese individuals, in our study with relatively lean subjects, neither a positive nor negative correlation could be found between LCN2 and BMI. We think this finding is important. Bone was shown to be the primary origin of circulating LCN2 in lean mice [10], whereas there were reports showing that the expression of Lcn2 was upregulated by more than three orders of magnitude during adipogenesis of 3T3-L1 preadipocytes [9]; and that adipose tissue expression of Lcn2 was significantly elevated both in obese mice and subjects [9, 11, 28]. More importantly, the increased adipose tissue content of LCN2 was positively associated with plasma LCN2 concentrations [28]. These findings, including ours, suggest that in overweight and obese individuals, the major contributor of circulating LCN2 is likely adipose tissue due to its much larger mass, whereas in lean subjects, the primary source may be the skeleton.
Circulating LCN2 has long been recognized as an early biomarker of acute kidney injury [29, 30] and was involved in chronic renal failure [12]. Both increased expression of Lcn2 in the renal tubular cells [12] and reduced clearance of LCN2 upon kidney injury accounted for the elevation of serum LCN2 in renal diseases. In the current study, the participants were generally with normal renal function, and a significant positive correlation between LCN2 and Scr was also observed. However, the positive correlation between LCN2 and CTx remained even after adjusting for Scr. It is unclear whether such a low-grade positive correlation between LCN2 and CTx may exacerbate and have bone-related consequences upon kidney injury, since there is evidence showing that in subjects with LCN2 levels above the median level (76.6 ng/ml), the risk of any osteoporotic fracture-related hospitalization in those with eGFR < 60 ml/min/1.73 m2 was 2.48 times higher than those with eGFR ≥ 60 ml/min/1.73 m2 [24].
It could be argued that the lack of meaningful associations between serum LCN2 and bone turnover markers, BMDs, and osteoporotic fractures, was due to the relative lower circulating concentrations of LCN2 in our study compared with others [24]. It has been demonstrated that LCN2 has relatively high stability in serum samples [31]. Some other factors may be responsible for the lower LCN2 concentrations in our study. First, the much younger age may account (ours vs. Lim et al. [24], 57.5 ± 9.8 vs. 75.3 ± 2.7 years of age), since age was positively related to LCN2 as revealed in our study and others [24]. Second, the subjects in our cohort had a lower BMI than in Lim et al. [24] (23.19 ± 3.27 vs. 27.1 ± 4.7 kg/m2). As discussed above, obesity is usually accompanied by the increased serum levels of LCN2 [9, 26, 27]. Third, ethnic/racial differences may also play a role. The body composition of Caucasians and Asians differs, Asian populations usually present smaller skeletal size and less subcutaneous adipose tissue [32], since both skeletal and adipose tissue were the important sources of LCN2, it is plausible to hypothesize that ethnicity/race is partially responsible for the lower circulating LCN2 in our cohort.
There are several limitations in the present study. First, it is a cross-sectional study, the level of evidence was low. As demonstrated in a prospective study, the association between LCN2 and hip fracture-related hospitalization was time-dependent; divergence began at approximately 36 months and increased steadily during follow-up in subjects with above-median LCN2 concentrations (76.6 ng/ml) compared with those below the median [24]. In addition, causality could not be determined. The positive correlation between LCN2 and CTx could not determine which came first and influenced the other or if this was just an epiphenomenon. Second, our study was performed in subjects with a large age range with most women being postmenopausal. However, in our multivariate stepwise regression analysis, the relationship between LCN2 and CTx did not change after adjusting for menopausal status.
In conclusion, we provide clues suggesting that in ambulatory healthy women under physiological conditions, the correlations among serum LCN2, BMDs, and OCN were confounded by age and Scr. Serum LCN2 concentration was very weakly associated with CTx and was not related to osteoporotic fractures. However, whether such a low positive correlation between LCN2 and CTx in the physiological condition would exaggerate and have clinical implications with aging or under certain pathological conditions such as unloading, long-term bed rest or renal injury is worthy of further study.
It is also noteworthy that bone-specific LCN2 could act as an appetite repressor in a hypothalamus-dependent manner [10]. The rise of serum LCN2 concentration with age and in unloading is likely to reflect reduced energy intake under these conditions [33]. Further studies on the relationship between bone–LCN2–energy metabolism will undoubtedly broaden our understanding of the role of LCN2 on bone and energy homeostasis.
References
M. Zaidi, Skeletal remodeling in health and disease. Nat. Med. 13, 791–801 (2007)
L.G. Raisz, Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J. Clin. Invest. 115, 3318–3325 (2005)
W. Wei, T. Motoike, J.Y. Krzeszinski, Z. Jin, X.J. Xie, P.C. Dechow, M. Yanagisawa, Y. Wan, Orexin regulates bone remodeling via a dominant positive central action and a subordinate negative peripheral action. Cell. Metab. 19, 927–940 (2014)
S.K. Ramasamy, A.P. Kusumbe, L. Wang, R.H. Adams, Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 507, 376–380 (2014)
K. Ikeda, S. Takeshita, Factors and mechanisms involved in the coupling from bone resorption to formation: how osteoclasts talk to osteoblasts. J. Bone Metab. 21, 163–167 (2014)
S. Hraba-Renevey, H. Turler, M. Kress, C. Salomon, R. Weil, SV40-induced expression of mouse gene 24p3 involves a post-transcriptional mechanism. Oncogene 4, 601–608 (1989)
L. Kjeldsen, A.H. Johnsen, H. Sengelov, N. Borregaard, Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 268, 10425–10432 (1993)
T.H. Flo, K.D. Smith, S. Sato, D.J. Rodriguez, M.A. Holmes, R.K. Strong, S. Akira, A. Aderem, Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432, 917–921 (2004)
Q.W. Yan, Q. Yang, N. Mody, T.E. Graham, C.H. Hsu, Z. Xu, N.E. Houstis, B.B. Kahn, E.D. Rosen, The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes 56, 2533–2540 (2007)
I. Mosialou, S. Shikhel, J.M. Liu, A. Maurizi, N. Luo, Z. He, Y. Huang, H. Zong, R.A. Friedman, J. Barasch, P. Lanzano, L. Deng, R.L. Leibel, M. Rubin, T. Nicholas, W. Chung, L.M. Zeltser, K.W. Williams, J.E. Pessin, S. Kousteni, MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature 543, 385–390 (2017)
Y. Wang, K.S. Lam, E.W. Kraegen, G. Sweeney, J. Zhang, A.W. Tso, W.S. Chow, N.M. Wat, J.Y. Xu, R.L. Hoo, A. Xu, Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin. Chem. 53, 34–41 (2007)
A. Viau, K. El Karoui, D. Laouari, M. Burtin, C. Nguyen, K. Mori, E. Pillebout, T. Berger, T.W. Mak, B. Knebelmann, G. Friedlander, J. Barasch, F. Terzi, Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J. Clin. Invest. 120, 4065–4076 (2010)
X. Leng, T. Ding, H. Lin, Y. Wang, L. Hu, J. Hu, B. Feig, W. Zhang, L. Pusztai, W.F. Symmans, Y. Wu, R.B. Arlinghaus, Inhibition of lipocalin 2 impairs breast tumorigenesis and metastasis. Cancer Res. 69, 8579–8584 (2009)
M. Mucha, A. Skrzypiec, E. Schiavon, B. Attwood, E. Kucerova, R. Pawlak, Lipocalin-2 controls neuronal excitability and anxiety by regulating dendritic spine formation and maturation. Proc. Natl. Acad. Sci. USA 108, 18436–18441 (2011)
S. Lindberg, S.H. Pedersen, R. Mogelvang, J.S. Jensen, A. Flyvbjerg, S. Galatius, N.E. Magnusson, Prognostic utility of neutrophil gelatinase-associated lipocalin in predicting mortality and cardiovascular events in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J. Am. Coll. Cardiol. 60, 339–345 (2012)
J.M. Warszawska, R. Gawish, O. Sharif, S. Sigel, B. Doninger, K. Lakovits, I. Mesteri, M. Nairz, L. Boon, A. Spiel, V. Fuhrmann, B. Strobl, M. Muller, P. Schenk, G. Weiss, S. Knapp, Lipocalin 2 deactivates macrophages and worsens pneumococcal pneumonia outcomes. J. Clin. Invest. 123, 3363–3372 (2013)
M. Capulli, A. Rufo, A. Teti, N. Rucci, Global transcriptome analysis in mouse calvarial osteoblasts highlights sets of genes regulated by modeled microgravity and identifies a “mechanoresponsive osteoblast gene signature”. J. Cell. Biochem. 107, 240–252 (2009)
N. Rucci, M. Capulli, S.G. Piperni, A. Cappariello, P. Lau, P. Frings-Meuthen, M. Heer, A. Teti, Lipocalin 2: a new mechanoresponding gene regulating bone homeostasis. J. Bone Miner. Res. 30, 357–368 (2015)
V. Veeriah, A. Zanniti, R. Paone, S. Chatterjee, N. Rucci, A. Teti, M. Capulli, Interleukin-1beta, lipocalin 2 and nitric oxide synthase 2 are mechano-responsive mediators of mouse and human endothelial cell-osteoblast crosstalk. Sci. Rep. 6, 29880 (2016)
M. Lu, L. Xia, Y.C. Liu, T. Hochman, L. Bizzari, D. Aruch, J. Lew, R. Weinberg, J.D. Goldberg, R. Hoffman, Lipocalin produced by myelofibrosis cells affects the fate of both hematopoietic and marrow microenvironmental cells. Blood 126, 972–982 (2015)
H.J. Kim, H.J. Yoon, K.A. Yoon, M.R. Gwon, S. Jin Seong, K. Suk, S.Y. Kim, Y.R. Yoon, Lipocalin-2 inhibits osteoclast formation by suppressing the proliferation and differentiation of osteoclast lineage cells. Exp. Cell. Res. 334, 301–309 (2015)
H.J. Kim, B. Ohk, W.Y. Kang, S.J. Seong, K. Suk, M.S. Lim, S.Y. Kim, Y.R. Yoon, Deficiency of lipocalin-2 promotes proliferation and differentiation of osteoclast precursors via regulation of c-Fms expression and nuclear factor-kappa B activation. J. Bone Metab. 23, 8–15 (2016)
P. Frings-Meuthen, J. Buehlmeier, N. Baecker, P. Stehle, R. Fimmers, F. May, G. Kluge, M. Heer, High sodium chloride intake exacerbates immobilization-induced bone resorption and protein losses. J. Appl. Physiol. 111, 537–542 (2011)
W.H. Lim, G. Wong, E.M. Lim, E. Byrnes, K. Zhu, A. Devine, N.J. Pavlos, R.L. Prince, J.R. Lewis, Circulating lipocalin 2 levels predict fracture-related hospitalizations in elderly women: a prospective cohort study. J. Bone Miner. Res. 30, 2078–2085 (2015)
C. Cervellati, G. Bonaccorsi, C. Bergamini, E. Fila, P. Greco, G. Valacchi, L. Massari, A. Gonelli, V. Tisato, Association between circulatory levels of adipokines and bone mineral density in postmenopausal women. Menopause 23, 984–992 (2016)
C.M. Paton, M.P. Rogowski, A.L. Kozimor, J.L. Stevenson, H. Chang, J.A. Cooper, Lipocalin-2 increases fat oxidation in vitro and is correlated with energy expenditure in normal weight but not obese women. Obesity 21, E640–E648 (2013)
N.M. Rashad, A.S. El-Shal, R.L. Etewa, F.M. Wadea, Lipocalin-2 expression and serum levels as early predictors of type 2 diabetes mellitus in obese women. IUBMB Life 69, 88–97 (2017)
T. Auguet, Y. Quintero, X. Terra, S. Martinez, A. Lucas, S. Pellitero, C. Aguilar, M. Hernandez, D. del Castillo, C. Richart, Upregulation of lipocalin 2 in adipose tissues of severely obese women: positive relationship with proinflammatory cytokines. Obesity 19, 2295–2300 (2011)
J. Mishra, C. Dent, R. Tarabishi, M.M. Mitsnefes, Q. Ma, C. Kelly, S.M. Ruff, K. Zahedi, M. Shao, J. Bean, K. Mori, J. Barasch, P. Devarajan, Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365, 1231–1238 (2005)
M. Haase, R. Bellomo, P. Devarajan, P. Schlattmann, A. Haase-Fielitz, and N.M.-a.I. Group, Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am. J. Kidney Dis. 54, 1012–1024 (2009)
J. Wang, H. Zhu, J. Xue, S. Wu, Z. Chen, Effects of storage conditions on the stability of serum CD163, NGAL, HMGB1 and MIP2. Int. J. Clin. Exp. Pathol. 8, 4099–4105 (2015)
D. Sulistyoningrum, D. Gasevic, S. Lear, J. Ho, A. Mente, A. Devlin, Total and high molecular weight adiponectin and ethnic-specific differences in adiposity and insulin resistance: a cross-sectional study. Cardiovasc. Diabetol. 12, 170 (2013)
J. Morley, Anorexia of aging physiologic and pathologic. Am. J. Clin. Nutr. 66, 760–773 (1997)
Acknowledgements
We thank Dr. Stavroula Kousteni and Ioanna Mosialou from the Department of Physiology and Cellular Biophysics, College of Physicians and Surgeons, Columbia University, New York, USA for their critical comments on this manuscript.
Funding
This work was supported by National Natural Science Foundation of China (grant number 81370977 and 81570796).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Dong-mei Liu and Hong-yan Zhao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, Dm., Zhao, Hy., Zhao, L. et al. The relationship among serum lipocalin 2, bone turnover markers, and bone mineral density in outpatient women. Endocrine 59, 304–310 (2018). https://doi.org/10.1007/s12020-017-1504-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-017-1504-1