Abstract
Hypothyroidism and thyrotoxicosis produce adverse effects in male reproduction by unknown mechanisms. We investigated whether triiodothyronine (T3) modulates luteinizing hormone (LH) and follicle stimulating hormone (FSH) synthesis/secretion, by inducing different thyroid states. In hypothyroidism, the content of Lhb and Fshb mRNAs was increased, while their association to ribosomes and the protein content were reduced and the serum LH and FSH concentrations were augmented and decreased, respectively. Thyrotoxicosis reduced Lhb mRNA and LH serum concentration, and increased Lhb mRNA translational rate. The Fshb mRNA content and its association to ribosomes were also increased, whereas FSH serum concentrations were comparable to euthyroid levels. Acute T3 treatment decreased the total content of Lhb and Fshb mRNAs, and increased their association to ribosomes, as well as the LHB and FSHB contents in secretory granules. This study shows that T3 acts on gonadotrophs, resulting in direct effects on LH and FSH synthesis/secretion of male rats, suggesting that some reproductive disorders observed in men may be associated with thyroid hormone imbalances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypothyroidism and thyrotoxicosis produce adverse effects in male reproduction in both humans and animals. In men, hypothyroidism is associated with hypogonadism and other alterations, such as reduction of serum testosterone [1] and other androgens [2], reduced conversion rate of androstenedione to testosterone [3], and reduction in sex hormone-binding globulin (SHBG) levels [4, 5]. Moreover, erectile dysfunction [6, 7] and sperm morphology abnormalities have been reported [6, 8]. Thyrotoxicosis induces abnormalities in sperm motility [7, 9] and is frequently associated with a reduction in the serum testosterone concentrations [10] and increased serum estradiol [11] and SHBG levels [4, 5] (for review see La Vignera et al. [12] and Krassas et al. [13]). Nevertheless, the regulation of gonadotroph activity and gonadotropin expression by thyroid hormone (TH) in males, as well as the mechanisms involved in these processes are still unclear [14].
We have previously reported that hypothyroidism induced in adult male rats causes an increase in Lhb mRNA expression associated with a reduction in its stability and translation rate [15], processes that depend on cytoskeleton assembly [16,17,18,19].
It is known that TH participates in the polymerization of actin filaments and microtubules in the brain [20] and pituitary [21, 22]. In hypothyroidism, there is a widespread disarrangement of the cytoskeleton in anterior pituitary cells [21, 22], and that acute triiodothyronine (T3) administration rapidly promotes its rearrangement throughout the anterior pituitary gland. This suggests that gonadotrophs may be affected by different thyroid states.
Considering that protein synthesis requires the recruitment of mRNA molecules and elongation factors into the ribosomes and that these processes are linked to the actin cytoskeleton, which is also involved with the mobilization and trafficking of vesicles toward the plasma membrane during the secretion process [23], we hypothesized that T3 could interfere with gonadotropic function, altering the synthesis and release of gonadotropin hormones.
To clarify this issue, several steps of luteinizing hormone (LH) and follicle stimulating hormone (FSH) synthesis and secretion were evaluated in adult male thyroidectomized rats, acutely treated with a physiological dose of T3, or treated for 5 days with a supraphysiological dose of T3 (thyrotoxicosis group).
Materials and methods
Chemicals
2-Mercapto-1-methylimidazole (MMI), T3, and FITC-conjugated secondary antibody were purchased from Sigma-Aldrich, Inc. (Saint Louis, MO, USA); Platinum SYBR green qPCR SuperMix UDG, Oligo dT, dNTP, BSA, protein molecular weight markers, 100 bp DNA ladder, and mercaptoethanol were purchased from Life Technologies Corp. (Camarillo, CA, USA); M-MLV Reverse Transcriptase was purchased from Promega Corp. (Madison, WI, EUA); anti-rat LHB and anti-rat FSHB antibodies were obtained from Dr. A. Parlow (National Institute of Diabetes and Digestive and Kidney Diseases, National Hormone and Pituitary Program; Torrance, CA, USA); anti-mouse GAPDH and the appropriate peroxidase-conjugated secondary antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA); Enhanced Chemiluminescence (ECL) kit was purchased from Amersham Biosciences (Little Chalfont, Buckinghamshire, UK); Coat-A-Count Total T3 and Total testosterone kit was purchased from Siemens (Deerfield, IL, USA); Hybridization nitrocellulose membranes and Luminex xMAP technology were purchased for LH and FSH from Millipore Corp. (Milliplex MAP rat pituitary panel, Billerica, MA, USA).
Animals and treatments
Sixty-day-old male Wistar rats (weighing 250–300 g) were used in the study. Some of the rats were subjected to hypothyroidism by surgical thyroidectomy under deep anesthesia with ketamine and xylazine, and were administered MMI 0.03% and CaCl2 0.05% in drinking water for 20 days, as previously described [15]. The animals were divided into the following groups: (1) Euthyroid: sham-operated animals; (2) Hypothyroid: thyroidectomized animals; (3) Thyrotoxicosis: hypothyroid animals intraperitoneally treated with T3 (1.5 μg/100 g of body weight—BW), twice a day, for 5 days; and (4) Hypo + 1XT3: hypothyroid animals intravenously treated with a physiological dose of T3 (0.3 μg T3/100 g BW) [24] 30 min before euthanasia. Group 4 was also evaluated for serum LH and FSH concentration 1, 2 and 3 h after T3 treatment. The effectiveness of the treatment was confirmed by heart weight, as well as serum levels of TSH and T3, as previously reported [15, 22]. A minimum of 10 animals per group were used, and each experiment was repeated at least three times. Animals were obtained from our breeding colony, maintained under a 12:12 h dark/light cycle in a temperature-controlled room (23 ± 1 °C) and fed rat chow ad libitum. All procedures are in accordance with the Brazilian College of Animal Experimentation and approved by the Institute of Biomedical Sciences, University of Sao Paulo—Ethical Committee for Animal Research (protocol 029/55/02).
Analysis of Lhb and Fshb mRNA expression by reverse transcription followed by real-time quantitative PCR (RT-qPCR)
Total RNA was extracted from pituitaries of the rats using the guanidine–phenol–chloroform method [25] and subjected to RT-qPCR, as previously described [26]. Briefly, 1.0 µg of total RNA was reverse transcribed at 65 °C for 10 min using 0.5 µg oligo (dT) and diethyl pyrocarbonate-treated (DEPC) water in a total volume of 5.0 µl. Subsequently, 25 U RNAse Out, 4.0 µl buffer (250 mM Tris–HCl, 375 mM KCl, 15 mM MgCl2, 50 mM DTT, pH 8.3), 1.0 µl dNTP (10 mM), 200 U M-MLV Reverse Transcriptase and DEPC-treated water were added to a final volume of 20 µl. The samples were then incubated for 60 min at 42 °C. Quantification of the mRNAs was performed by qPCR using the Platinum SYBR Green qPCR SuperMix UDG kit (Life Technologies, USA), according to the manufacturer’s instructions, and the Rotor-Gene thermocycler (Corbett Life Sciences, Australia). At the end of the reaction, a melting curve was generated and analyzed to confirm the specificity of the amplification. The quantification was performed by the ΔΔCt method, as described [27, 28]. Rpl19 was used as the reference gene. The primers used had the following sequences: (1) Lhb (NM_012858): Forward 5′-TGAGTTCTGCCCAGTCTGC-3′ and Reverse 5′-GTGGGTGGGCATCAGAAGAG-3′; (2) Fshb (NM_001007597): Forward 5′-AAGTCGATCCAGCTTTGCAT-3′ and Reverse 5′-GTCCCAGGCCTCTTACAGTG-3′; (3) Rpl19 (NM_031103.1): Forward 5′-CAATGAAACCAACGAAATCG-3′ and Reverse: 5′-TCAGGCCATCTTTGATCAGCT-3′. The primers efficiencies were 0.9, 1.1, and 1.0 for Lhb, Fshb, and Rpl19, respectively.
Evaluation of the polysome profile
The expression of gonadotropin mRNA bound to ribosomes was evaluated according to the method previously described [15]. The UV absorbance of the resulting gradients was monitored at 254 nm (Monitor UVis-920; GE HealthcareBio-Sciences AB, Uppsala, Sweden). The gradients were separated into 12 fractions of ~1 ml each for RNA isolation, immediately frozen in dry ice, and then stored at −70 °C. The polysomal fractions were estimated using spectrophotometry and the integrity of RNAs was verified on a 1% agarose–TBE gel (Supplementary Figure 1). These procedures allowed for the quantification of the fractions that corresponded to Lhb and Fshb mRNA attached to polysomes by RT-qPCR [29].
Western blotting
The analysis of LHB and FSHB pituitary content was performed, as previously described [26]. Following electrophoresis and protein transfer, the nitrocellulose membranes were incubated with blocking buffer (5% nonfat dry milk, PBS buffer, and 0.1% Tween 20) to reduce nonspecific binding overnight at 4 °C, and subsequently incubated with anti-LHB antibody (1:75,000), anti-FSHB antibody (1:2500), or anti-GAPDH antibody (1:1000) diluted in blocking buffer for 3 h at room temperature (RT) (Table 1). The band detection was performed using the appropriate peroxidase-conjugated secondary antibody (1:5000) diluted in the same primary antibody buffer, for 1 h at RT, and developed using the ECL kit. The labeling specificity was confirmed by comparison with the protein molecular weight markers and was analyzed with ImageJ 1.47v software (National Institutes of Health, NIH, USA).
Immunofluorescence
Pituitaries were fixed in 2% paraformaldehyde solution in 2% v/v saline phosphate buffer (PBS; 2.7 mM KCl, 137 mM NaCl, 8 mM NaHPO4, 1.4 mM KPO4, pH 7.4), for 30 min at 4 °C, embedded in tissue freezing medium (Triangle Biomedical Sciences, Durham, NC, USA) and rapidly frozen in cold 2-methylbutane (Spectrum, Gardena, CA, USA), as previously described [22]. The tissue was then sliced into 5-μm-thick sections and transferred to gelatin-coated slides. The slides were incubated with normal goat serum diluted with an equal proportion of 10% PBS/BSA for 1 h at RT, to prevent nonspecific binding. Incubation with the rabbit primary antibody against rat LHB or FSHB (diluted in PBS/0.3% Tween 20) was performed overnight at 4 °C (Table 1). After rinsing, the slides were incubated with the FITC-conjugated secondary antibody against rabbit (1:200, Fluorescein isothiocyanate conjugated; Sigma) for 90 min at RT. Prolong Gold with 4,6-diamino-2-phenylindole (DAPI, Life Technologies, Camarillo, CA, USA) was added for nuclear staining and antifade protection, and coverslips were mounted. Negative controls were obtained by omitting the primary antibodies from the protocol (Supplementary Figure 2).
The slides were visualized with a Nikon Eclipse E600 microscope using the appropriate filters and images were digitalized using a Cool SNAP-Procf color digital camera (Roper Scientific, Trenton, New Jersey, USA) and Image-Pro Plus Software (Media Cybernetics, Silver Spring, MD, USA). Figures were prepared in Adobe Photoshop CS4 Version 11.0 (Adobe Systems Inc., Mountain View, CA, USA).
Hormone dosages
LH and FSH were measured using Luminex xMAP technology (Milliplex MAP rat pituitary panel, Millipore, USA). TSH, testosterone, and T3 serum concentrations were measured by radioimmunoassay (TSH—National Hormone and Peptide Program/NIDDK, Bethesda, MD, USA; total testosterone and total T3 Coat-A-Count, Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA).
Statistical analysis
The variables studied were first submitted to normality tests using the Kolmogorov–Smirnov test and homoscedasticity tests using the Bartlet test. Then the results were analyzed by the one-way ANOVA and post hoc Tukey test, using the Statistic 7.0 software (Statsoft Inc., Tulsa, OK, USA). Individual comparisons between euthyroid and hypothyroid groups presented in Fig. 1 were performed with the Student's t-test. Statistical differences were considered significant when the value of p was equal to or lower than 0.05. The values were expressed as means and standard error of the means (±SEM).
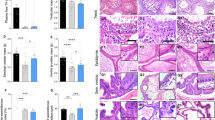
FSH expression in euthyroid and hypothyroid male rats. The following parameters were observed: a Total Fshb mRNA content, b Fshb mRNA associated to ribosomes, c FSHB protein content, and d serum FSH concentrations. The values were expressed as means ± SEM; Student's t-test, *p < 0.05, **p < 0.01; n = 10 animals/group (A, C, and D); n = 27 animals/group (B)
Results
Characterization of the experimental model
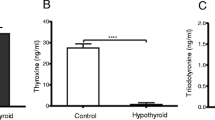
The animals used in this study were the same as those used in previous reports from our group [15, 22]. In brief, the hypothyroid group presented overt hypothyroidism as shown by the high serum TSH and low T3 levels. The hyperthyroid group presented low TSH and high serum T3 levels. The hypothyroid rats acutely treated with T3 presented high serum TSH and T3 levels, as compared to the euthyroid group.
Profile of pituitary Fshb mRNA, FSH expression, and serum FSH concentration of male rats under different thyroid states
The alterations of Fshb mRNA content and its association to ribosomes, FSHB content, and serum FSH concentrations were evaluated in male rats subjected to hypothyroidism (Fig. 1), thyrotoxicosis, and acute administration of T3 (Fig. 2). The distribution of FSHB labeling in pituitaries of euthyroid, hypothyroid, thyrotoxicosis, and Hypo + 1XT3 groups is shown in Fig. 3, and the serum FSH concentration values 1, 2, and 3 h after a physiological dose of T3 are shown in Fig. 4. Representative Western blots are presented in Fig. 5.
FSH expression in different thyroid hormone conditions: hypothyroid, thyrotoxicosis, and T3 acute group (Hypo + 1XT3). The following parameters were observed: a Total Fshb mRNA content, b Fshb mRNA associated to ribosomes, c FSHB protein content, and d serum FSH concentrations. The dashed horizontal line indicates the euthyroid levels. The values were expressed as means ± SEM; one-way ANOVA, and post hoc Tukey test; *p < 0.05, **p < 0.01, and ***p < 0.001 vs. euthyroid group (dashed line); #vs. hypothyroid (p < 0.01) and vs. thyrotoxicosis (p < 0.05); n = 10 animals/group (A, C, and D); n = 27 animals/group (B)
FSH distribution in gonadotrophs in different thyroid hormone conditions: euthyroid, hypothyroid, thyrotoxicosis, and T3 acute group (Hypo + 1XT3). FSHB immunolabeling is shown in green by immunofluorescence and the cells nuclei are seen in blue with DAPI. FSHB labeling at the membrane of the gonadotrophs is indicated by the arrow and in the cytoplasm by the asterisk. n = 10 animals/group. Scale bars: 10 μm. BV blood vessel
Serum FSH concentrations in hypothyroid and animals treated with a physiological dose of T3 for 1 h (Hypo + 1XT3 1 h), 2 h (Hypo + 1XT3 2 h), and 3 h (Hypo + 1XT3 3 h). The dashed line indicates the euthyroid levels. The values were expressed as means ± SEM; one-way ANOVA and post hoc Tukey test; *p < 0.05 vs. euthyroid group (dashed line); n = 10 animals/group
It was shown that hypothyroidism increased Fshb mRNA expression (Fig. 1a), and reduced its association to ribosomes (Fig. 1b), the FSHB protein content (Fig. 1c), as well as the serum FSH concentration (Fig. 1d). In the immunofluorescence studies (Fig. 3), the hypothyroid group presented enlarged blood vessels (BV) and the FSHB labeling was predominantly close to the plasma membrane of the gonadotrophs, as indicated by the arrow. In contrast, the FSHB labeling in the euthyroid group was scattered through the entire cytoplasm, as indicated by the asterisk (Fig. 3).
In the thyrotoxicosis state there was also an increase in Fshb mRNA expression (Fig. 2a), while its association to ribosomes was comparable to the values of the euthyroid group (dashed line) (Fig. 2b). The FSHB protein content was increased (Fig. 2c), but the serum FSH concentration was not altered (Fig. 2d). The FSHB labeling was stronger than that observed in the euthyroid group, scattered throughout the cytoplasm (asterisk), but also close to the plasma membrane (arrow) (Fig. 3)
The acute T3 treatment of hypothyroid rats altered all of the parameters evaluated. The mRNA content was markedly reduced to about 20% of the euthyroid levels (dashed line) and was 10-fold lower when compared to the hypothyroid and thyrotoxicosis groups (Fig. 2a). The association to ribosomes was similar to the values observed in the euthyroid group (dashed line), but higher than the values observed in the hypothyroid group (Fig. 2b). The FSHB content (Fig. 2c) and serum FSH concentration (Fig. 2d) were rapidly increased when compared to hypothyroidism, and returned to the levels observed in the euthyroid group (dashed line). The FSHB labeling was increased and dispersed throughout the entire cytoplasm compared to the hypothyroid and euthyroid groups (Fig. 3). The serum FSH concentrations were similar to euthyroid values (dashed line) from 1 h up to 3 h after T3 administration (Fig. 4).
Profile of pituitary Lhb mRNA, LH expression, and serum LH concentration of male rats under different thyroid states
The alterations in Lhb mRNA expression and its association to ribosomes, LHB content, and serum LH concentrations were evaluated in male rats subjected to thyrotoxicosis and acute T3 administration, and the data are presented in Fig. 6. The distribution of LHB labeling in the pituitary is shown in Fig. 7, and the serum LH concentrations 1, 2, and 3 h after T3-treatment (1X) is shown in Fig. 8. Representative Western blots are presented in Fig. 5.
LH expression in different thyroid hormone conditions: hypothyroid, thyrotoxicosis, and T3 acute group (Hypo + 1XT3). The following parameters were observed: a Lhb mRNA content, b Lhb mRNA associated to ribosomes, c LHB protein content, and d serum LH concentrations. The dashed line indicates the euthyroid levels. The values were expressed as means ± SEM; one-way ANOVA and post hoc Tukey test; *p < 0.05 and **p < 0.01 vs. euthyroid group (dashed line); # vs. thyrotoxicosis (a p < 0.05; b p < 0.01; c p < 0.0001; d p < 0.01) and vs. Hypo + 1XT3 (a and c p < 0.01); @ vs. hypothyroid (p < 0.01) and Hypo + 1XT3 (p < 0.05); & vs. hypothyroid and Hypo + 1XT3 (p < 0.01); n = 10 animals/group (a, c, and d); n = 27 animals/group (B)
LH distribution in gonadotrophs in different thyroid hormone conditions: euthyroid, hypothyroid, thyrotoxicosis, and T3 acute group (Hypo + 1XT3). LHB immunolabeling is shown in green by immunofluorescence and the cells nuclei are seen in blue with DAPI. LHB labeling at the membrane of the gonadotrophs is indicated by the arrow and in cytoplasm by the asterisk. Scale bars: 10 μm. n = 10 animals/group
Serum LH concentrations in hypothyroid and animals treated with physiological dose of T3 for 1 h (Hypo + 1XT3 1 h), 2 h (Hypo + 1XT3 2 h), and 3 h (Hypo + 1XT3 3 h). The dashed line indicates the euthyroid levels. The values were expressed as means ± SEM; one-way ANOVA and post hoc Tukey test; **p < 0.01 vs. euthyroid group (dashed line); # vs. hypothyroid (p < 0.05) and Hypo + 1XT3 1 h (p < 0.01); n = 10 animals/group
With respect to hypothyroidism, the results of Lhb mRNA content and its association to ribosomes, LH serum levels, and LHB content in the pituitary of euthyroid vs. hypothyroid groups were previously published [15], and included in Fig. 6, as a comparison with the thyrotoxicosis and Hypo + 1XT3 groups (euthyroid group in dashed lines), since these animals/samples were part of the same study cited above. The distribution of LHB labeling in pituitaries of euthyroid, hypothyroid, thyrotoxicosis, and Hypo + 1XT3 group is shown in Fig. 7. The immunofluorescence studies of pituitary tissue sections revealed that the LHB labeling in the hypothyroid group was usually close to the nucleus, in contrast to the euthyroid group, in which the LHB labeling was observed mainly scattered throughout the cytoplasm (asterisk), and also close to the plasma membrane (arrow) (Fig. 7).
Thyrotoxicosis did not alter the Lhb mRNA content (Fig. 6a), the LHB protein content (Fig. 6c) or serum LH concentration (Fig. 6d), but the association of Lhb mRNA with the ribosome (Fig. 6b) was three-fold higher than the values of the euthyroid group (dashed line). The LHB staining was distributed throughout the gonadotroph cytoplasm and was more intense than the euthyroid group (Fig. 7).
The acute T3 treatment of hypothyroid rats (Hypo + 1XT3) rapidly reestablished the Lhb mRNA content (Fig. 6a), its association to ribosomes (Fig. 6b), and the LHB protein content in the pituitary (Fig. 6c) to values comparable to euthyroid animals (dashed line). However, the elevated serum LH values observed in the hypothyroid group remained high after acute T3 treatment (Fig. 6d). The LHB labeling after acute T3 treatment was increased compared to the hypothyroid group and scattered throughout the whole cytoplasm, as observed in the euthyroid group (Fig. 7). Three hours after T3-treatment, the serum LH concentration was decreased to values similar to those observed in the euthyroid group (Fig. 8 dashed line).
When hypothyroid and thyrotoxicosis groups are compared to each other, opposite results were observed for the mRNA content (Fig. 6a), its association to ribosomes (Fig. 6b), LHB protein content in the pituitary (Fig. 6c) and serum LH values (Fig. 6d).
Serum testosterone concentration of male rats under different thyroid states
Serum testosterone concentration was measured in male rats subjected to thyrotoxicosis and acute T3 administration and the data are shown in Fig. 9. Hypothyroidism reduced the serum testosterone concentration ~10-fold compared to the euthyroid group (dashed line), and acute T3 administration did not change this parameter. In thyrotoxicosis, the serum testosterone levels were below the detectable limit for the method (<8 pg/mL).
Total serum testosterone concentrations in euthyroid, hypothyroid, thyrotoxicosis, and T3 acute group (Hypo + 1XT3). The dashed line indicates the euthyroid levels. The values were expressed as means ± SEM; one-way ANOVA and post hoc Tukey test; **p < 0.01 vs. euthyroid group (dashed line); n = 10 animals/group
Discussion
Infertility has become a problem of global proportions and affects 20% of couples trying to conceive [30]. Among the couples undergoing infertility treatment, about 50% present male reproductive disorders [30,31,32]. Many factors are involved in male infertility, but the most common issue is sperm abnormalities [30, 33]. Previous studies, in men, have shown that hypothyroidism is associated with sperm morphology defects, and thyrotoxicosis is associated with decreased sperm motility. However, once thyroid function is normalized all of the disorders are reversed [4, 7, 8, 12, 13]. Recently, subclinical hyperthyroidism and subclinical hypothyroidism were identified in 3.7 and 7.4%, respectively, of men seeking medical care for infertility [34]. In rats, hypothyroidism, but not thyrotoxicosis decreases the sperm production, whereas sperm functionality, which is related to the fertilizing ability of the sperm, is negatively affected by both hypothyroidism and thyrotoxicosis [35]. All these data strongly suggest TH participation in male reproductive physiology, although the regulation of gonadotroph activity and the mechanisms involved in the regulation of gonadotropin expression by TH in males are still unclear [14].
In men with primary hypothyroidism and hypogonadism the levels of gonadotropins were reported to be decreased (hypogonadotrophic), increased (hypergonadotrophic), or even normal, and these findings were also reported in men with high prolactin levels [11]. However, the prolactin in hypothyroid men is not elevated, which suggests that the defect in gonadal function in primary hypothyroidism is at the hypothalamus and/or pituitary level rather than at the gonadal level. Supporting this, in primary hypothyroidism the pituitary did not respond to exogenous GnRH administration and free testosterone concentrations are reduced [11]. Interestingly, contrary to what is observed in hypothyroid men, the response to an exogenous administration of GnRH is exaggerated in the pituitary of hyperthyroid men, and Leydig cells do not respond to hCG [11].
The pituitary is a key regulator of testicular function by the action of LH and FSH [36,37,38,39,40,41]. In this context, the present study aimed to evaluate whether T3 regulates different steps of LH and FSH synthesis and secretion in adult male rats, which could point out a cross talk between the hypothalamus–pituitary–thyroid (HPT) and HPG axes, at the molecular level. The results for mRNA and pituitary protein content were assessed by the evaluation of the beta-specific subunit, which is unique for each hormone, LH and FSH, and guarantees biological specificity [42].
Firstly, we evaluated the pituitary function in hypothyroidism triggered in adulthood. Our data indicate the importance of thyroid hormones on the post-transcriptional regulation of FSH synthesis. During the hypothyroid state, even though the Fshb mRNA content was high, its association to ribosomes was markedly reduced, which in turn caused a decrease in the FSHB translation rate, FSHB protein content, and consequently in serum FSH concentration. Besides the reduction observed in the synthesis of FSHB, the secretion process appeared to be preserved, as evidenced by the FSHB immunolabeling close to plasma membrane of gonadotrophs. Thus, we may infer that the lower amount of FSH produced in the gonadotrophs is poorly retained by the secretory vesicles, and promptly secreted, as depicted by the diffuse labeling of FSHB throughout the cytoplasm.
Interestingly, as previously described [15], in the same hypothyroid condition, several steps of LH synthesis are altered as shown by the elevated Lhb mRNA content, which seemed to be sufficient to guarantee the higher levels of serum LH concentration, even with a noticeable reduction in the polyadenylation and the association of Lhb mRNA to the ribosomes, and consequently in LHB translation. The immunolabeling analyses supported the hypothesis that the synthesis and secretion of LHB during hypothyroidism are increased, since the LHB labeling was predominantly observed at perinuclear regions of LHB-positive cells and reduced in the cytoplasm.
During hypothyroidism the serum testosterone concentration is reduced, decreasing its negative feedback effect at the hypothalamus and pituitary levels, thus increasing the release of GnRH and consequently raising the Lhb and Fshb transcriptional and secretion rates [43]. The absence of a negative testosterone effect in gonadotrophs is evidenced by the increased serum LH concentration in hypothyroid animals. However, the serum FSH concentration was reduced. This discrepancy could be due to an increase in the synthesis and secretion of inhibin/activin subunits in response to the high GnRH environment.
Additionally, SHBG is a plasma protein that binds testosterone and other sex steroid hormones with high affinity and selectivity transports them through the plasma to target tissues [5]. Therefore, SHBG is responsible for carrying testosterone secreted from the testis to the brain region, where it will dissociate from SHBG and allow the free testosterone to cross the blood–brain barrier, thus making it available to perform in the negative feedback mechanism for LH synthesis and secretion [40]. In rodents, the role of SHBG in the control of free circulating testosterone levels is still under investigation [44, 45]. Hypothyroidism decreases the synthesis of SHBG in the liver and reduces the serum levels, compromising the transport of sex steroids in the plasma and consequently the negative feedback mechanism [5, 11, 12]. This finding, plus the fact, that testosterone levels were markedly reduced, could explain why the serum LH concentration is elevated in the hypothyroid group.
Besides the increase in gonadotropin mRNA content, the transcripts association to ribosomes and the LH and FSH translation rate are reduced, which seems to be an effect due to the absence of TH, since an increase in the translational rate should be expected in response to the reduced testosterone signaling and SHBG content observed in hypothyroid conditions.
To clarify the role of TH on the HPG axis, hypothyroid male rats were injected with a single physiological dose of T3, and gonadotrophs were evaluated after 30 min. The alterations observed in gonadotroph activity during hypothyroidism were promptly reversed by T3 treatment, as shown by the decrease in Fshb and Lhb mRNA expression and the reestablishment of their association to ribosomes, as well as of FSHB and LHB pituitary protein content to euthyroid levels. Additionally, the pattern of FSHB and LHB distribution after acute T3 treatment was very similar to euthyroid pituitaries, as shown by the immunohistochemical studies (Figs. 3 and 7). Serum FSH concentration also returned to euthyroid levels after acute T3-treatment (30 min), while serum LH concentration remained high for up to 2 h after the T3 administration, and returned to euthyroid values after 3 h. Therefore, physiological administration of T3 reestablishes the studied parameters to those observed in the euthyroid group, thus indicating that T3 is a modulator of the HPG axis, at the pituitary level.
Furthermore, these findings suggest that posttranscriptional mechanisms at different steps are triggered by T3, which would lead to the differential secretion pattern for LH and FSH. It is noteworthy that the results, for both LH and FSH, observed after acute T3 treatment were not dependent on the dose of T3 administered, since similar results were observed when higher doses of T3 were tested (data not shown). Hence, these data point out that the rapid effects of T3 on gonadotropin synthesis and secretion were not correlated to the degree of occupancy of thyroid hormones receptors, but possibly triggered by non-genomic pathways [46]. In this sense, we have previously reported that the T3 rapidly increases the total protein content and the chain glycoprotein alpha (CGA) labeling in both thyrotrophs and gonadotrophs [47].
It is worth mentioning that the TH-induced increase in the SHBG levels is only observed 24 h after the treatment [48], and the testosterone concentration was not affected by acute T3 treatment. The rapid effects observed in gonadotroph activity is exclusively due to T3, with low or no interference from SHBG and testosterone in this model. All these data strongly reinforce the notion that T3 modulates the secretion of the mature LH and FSH glycoproteins.
The synthesis and secretion of gonadotropins were also studied in conditions of excess of TH, in thyrotoxic male rats. FSH expression, Fshb mRNA and consequently the synthesis of FSHB were all shown to be increased in thyrotoxicosis. However, the serum FSH concentration was kept within the euthyroid group values, suggesting that the FSH secretion might be modulated by excess T3. For LH, the thyrotoxicosis markedly increased the amount of Lhb mRNA associated to ribosomes. This augment might raise the LHB translation rate and could be responsible for the elevated LHB protein content, which was corroborated by the immunohistochemical studies. However, no change was observed in serum LH concentration, when compared to the euthyroid group, suggesting that T3 could exert a modulating influence on LH secretion, as previously observed for TSH [22].
An interesting observation was the dilated BV in the pituitary gland of hypothyroid animals. Thyroid hormones are known to increase eNOS expression, thus augmenting the capillaries diameter and decreasing the blood pressure [49,50,51,52,53]. Therefore, our finding suggests that other mechanisms could be involved in the dilation of BV in the hypothyroid condition.
In fact, another member of the NOS family, the neuronal NOS (nNOS), which is expressed in several cell types in the central nervous system (CNS), is also known to induce vasodilatation, thus participating in the regulation of the CNS blood pressure [54], and is constitutively found in the pituitaries of rats and in larger amounts than eNOS [55]. Moreover, it was shown that in the liver of hypothyroid rats the mRNA and protein levels of nNOS are increased, resulting in an enhanced nitric-oxide synthase activity [56]. It is also well known that the number and activity of thyrotrophs are increased in hypothyroid conditions, as well as the secretion of TRH, which has been shown to stimulate nNOS activity via calcium/calmodulin-dependent protein kinase (CaMKs) pathway [57]. In this sense, both TRH and the higher activity of thyrotrophs could contribute to the increase in the nNOS activity in the pituitary, increasing the NOS synthesis and modulating the blood flow. Therefore, it could be suggested that the enlarged BV in the pituitary of hypothyroid rats may be associated with the increase in the nNOS expression and activity, when TH levels are reduced.
Rapid and long-term changes in LH and FSH synthesis and secretion observed in the Hypo + 1XT3 and thyrotoxicosis groups, respectively, were exclusively due to T3, since the serum testosterone concentration remained reduced in these groups, as observed in the hypothyroid group. Taken together, this excludes the effects of testosterone on its own axis, and reinforces the hypothesis that there is an important cross-talk between the HPT and HPG axes. The opposite effects on the LH parameters (mRNA content, association to ribosomes, LHB content in the pituitary and serum LH values) observed in hypothyroidism vs. thyrotoxicosis also contributes to this understanding.
Conclusion
The findings presented herein suggest that physiological levels of T3 are necessary for the regulation of the HPG axis and provide novel insights into a potential mechanism by which the gonadotrophin subunits are modulated by thyroid hormones at posttranscriptional and secretion levels. Moreover, the results obtained are thought to reinforce the link between thyroid dysfunction and infertility.
References
P. Donnelly, C. White, Testicular dysfunction in men with primary hypothyroidism; reversal of hypogonadotrophic hypogonadism with replacement thyroxine. Clin. Endocrinol. 52(2), 197–201 (2000)
N. Tagawa, T. Takano, S. Fukata, K. Kuma, H. Tada, Y. Izumi, Y. Kobayashi, N. Amino, Serum concentration of androstenediol and androstenediol sulfate in patients with hyperthyroidism and hypothyroidism. Endocr. J. 48(3), 345–354 (2001)
A. Iranmanesh, G. Lizarralde, M.L. Johnson, J.D. Veldhuis, Dynamics of 24-hour endogenous cortisol secretion and clearance in primary hypothyroidism assessed before and after partial thyroid hormone replacement. J. Clin. Endocrinol. Metab. 70(1), 155–161 (1990). https://doi.org/10.1210/jcem-70-1-155
J. Nielsen, R.B. Jensen, A. Juul, Increased sex hormone-binding globulin levels in children and adolescents with thyrotoxicosis. Horm. Res. Paediatr. 79, 157–61 (2013). https://doi.org/10.1159/000348837
M. Pugeat, N. Nader, K. Hogeveen, G. Raverot, H. Dechaud, C. Grenot, Sex hormone-binding globulin gene expression in the liver: drugs and the metabolic syndrome. Mol. Cell. Endocrinol. 316(1), 53–59 (2010). https://doi.org/10.1016/j.mce.2009.09.020
M.R. Nikoobakht, M. Aloosh, N. Nikoobakht, A.R. Mehrsay, F. Biniaz, M.A. Karjalian, The role of hypothyroidism in male infertility and erectile dysfunction. Urol. J. 9(1), 405–409 (2012)
G.E. Krassas, K. Tziomalos, F. Papadopoulou, N. Pontikides, P. Perros, Erectile dysfunction in patients with hyper- and hypothyroidism: how common and should we treat? J. Clin. Endocrinol. Metab. 93(5), 1815–1819 (2008). https://doi.org/10.1210/jc.2007-2259
G.E. Krassas, F. Papadopoulou, K. Tziomalos, T. Zeginiadou, N. Pontikides, Hypothyroidism has an adverse effect on human spermatogenesis: a prospective, controlled study. Thyroid 18(12), 1255–1259 (2008). https://doi.org/10.1089/thy.2008.0257
G.E. Krassas, N. Pontikides, Male reproductive function in relation with thyroid alterations. Best Pract. Res. Clin. Endocrinol. Metab. 18(2), 183–195 (2004). https://doi.org/10.1016/j.beem.2004.03.003
S. Rojdmark, A. Berg, G. Kallner, Hypothalamic-pituitary-testicular axis in patients with hyperthyroidism. Horm. Res. 29(5–6), 185–190 (1988)
A.W. Meikle, The interrelationships between thyroid dysfunction and hypogonadism in men and boys. Thyroid 14(3, Suppl. 1), 17–25 (2004)
S. La Vignera, R. Vita, R.A. Condorelli, L.M. Mongioì, S. Presti, S. Benvenga, A.E. Calogero, Impact of thyroid disease on testicular function. Endocrine 58(3), 397–407 (2017). https://doi.org/10.1007/s12020-017-1303-8
G.E. Krassas, K. Poppe, D. Glinoer, Thyroid function and human reproductive health. Endocr. Rev. 31(5), 702–755 (2010). https://doi.org/10.1210/er.2009-0041
E. Krajewska-Kulak, P. Sengupta, Thyroid function in male infertility. Front. Endocrinol. 4, 174 (2013). https://doi.org/10.3389/fendo.2013.00174
R.M. Romano, P. Bargi-Souza, E.L. Brunetto, F. Goulart-Silva, M.C. Avellar, C.A. Oliveira, M.T. Nunes, Hypothyroidism in adult male rats alters posttranscriptional mechanisms of luteinizing hormone biosynthesis. Thyroid 23(4), 497–505 (2013). https://doi.org/10.1089/thy.2011.0514
K. Czaplinski, R.H. Singer, Pathways for mRNA localization in the cytoplasm. Trends Biochem. Sci. 31(12), 687–693 (2006). https://doi.org/10.1016/j.tibs.2006.10.007
J. Hesketh, Translation and the cytoskeleton: a mechanism for targeted protein synthesis. Mol. Biol. Rep. 19(3), 233–243 (1994)
S. Kindler, H. Wang, D. Richter, H. Tiedge, RNA transport and local control of translation. Annu. Rev. Cell Dev. Biol. 21, 223–245 (2005). https://doi.org/10.1146/annurev.cellbio.21.122303.120653
Y. Funakoshi, Y. Doi, N. Hosoda, N. Uchida, M. Osawa, I. Shimada, M. Tsujimoto, T. Suzuki, T. Katada, S. Hoshino, Mechanism of mRNA deadenylation: evidence for a molecular interplay between translation termination factor eRF3 and mRNA deadenylases. Genes Dev. 21(23), 3135–3148 (2007). https://doi.org/10.1101/gad.1597707
J.L. Leonard, Non-genomic actions of thyroid hormone in brain development. Steroids 73(9–10), 1008–1012 (2008). https://doi.org/10.1016/j.steroids.2007.12.016
F.G. Silva, G. Giannocco, M.F. Santos, M.T. Nunes, Thyroid hormone induction of actin polymerization in somatotrophs of hypothyroid rats: potential repercussions in growth hormone synthesis and secretion. Endocrinology 147(12), 5777–5785 (2006). https://doi.org/10.1210/en.2006-0110
P. Bargi-Souza, R.M. Romano, M. Salgado Rde, F. Goulart-Silva, E.L. Brunetto, T.M. Zorn, M.T. Nunes, Triiodothyronine rapidly alters the TSH content and the secretory granules distribution in male rat thyrotrophs by a cytoskeleton rearrangement-independent mechanism. Endocrinology 154(12), 4908–4918 (2013). https://doi.org/10.1210/en.2013-1508
R. Regazzi. Molecular Mechanisms of Exocytosis. (Landes Bioscience and Springer Science, New York, 2007)
W.H. Dillmann, S. Berry, N.M. Alexander, A physiological dose of triiodothyronine normalizes cardiac myosin adenosine triphosphatase activity and changes myosin isoenzyme distribution in semistarved rats. Endocrinology 112(6), 2081–2087 (1983)
P. Chomczynski, N. Sacchi, Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162(1), 156–159 (1987). https://doi.org/10.1006/abio.1987.9999
M.A. Romano, R.M. Romano, L.D. Santos, P. Wisniewski, D.A. Campos, P.B. de Souza, P. Viau, M.M. Bernardi, M.T. Nunes, C.A. de Oliveira, Glyphosate impairs male offspring reproductive development by disrupting gonadotropin expression. Arch. Toxicol. 86(4), 663–673 (2012). https://doi.org/10.1007/s00204-011-0788-9
K.J. Livak, T.D. Schmittgen, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4), 402–408 (2001). https://doi.org/10.1006/meth.2001.1262
M.W. Pfaffl, A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29(9), e45 (2001)
M.J. del Prete, R. Vernal, H. Dolznig, E.W. Mullner, J.A. Garcia-Sanz, Isolation of polysome-bound mRNA from solid tissues amenable for RT-PCR and profiling experiments. RNA 13(3), 414–421 (2007). https://doi.org/10.1261/rna.79407
P. Szkodziak, S. Wozniak, P. Czuczwar, E. Wozniakowska, P. Milart, A. Mroczkowski, T. Paszkowski, Infertility in the light of new scientific reports—focus on male factor. Ann. Agric. Environ. Med. 23(2), 227–230 (2016). https://doi.org/10.5604/12321966.1203881
P. Patrizio, F. Sanguineti, D. Sakkas, Modern andrology: from semen analysis to postgenomic studies of the male gametes. Ann. N. Y. Acad. Sci. 1127, 59–63 (2008). https://doi.org/10.1196/annals.1434.021
M. Punab, O. Poolamets, P. Paju, V. Vihljajev, K. Pomm, R. Ladva, P. Korrovits, M. Laan, Causes of male infertility: a 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum. Reprod. 32(1), 18–31 (2017). https://doi.org/10.1093/humrep/dew284
C. Krausz, Male infertility: pathogenesis and clinical diagnosis. Best Pract. Res. Clin. Endocrinol. Metab. 25(2), 271–285 (2011). https://doi.org/10.1016/j.beem.2010.08.006
F. Lotti, E. Maseroli, N. Fralassi, S. Degl’Innocenti, L. Boni, E. Baldi, M. Maggi, Is thyroid hormones evaluation of clinical value in the work-up of males of infertile couples? Hum. Reprod. 31(3), 518–529 (2016). https://doi.org/10.1093/humrep/dev338
R.M. Romano, S.N. Gomes, N.C. Cardoso, L. Schiessl, M.A. Romano, C.A. Oliveira, New insights for male infertility revealed by alterations in spermatic function and differential testicular expression of thyroid-related genes. Endocrine 55(2), 607–617 (2017). https://doi.org/10.1007/s12020-016-0952-3
U.B. Kaiser, A. Jakubowiak, A. Steinberger, W.W. Chin, Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro 1. Endocrinology 138(3), 1224–31 (1997)
L. Nagirnaja, K. Rull, L. Uusküla, P. Hallast, M. Grigorova, M. Laan, Genomics and genetics of gonadotropin beta-subunit genes: unique FSHB and duplicated LHB/CGB loci. Mol. Cell. Endocrinol. 329(1), 4–16 (2010)
A.W. Norman, H.L. Henry, Hormones (Academic Press, San Diego, 2014)
L. O’Donnell, S.J. Meachem, P.G. Stanton, R.I. McLachlan, in Chapter 21: Endocrine Regulation of Spermatogenesis. ed. by J.D. Neill, T.M. Plant, D.W. Pfaff, J.R.G. Challis, D.M. de Kretser, J.S. Richards, P.M. Wassarman. Knobil and Neill’s Physiology of Reproduction, vol 1 (Academic Press, St. Louis, 2006), pp. 1017–1069
K.-H. Jeong, U.B. Kaiser, in Chapter 31: Gonadotropin-Releasing Hormone Regulation of Gonadotropin Biosynthesis and Secretion. ed. by J.D. Neill, T.M. Plant, D.W. Pfaff, J.R.G. Challis, D.M. de Kretser, J.S. Richards, P.M. Wassarman (Academic Press, St. Louis, 2006), pp. 1635–1701
A.E. O’Connor, D.M. De Kretser, Inhibins in normal male physiology. Semin. Reprod. Med. 22(3), 177–185 (2004). https://doi.org/10.1055/s-2004-831893
J.G. Pierce, T.F. Parsons, Glycoprotein hormones: structure and function. Annu. Rev. Biochem. 50(1), 465–495 (1981)
F.J. Hayes, S. DeCruz, S.B. Seminara, P.A. Boepple, W.F. Crowley Jr., Differential regulation of gonadotropin secretion by testosterone in the human male: absence of a negative feedback effect of testosterone on follicle-stimulating hormone secretion. J. Clin. Endocrinol. Metab. 86(1), 53–58 (2001). https://doi.org/10.1210/jcem.86.1.7101
M.R. Laurent, G.L. Hammond, M. Blokland, F. Jardi, L. Antonio, V. Dubois, R. Khalil, S.S. Sterk, E. Gielen, B. Decallonne, G. Carmeliet, J.M. Kaufman, T. Fiers, I.T. Huhtaniemi, D. Vanderschueren, F. Claessens, Sex hormone-binding globulin regulation of androgen bioactivity in vivo: validation of the free hormone hypothesis. Sci. Rep. 6, 35539 (2016). https://doi.org/10.1038/srep35539
Y. Li, X. Li, H. Fan, X. Li, Y. Zhong, J. Cao, D. Yu, M. Zhang, J.G. Wen, L. Geng, Z. Suo, Age-dependent sex hormone-binding globulin expression in male rat. Ultrastruct. Pathol. 39(2), 121–130 (2015). https://doi.org/10.3109/01913123.2015.1009222
P.J. Davis, F. Goglia, J.L. Leonard, Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 12(2), 111–121 (2016). https://doi.org/10.1038/nrendo.2015.205
P. Bargi-Souza, R.M. Romano, F. Goulart-Silva, E.L. Brunetto, M.T. Nunes, T(3) rapidly regulates several steps of alpha subunit glycoprotein (CGA) synthesis and secretion in the pituitary of male rats: potential repercussions on TSH, FSH and LH secretion. Mol. Cell. Endocrinol. 409, 73–81 (2015). https://doi.org/10.1016/j.mce.2015.04.002
W. Rosner, D.P. Aden, M.S. Khan, Hormonal influences on the secretion of steroid-binding proteins by a human hepatoma-derived cell line. J. Clin. Endocrinol. Metab. 59(4), 806–808 (1984). https://doi.org/10.1210/jcem-59-4-806
Y. Cai, M.M. Manio, G.P. Leung, A. Xu, E.H. Tang, P.M. Vanhoutte, Thyroid hormone affects both endothelial and vascular smooth muscle cells in rat arteries. Eur. J. Pharmacol. 747, 18–28 (2015). https://doi.org/10.1016/j.ejphar.2014.11.036
A.L. de Castro, A.V. Tavares, R.O. Fernandes, C. Campos, A. Conzatti, R. Siqueira, T.R. Fernandes, P.C. Schenkel, C.L. Sartorio, S. Llesuy, A. Bello-Klein, A.S. da Rosa Araujo, T3 and T4 decrease ROS levels and increase endothelial nitric oxide synthase expression in the myocardium of infarcted rats. Mol. Cell. Biochem 408(1–2), 235–243 (2015). https://doi.org/10.1007/s11010-015-2501-4
D.J. Grieve, S. Fletcher, A.A. Pitsillides, K.M. Botham, J. Elliott, Effects of oral propylthiouracil treatment on nitric oxide production in rat aorta. Br. J. Pharmacol. 127(1), 1–8 (1999). https://doi.org/10.1038/sj.bjp.0702501
L. Tian, L. Zhang, J. Liu, T. Guo, C. Gao, J. Ni, Effects of TSH on the function of human umbilical vein endothelial cells. J. Mol. Endocrinol. 52(2), 215–222 (2014). https://doi.org/10.1530/jme-13-0119
E. Bussemaker, R. Popp, B. Fisslthaler, C.M. Larson, I. Fleming, R. Busse, R.P. Brandes, Hyperthyroidism enhances endothelium-dependent relaxation in the rat renal artery. Cardiovasc. Res. 59(1), 181–8 (2003)
U. Förstermann, W.C. Sessa, Nitric oxide synthases: regulation and function. Eur. Heart J. 33(7), 829–837 (2012). https://doi.org/10.1093/eurheartj/ehr304
R. Ramachandran, K.B. Ploug, A. Hay-Schmidt, J. Olesen, I. Jansen-Olesen, S. Gupta, Nitric oxide synthase (NOS) in the trigeminal vascular system and other brain structures related to pain in rats. Neurosci. Lett. 484(3), 192–196 (2010). https://doi.org/10.1016/j.neulet.2010.08.050
M.C. Franco, V.G. Antico Arciuch, J.G. Peralta, S. Galli, D. Levisman, L.M. Lopez, L. Romorini, J.J. Poderoso, M.C. Carreras, Hypothyroid phenotype is contributed by mitochondrial complex I inactivation due to translocated neuronal nitric-oxide synthase. J. Biol. Chem. 281(8), 4779–4786 (2006). https://doi.org/10.1074/jbc.M512080200
T. Song, N. Hatano, T. Kambe, Y. Miyamoto, H. Ihara, H. Yamamoto, K. Sugimoto, K. Kume, F. Yamaguchi, M. Tokuda, Y. Watanabe, Nitric oxide-mediated modulation of calcium/calmodulin-dependent protein kinase II. Biochem. J. 412(2), 223–231 (2008). https://doi.org/10.1042/bj20071195
Acknowledgements
The authors thank Leonice Lourenço Poyares for the excellent technical assistance.
Funding
This work was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP, (2008/50977-2, 2009/17822-8, 2013/05629-4) and Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (305936/2013-1), Brazil. P.B.S., R.M.R., and E.L.B. are the recipients of a FAPESP fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing of interests.
Ethical approval
All procedures are in accordance to the Brazilian College of Animal Experimentation and approved by the Institute of Biomedical Sciences, University of Sao Paulo—Ethical Committee for Animal Research (protocol 029/55/02).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Romano, R.M., Bargi-Souza, P., Brunetto, E.L. et al. Triiodothyronine differentially modulates the LH and FSH synthesis and secretion in male rats. Endocrine 59, 191–202 (2018). https://doi.org/10.1007/s12020-017-1487-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-017-1487-y