Abstract
Exendin-4, a glucagon-like peptide-1 receptor agonist, is currently regarded as an effective therapeutic strategy for type-2 diabetes. Previous studies indicated that exendin-4 promoted β cell proliferation. However, the underlying mechanisms remain largely unknown. Recently it was reported that exendin-4 promoted pancreatic β cell proliferation by regulating the expression level of Wnt4. The present study was designed to investigate whether other Wnt isoforms take part in accommodation of β-cell proliferation. We found that exendin-4 promotes the proliferation and suppresses the expression of Wnt5a in INS-1 cell line and C57Bl/6 mouse pancreatic β-cells. Further mechanistic study demonstrated that exendin-4 promoted INS-1 cell proliferation partly through down-regulating the expression of Wnt5a. Furthermore, Wnt5a could induce the activation of calmodulin-dependent protein kinase II in INS-1 cells, thereby decreasing the cellular stable β-catenin and its nuclear translocation, and finally reduce the expression of cyclin D1. In addition, we also found that both of the receptors (Frz-2 and Ror-2) mediated the effect of Wnt5a on β cell line INS-1 proliferation. Taken together, this study suggests that Wnt5a plays a critical role in exendin-4-induced β-cell proliferation, indicating that Wnt5a might be a novel regulator in counterbalance of β cell mass.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance and defective insulin secretion [1]. The prevalence of T2D is growing fast worldwide, and it has become a serious social health problem [2]. It’s well-known that the lack of functional pancreatic β-cells leads to hyperglycemia and diabetes [3]. Therefore, it is critically important to find ways to increase functional β-cells mass. Recent studies showed that induction of β-cell proliferation was a major approach to increase β-cell mass [2], which demands further exploration of the molecular mechanisms involved in regulating β cell proliferation.
Exendin-4 (Ex-4), a glucagon-like peptide-1 receptor agonist, has been used in treatment for T2DM popularly [4]. Ex-4 stimulates insulin and somatostatin secretion as well as inhibits glucagon secretion. In addition, it can also promote islet β-cell proliferation and differentiation, and effectively improve their functions [5]. However, the mechanisms involved in the proliferative role of Ex-4 in pancreatic β cell are still needed to be further studied.
Wnt genes family, which includes at least 19 family members, plays crucial roles in many biological processes, including embryonic development, cell proliferation, cell fate, cell migration, and tumor progression [6]. Wnt factors, interacting with the Frizzled receptors, modulate two distinct signaling pathways: the canonical Wnt/β-catenin pathway and the noncanonical β-catenin-independent pathways. The canonical Wnt/β-catenin pathway is characterized by translocation of β-catenin into nucleus and promoting expression of genes that regulate cell proliferation, such as c-myc, cyclin D1, and β-catenin itself [7]. The noncanonical β-catenin-independent pathways include the calcium/calmodulin-dependent kinase II (CaMKII)-mediated Wnt/Ca2+ pathway and the small GTPase RhoA- and Jun N-terminal kinase (JNK)-dependent planar cell polarity pathway [8, 9]. Emerging evidence has suggested that Wnt genes family is also involved in the regulation of β cell proliferation and insulin secretion [10–12] . For example, Wnt3a was reported to promote β cell proliferation by activating the canonical pathway [13, 14]. Moreover, Heller C et al. showed that Wnt4 can promote INS-1 cell proliferation [15], but they failed to explain the underlying mechanism. However, the role of other Wnt isoforms in regulating adult pancreatic β cell proliferation and its underlying mechanisms are still unclear.
Here, we show that Ex-4 promotes β-cell proliferation by regulating the expression of Wnt5a. Moreover, our data suggest that Wnt5a inhibits the noncanonical CaMKII pathway via interacting with receptors Frizzled-2 (Frz-2) and Ror-2.
Materials and methods
Cell culture and treatments
INS-1 cells were cultured in RPMI1640 medium containing 10 % heated-inactivated fetal bovine serum supplemented with 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 10 mM HEPES, 100 IU/ml penicillin and 100 IU/ml streptomycin. Ex-4 (Sigma Chemical, St Louis, MO) was dissolved in sterilized phosphate buffered saline to a concentration of 100 μM as stock solution. Recombinant Human/Mouse Wnt5a (R&D system, Wiesbaden-Nordenstadt, Germany) was dissolved with sterilized PBS to 100 μg/ml as a stock solution. KN-62 was dissolved with sterilized dimethyl sulfoxide (DMSO), and made up to 1 mM as a stock solution.
Animal experiments
Animal studies were approved by the Animal Laboratory center of Sun Yat-sen University. All animal studies were conducted with male C57Bl/6J mice, 6 weeks of age and weighing 12 ~ 13 g (Animal center of Guangdong province). C57Bl/6J mice were treated with Ex-4 in 100 μg. kg1 day−1 i.p (n = 6) or PBS (n = 5) for 14 days and 21 days.
Immunofluorescence histology and islet morphometry
Immunofluorescence and image analyses were performed on paraffin sections as described [16]. The primary antibodies used were mouse monoclonal Wnt-5a, rabbit polyclonal Insulin antibody, and goat anti-mouse Ki-67 (M-19) (Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies were mouse anti-rabbit IgG-FITC (Santa Cruz Biotechnology, Santa Cruz, CA), anti-mouse IgG-TRITC, and anti-goat IgG (whole molecule)-TRITC (Sigma-Aldrich, St Louis, MO). Fluorescence nuclear counterstaining was performed with DAPI (4′, 6′-diamidino-2-phenylindole). Fluorescent images were captured on NIKON ECLIPSE TI-SR inverted fluorescence microscope at an optical depth of 1 μm (magnification, 200× or 400×). Islet area and size were determined using Image-Pro Plus (Medica Cybernetics, Silver Spring, MD) imaging software. The software was calibrated to calculate area in μm2. Pancreata from all of the mice in experiments were examined. At least three 5-μm sections per mouse, at least 150 μm apart, were analyzed in their entirety. The number of positive cells per section were measured by counting expressing cells for each individual pancreas (all sections containing pancreatic tissue were examined).
Wnt signaling luciferase reporter assay (TOPflash)
The TOPFLASH plasmid is a luciferase reporter plasmid which contains two sets of three copies of the TCF-binding site upstream of the thymidine kinase minimal promoter [12]. The plasmid FOPFLASH contains mutated TCF binding-sites, serving as a negative control. Both plasmids are commercially available (Upstate, Cell signaling solutions, Charlottesville, VA, USA). The plasmid pRL-TK (Promega, Mannheim, Germany) served as an internal control for transfection efficiency. In all experiments, INS-1 cells were plated into 24-well dishes 24 h before transfection with FOPFLASH (1 μg/well) or TOPFLASH (1 μg/well) using LipofectamineTM 2000 (Invitrogen, Carlsbad, CA). Ex-4 or recombinant Wnt5a were added to the culture medium 12 h following transfection. The luciferase assay was performed as described previously [12].
Small interfering RNA (siRNA)-mediated knockdown of target gene expression
The sequences of siRNAs are as following: Wnt5a-RNAi#1: forward, 5′- CAAGGAAUUCGUGGACGCACGAGAAdTd-3′, and reverse, 5′-UUCUCGUGCGUCCACGAAUUCCUU GdTd-3′; Wnt5a-RNAi#2: forward, 5′-GCAUCCUCAUGA ACUUGCACAACAAdTd-3′, and reverse, 5′-UUGUUGU GCAAGUUCAUGAGGAUGCdTd-3′; Wnt5a-RNAi#3: forward, 5′-GGACAGUAUACAACCUGGCAGAUGUdTd-3′, and reverse, 5′-ACAUCUGCCAGGUUGUAUACUGUCCd Td-3′; Frz-2-RNAi#1: forward, 5′-UGCAUCAAUUCUACCC GCUGGUGAAdTd-3′, and reverse, 5′-UUCACCAGCGGGU AGAAUUGAUGCAdTd-3′; Frz-2-RNAi#2: forward, 5′-CGU CCUAUCUCAGCUAUAAGUUUCUdTd-3′, and reverse, 5′-AGAAACUUAUAGCUGAGAUAGGACGdTd-3′; Frz-2-RNAi#3: forward, 5′-CACCAUGGUGUCAGUGGCCUACA UUdTd-3′, and reverse, 5′-AAUGUAGGCCACUG ACACC AUGGUdTd-3′; Ror-2-RNAi#1: forward, 5′-CCAUUGACAC CUUGGGACAACUUGAdTd-3′, and reverse, 5′-UCAAGUU GUCCCAAGGUGUCAAUGGdTd-3′; Ror-2-RNAi#2: 5′-CCAUUACCGCCACUGGUGUUCU GUAdTd-3′, and reverse, 5′-UACAGAACACCAGUGGCGG UAAUGGdTd-3′; Ror-2-RNAi#3: 5′-CCAGCACAAACAGGCCAAACUU AAAdTd-3′, and reverse, 5′-UUUAAGUUUGGCCUGUU UGUGCUGGdTd-3′. Complexes of siRNA (specific siRNA targeted against three different areas of target gene mRNA, while nonsense siRNA served as a negative control) and LipofectamineTM 2000 (Invitrogen, Carlsbad, CA) were prepared according to the manufacturer’s instructions using 1 μg/well siRNA of a 24-well cell culture plate for Edu proliferation detection and using siRNA of the same concentration in 6-well or 6 cm culture plate for Western blot (WB). Samples were harvested and analyzed after an incubation period of 36 h or 48 h. All experiments were carried out in triplicate.
5-ethynyl-2’-deoxyuridine (EdU) incorporation assay
The EdU assay (RiboBio Co., Guangzhou, China) was conducted to evaluate INS-1 cell proliferation. 2 ~ 4 × 104 cells were seeded in 24-well plates. Then they were exposed to 50 μM EdU for 2 h. Next, they were fixed in 4 % paraformaldehyde and permeabilized in 0.5 % Triton X-100. Images were taken using a fluorescent microscope at 488 nm excitation. For calculation of proliferation rate, EdU-positive cells were counted at least three random separate fields.
RNA extraction and real-time reverse transcription-polymerase chain reaction
INS-1 cells were treated with or without 50 nM Ex-4 for 12 h and 24 h. Total RNA was extracted using the EastepTM Universal RNA Extraction Kit (Promega, Madison, WI) and transversely transcribed with the GoScriptTM Reverse Transcription System (Promega, Madison, WI) according to the manufacturer’s instruction. Real-time PCR was performed in triplicate with GoTaq® qPCR Master Mix (Promega, Madison, WI) and run on the ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Framingham, MA). Relative quantification of mRNA expression level was performed using the 2−ΔΔCt method. The mRNA level of the housekeeping gene GAPDH was used as a control.
Western blotting
The cells were washed with PBS and lysed in cell lysis buffer with the addition of protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO). The proteins (40 μg) were separated by SDS-PAGE and transferred onto PVDF membranes. Then the members were blotted with the following primary antibodies: anti-Wnt5a, anti-Ror-2 (Cell Signal Technology, Beverly, Mass), anti-Wnt4, anti-p-CaMK II, anti-β-catenin, anti-cyclin D1, and anti-GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA) at 4 °C overnight. Blots were incubated with HRP-conjugated secondary antibody for 1 h. The proteins were visualized using the ECL Plus WB detection system (Pierce, Rockford, IL).
Statistical analysis
All values are reported as mean ± standard deviation (SD). Paired t-test and one-way analysis of variance were performed for statistical analysis, as appropriate, using the SPSS13.0 software (SPSS Inc., Chicago, IL). A value of P < 0.05 was considered statistically significant.
Results
Ex-4 promotes the proliferation of INS-1 cells and β cells of C57Bl/6J islet
In order to explore the role of Ex-4 in INS-1 cell proliferation, varying doses of Ex-4 were added to the culture medium for 24 h. Then cell proliferation rate was determined using the Edu incorporation assay. As shown in Fig. 1a,b, a significant raise of proliferation rate was first observed at the minimal concentration of 10 nM, with the largest increase at a concentration of 50 nM.
In vivo, C57Bl/6J mice were injected with Ex-4 in 100 μg kg1 day−1 i.p for 14 days or 21 days. Islet area expanded by ~1.7 and ~1.9-fold in Ex-4-treated mice of 14 and 21-day-group respectively (Fig. 2a–g), consistent with previous observations [17]. This increase is due to β-cell replication and not hypertrophy because β-cell size did not change (Fig. 2a–h). β-cells of active hyperplasia per islet increased by ~ 1.6 and ~ 1.9-fold after Ex-4 treatment in two groups (Fig. 2f). Consistent with previous studies, these data suggest that Ex-4 exerts a pro-proliferative effect in INS-1 cells and β cells of C57Bl/6J islet.
Ex-4 treatment results in increased β-cell proliferation and islet size in vivo. Representative fluorescence photomicrographs of islet sections of C57Bl/6J mice treated with PBS (n = 5) /Ex-4(n = 6) for 14 days or 21 days (a–d) (400× magnification). a, b, c and d are partitioned as follows: top left, DAPI nuclear stain (blue); top right, fluorescein isothiocyanate (FITC) immunostain for insulin (green); bottom left, TRITC immuno-stain for Ki67 (red); and bottom right, a merged image of the three preceding panels with white arrows pointing out Ki67 positive beta cells. Compared with their controls (a, c), Ex-4-treated mice exhibited increased islet area in both groups e, β-cell replication as assessed by Ki67 in insulin-positive β-cells g, β-cells per islet f and unchanged calculated β-cell size h. Data are reported as mean ± SD in graphs. * P < 0 .05
Ex-4 suppresses the expression of Wnt5a in INS-1cells and C57Bl/6J islet
Earlier reports have shown that Ex-4 may promote the proliferation of pancreatic β cells by regulating the expression level of Wnt4. To further study whether other Wnt isoforms take part in accommodation of β-cell proliferation, the mRNA expression levels of all 19 Wnt gene family members in INS-1cells exposed to 50 nM Ex-4 for 12 h were analyzed by RT-PCR. As illustrated in Fig. 3a and Table. 1, Ex-4 altered the transcription of five Wnts (Wnt4, 5a, 7a, 9b, 16). Of those, Wnt-7a, 9b and16 were expressed only at very low levels in both control and treatment group, whereas Wnt4 and Wnt5a expressed at higher levels. In consideration of the fact that Wnt4 has been reported to be regulated by Ex-4, we chose Wnt5a as an important candidate for further investigation. We next detected the protein expression level of Wnt5a by WB in 50 nM Ex-4 time course experiment. As expected, Ex-4 significantly suppressed the expression of Wnt5a on the protein level (Fig. 3b). In INS-1 cell line, Ex-4 was observed to suppress the Wnt5a expression on both mRNA and protein levels.
Ex-4 inhibits the expression of Wnt5a. a qRT-PCR was performed to detect the mRNA expression levels of Wnt5a in INS-1 cells stimulated with 50 nM Ex-4 as indicated. GAPDH was used as a house keeping gene control. b WB analysis was conducted to detect the expression levels of Wnt5a in INS-1 cells stimulated with 50 nM Ex-4 as indicated. GAPDH was used as a loading control. For a, b, results derived from three independent experiments are expressed as mean ± SD. *P < 0.05
In human islets, Wnt5a was reported to be one of the most highly expressed Wnt factors [18]. In our study, we found that the expression level of Wnt5a in situ was rather high in C57Bl/6J mice islet (Fig. 4a, c). Surprisingly, both 14-day and 21-day Ex-4 treatment decreased the expression of Wnt5a in mice islet (Fig. 4a–d).
Ex-4 treatment suppresses the expression of Wnt5a in C57Bl/6J islet in vivo. Immunofluorescence staining of C57Bl/6J pancreas sections with DAPI (blue), insulin (green), Wnt5a (red) (200×magnification). C57Bl/6J mice were treated with PBS (n = 5) (a and c) or Ex-4 (n = 6) (b, d) for 14 days (a, b) and 21 days (c, d). In both 14 days and 21 days groups, two vehicle control groups present a similar high Wnt5a expression level (a, c), while Ex-4 treatment observably suppresses the expression of Wnt5a in islet (b, d)
Collectively, these results indicate that Ex-4 suppresses the expression of Wnt5a in both INS-1 cell line and C57Bl/6J islet.
Wnt5a mediates the stimulatory effect of Ex-4 on INS-1 cell proliferation
To investigate the function of Wnt5a in β cell proliferation, we knocked down Wnt5a expression in INS-1 cells using siRNAs (Fig. 5a). Initially, we assessed the effect of Wnt5a on replication of INS-1 cells by performing EdU incorporation assays. As shown in Fig. 5b, the proliferation rate of Wnt5a-silenced cells was significantly higher than that of cells transfected with nonsense siRNA. To further confirm the effect of Wnt5a on INS-1 cells proliferation, we next added various concentrations (0, 50, 100, 250, 500, 1000 ng/ml) of recombinant Wnt5a protein to the culture medium. The suppressive effect of recombinant Wnt5a protein on β-cell proliferation is dose-dependent (Fig. 5c). Moreover, to assess whether Wnt5a medicated the pro-proliferative role of Ex-4 in INS-1 cells, we added various dose of recombinant Wnt5a protein in the INS-1 cells co-stimulated with Ex-4. Figure 5d indicated that the pro-proliferative role of Ex-4 (50 nM) was significantly weakened by recombinant Wnt5a in a dose-dependent manner. Taken together, Wnt5a acts as a suppressor in proliferation of β-cell line INS-1, and Ex-4 promotes the replication of INS-1 cells partly via down-regulating the expression of Wnt5a.
Wnt5a mediates the stimulatory effect of Ex-4 on INS-1 cell Proliferation. a Protein expression of Wnt5a in indicated cells was analyzed by WB assay. GAPDH was used as a loading control. b EdU incorporation assay was conducted to investigate the effect of silencing Wnt5a on the proliferation of INS-1 cells. For a and b, NC, nonsense siRNA served as negative control. Nonsense siRNA or siRNA-Wnt5a of three different sequences numbered 1, 2, 3 were transfected into INS-1 cells for 36 h. c, d Recombinant Wnt5a protein decreased the proliferation of INS-1 cells in a dose-dependent manner. INS-1 cells were co-cultured with recombinant Wnt5a protein in the presence or absence of 50 nM Ex-4 for 24 h. Representative images and relative quantification of EdU incorporation assays are both shown in (c, d). All data are reported as mean ± SD of three independent experiments. * P < 0.05
Wnt5a inhibits β-catenin/TCF/LEF pathway and down-regulates the expression of cyclin D1 via activation of CaMKII signaling
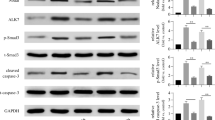
Wnt5a is well-known as the activator of noncanonical Wnt pathway. To investigate the underlying signaling mechanism of how Wnt5a inhibits the proliferation of INS-1 cells, we first focused on the CaMKII pathway. We examined the expression level of p-CaMKII at Thr286 by WB. As shown in Fig. 6a, CaMKII phosphorylation was observed to increase at as early as 0.5 h after 200 ng/ml recombinant Wnt5a stimulation and reached a peak at 1 h. Furthermore, we explored whether Wnt5a regulates the level of stable β-catenin in whole cell. The whole-cell stable level of β-catenin dramatically decreased after treatment with recombinant Wnt5a (Fig. 6a). Cyclin D1 is a well-known pro-proliferation target gene of Wnt signaling. In light of our finding that Wnt5a activated Wnt signaling in INS-1 cells, we determined whether Wnt5a modulated the expression of cyclin D1. By WB, we found that the protein levels of cyclin D1 were significantly reduced after Wnt5a recombinant protein stimulation (Fig. 6a).
Wnt5a inhibits the activation of β-catenin-dependent Wnt signaling pathway and down-regulates the expression of cyclin D1 via activation of CaMK II signaling. a WB analysis was performed to detect the protein level of p-CaMKII, β-catenin and cyclin D1 in INS-1 cells stimulated by 200 ng/ml recombinant Wnt5a for indicated time. GAPDH was used as a loading control. b The effect of Wnt5a on inhibiting β-catenin and cyclin D1 expression could be blocked by KN-62. INS-1 cells were treated with 10 μM KN-62 or solvent (DMSO and PBS) for 24 h. 200 ng/ml Wnt5a recombinant protein was added to medium 2 h before harvest. c Wnt5a antagonizes the activation of TCF-dependent Wnt signaling pathway mediated by Ex-4. d Wnt5a inhibits TCF-dependent Wnt signaling pathway in INS-1 cells in a time dependent manner. For c, d, INS-1 cells were transiently transfected with the TOPFLASH plasmid for 12 h, then INS-1 cells were stimulated with recombinant Wnt5a protein in the presence or absence of Ex-4 as indicated. TCF reporter gene activity is depicted relative to vehicle-treated controls. Values are means + SD of 3 independent experiments, each performed in triplicate. e Ex-4 down-regulated the activation of CaMK II in INS-1 cells. WB analysis was performed to detect the protein level of p-CaMKII in INS-1 cells stimulated by 50 nM Ex-4 for indicated time. GAPDH was used as a loading control. For a, b and e, Values are means + SD of three independent experiments
To further examine whether CaMKII activation is necessary for Wnt5a-induced β-catenin degradation and cyclin D1 down-regulation, we used KN-62 to inhibit the activity of CaMKII. The level of whole cell β-catenin and cyclin D1 expression in KN-62-treated group was higher than that in control group (Fig. 6b).
To confirm the effect of CaMKII activation on β-catenin nuclear translocation in INS-1 cells, we transiently transfected INS-1 cells with the TOPFLASH plasmid. Recombinant Wnt5a protein decreased the TOPFLASH transcription in INS-1 cells significantly in a dose-dependent manner. Consistent with previous study [19], 50 nM Ex-4 led to a strong activation of canonical Wnt signaling ( to 3.32 ± 0.29 fold ). Recombinant Wnt5a could inhibit this effect significantly in a dose-dependent manner (Fig. 6c). In time course experiment, 200 ng/ml recombinant Wnt5a gradually decreased the TOPFLASH transcription in INS-1 cells (Fig. 6d).
So far, we found that Ex-4 down-regulated the expression of Wnt5a and explored the subcellular mechanism of Wnt5a modulating the proliferation of INS-1 cells. It is necessary to test the effect of Ex-4 on downstream signaling of Wnt5a. Previous investigation has indicated that Ex-4 could enhance phosphorylation of β-catenin on the stabilizing PKA site, Ser-675 [19]. Cyclin D1 and c-Myc, were observed up-regulated by Ex-4 in the same investigation. Here, INS-1 cells were stimulated by 50 nM Ex-4 in time course (0, 1, 3, 6, 12, 24 h, Fig. 6e), and the phosphorylation of CaMKII was significantly suppressed by Ex-4.
Therefore, we consider that Wnt5a inhibits the β-catenin-dependent Wnt signaling and down-regulates the expression of cyclin D1 through activation of CaMKII pathway. Ex-4 modulates the canonical Wnt pathway and replication of INS-1 cells at least partially via down-regulating the expression of Wnt5a, which was illustrated by a summary cartoon at Fig. 8.
Both receptor Frz-2 and Ror-2 involve in mediating Wnt5a to accommodate the proliferation of β cells. a Knockdown the expression of Frz-2 in INS-1 cells was confirmed by WB assays. GAPDH was used as a loading control. b Depletion of Ror-2 in INS-1 cells. GAPDH was used as a loading control. c, d The siRNAs of Frz-2 or Ror-2 was transfected alone (c) or co-transfected (d) into the INS-1 cells in vitro. The cell proliferation was detected with EdU staining after transfection for 36 h. e, f INS-1 cells were transfected with siRNAs of Frz-2 (e) or Ror-2 (f) for 36 h. Then the cells were treated with 500 ng/ml recombinant Wnt5a for 12 h. The cell proliferation rate was determined by EdU assay. NC, nonsense siRNA served as negative control. siRNAs targeted on specific gene of three different sequences were numbered 1, 2, 3. All data are reported as mean ± SD of 3 independent experiments. * P < 0.05
Both receptor Frz-2 and Ror-2 involve in mediating Wnt5a to accommodate the proliferation of β cells
Given that the Frz-2 and Ror-2 play key roles in Wnt5a signaling, we assessed their role on Wnt5a-stimulated activation of CaMKII in INS-1 cells. To confirm the role of these two receptors on INS-1 cells proliferation, valid siRNAs targeting on Frz-2/Ror-2 were transfected into INS-1 cells respectively (Fig. 7a,b). Data shown in Figs. 7c,d demonstrate that silencing Frz-2 or Ror-2 alone could not cause significant alteration of cell proliferation rate, while simultaneous knockdown of two receptors led to a significant increase of proliferation rate in comparison with negative control (P < 0.05). To further explore the effect of Frz-2 or Ror-2 on proliferation under exogenous Wnt5a stimulation, we transfected the siRNA of Frz-2 or Ror-2 to INS-1 cells respectively and 36 h later, 500 ng/ml recombinant Wnt5a was added to the medium for another 12 h culture. As shown in Fig. 7e,f, the knockdown of Frz-2 or Ror-2 alone attenuated the suppressive effect of exogenous Wnt5a on INS-1 cells proliferation (P < 0.05). Moreover, Frz-2 showed more compelling effect on reduction of replication with exogenous Wnt5a stimulation. These data suggest that both Frz-2 and Ror-2 take part in mediating Wnt5a to suppress replication of INS-1 cells, and these two receptors could compensate for each other on the condition of Wnt5a expressed at basal level. However, when this compensation between two receptors is broken by increase of ligand quantity, exogenous Wnt5a stimulation reveals that Frz-2 plays more important role in regulating β-cell proliferation than Ror-2.
Discussion
This study demonstrated that Ex-4 promoted β-cell proliferation through down-regulating the expression of Wnt5a in both INS-1 cell line and mouse islet. Moreover, Wnt5a was found to activate the CaMKII pathway to decrease whole cell stable β-catenin and its nuclear translocation, suppressing the expression of cyclin D1 and proliferation of INS-1 cells. To note, Wnt5a is firstly reported as a novel negative regulator on β-cell proliferation.
A large number of previous studies have shown that Ex-4 increases β cell proliferation and mass in vitro and in vivo [20–23]. In line with the past study, we also found that Ex-4 promoted β cell proliferation. However, the underlying molecular mechanisms are not completely understood.
To further address the molecular basis involved in Ex-4-mediated promotion of β cell proliferation, we focused on the Wnt family, since recent studies have demonstrated that some of Wnt isoforms took part in regulating β cell proliferation. For example, Wnt3a was previously reported as a representative ligand to activate canonical Wnt/β-catenin pathway and then facilitate the mitosis of β cells [10]. Moreover, Heller C et al. reported that Wnt4 is also a positive regulator in pancreatic β cell replication [15]. To figure out the specific Wnt isoform that involved in the proliferation of β cell, we analyzed the mRNA expression pattern of all 19 members of Wnt genes family in β-cell line INS-1 treated with Ex-4. For the first time, we found that Wnt5a was significantly down-regulated by Ex-4, which was verified in mouse islet. Meanwhile, Wnt5a was proved to mediate the proliferative effect of Ex-4 on INS-1 cells.
However, for the mechanism of Ex-4 regulating the expression of Wnt5a, there are no clues evidenced in primary β cells or β cell line. Previous investigations provided pieces of information in other tissue and cells: Wnt5a was identified as a transcriptional target of TGF-β in airway smooth muscle cells [24]. Another target of TGF-β, transcription factor CUTL1, enhanced cell migration and invasion in breast cancer patients [25], and Wnt5a was subsequently shown to be one of the transcriptional targets of CUTL1 [26]. Cheol Whee Park et al. treated db/db mice with 1 nmol/kg Ex-4 and observed significantly decreased expression of TGF-β1 in kidney [27]. Meanwhile, Ex-4 was reported to inhibit the expression of TGF-β1 in human mesangial cells [28]. The mechanism Ex-4 regulating expression of Wnt5a in β-cells is currently not understood and will be worth studying in the future.
Here, we focused on exploring the subcellular mechanisms through which Wnt5a regulates proliferation in INS-1 cells. Previous studies indicated that Wnt5a bound to its receptors and triggered the downstream noncanonical signaling cascades, including CaMKII [29]. CaMKII is a multifunctional serine (Ser)/threonine (Thr) kinase. CaMKII is activated when phosphorylation at T286 occurs [30]. CaMKII plays an integral role in the regulation of learning, memory, cytoskeletal organization, ion channel activity, cell growth, and cancer progression [31–35]. However, little is known about the function of CaMKII pathway in β cells. Here, we hypothesized that Wnt5a affected the proliferation of INS-1 cells via activation of the CaMKII signaling pathway. As predicted, we found that exogenous Wnt5a activated the CaMKII pathway. Moreover, KN-62, a specific CaMKII inhibitor, blocked the activity of Wnt5a in phosphorylation or activation of CaMKII. Also, Wnt5a has been frequently shown to inhibit the β-catenin pathway [36], and cyclin D1 is a well-known target of β-catenin pathway [37]. So we wondered whether Wnt5a influenced the stable β-catenin level or its nuclear translocation and expression of cyclin D1. Our results indicated that Wnt5a significantly decreased the expression of cyclin D1 in INS-1 cells, via inhibiting β-catenin/TCF/LEF pathway. Importantly, Wnt5a was observed to inhibit the canonical Wnt signaling pathways by activating the noncanonical Wnt signaling pathways. As expected, our data demonstrate that CaMKII pathway is involved in the anti-proliferative role of Wnt5a in INS-1 cells. Besides, CaMKII-mediated non-canonical Wnt pathway is known to phosphorylate Lef via Tak1/NLK signaling to block TCF/LEF transcription factor [38]. Whether CaMKII could regulate cyclin D1 expression via Tak1/NLK/TCF/LEF pathway apart from inhibiting β-catenin nuclear translocation in INS-1 cells remains unclear, and further investigations are required. In several earlier studies, Wnt5a has been reported to show distinct impact on cell mitosis in multiple cell types [39–43], which has been proposed that diversity of receptors leads to accommodation of various intracellular pathways. Hence, we are interested in figuring out the specific receptor which mediates Wnt5a to suppress proliferation of β cell line. It is noticed that receptor Frz-2, but not Frz-1, could mediate Wnt ligand to activate Ca2+/ CaMKII pathways [9, 44]. Besides, Wnt5a has been shown to interact with Ror-2 to inhibit Wnt/β-catenin signaling [45–47]. Hence, we focused on investigating the role of Frz-2 and Ror-2 on Wnt5a signaling in β cells. Similar with the earlier studies, our data indicated that both of the receptors Frz-2 and Ror-2 mediated Wnt5a to suppress β cell line proliferation. Furthermore, both receptors are found to compensate for each other when Wnt5a is expressed at basal level, while Frz-2 showed more compelling effect on reduction of INS-1 cells replication with exogenous Wnt5a stimulation.
Together, our work demonstrates that Wnt5a is down-regulated by Ex-4 in both β-cell line and mouse islet, and Wnt5a inhibits proliferation through regulating CaMKII pathway in β-cell line INS-1. The current study provides a probable new insight of the deep mechanisms of Ex-4 and Wnt5a in counterbalance of β-cell mass.
References
M. Cnop, N. Welsh, J.C. Jonas, A. Jorns, S. Lenzen, D.L. Eizirik, Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54(Suppl 2), S97–S107 (2005)
A. Vetere, A. Choudhary, S.M. Burns, B.K. Wagner, Targeting the pancreatic beta-cell to treat diabetes. Nat. Rev. Drug. Discov. 13, 278–289 (2014)
D. Mathis, L. Vence, C. Benoist, Beta-Cell death during progression to diabetes. Nature 414, 792–798 (2001)
D.J. Drucker, J.B. Buse, K. Taylor, D.M. Kendall, M. Trautmann, D. Zhuang, L. Porter; Group D-S, Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 372, 1240–1250 (2008)
W. Kim, J.M. Egan, The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol. Rev. 60, 470–512 (2008)
M.D. Gordon, R. Nusse, Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem. 281, 22429–22433 (2006)
O.R. Bandapalli, S. Dihlmann, R. Helwa, S. Macher-Goeppinger, J. Weitz, P. Schirmacher, K. Brand, Transcriptional activation of the beta-catenin gene at the invasion front of colorectal liver metastases. J. Pathol. 218, 370–379 (2009)
K. Wunnenberg-Stapleton, I.L. Blitz, C. Hashimoto, K.W. Cho, Involvement of the small GTPases XRhoA and XRnd1 in cell adhesion and head formation in early Xenopus development. Development 126, 5339–5351 (1999)
M. Kuhl, L.C. Sheldahl, C.C. Malbon, R.T. Moon, Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J. Biol. Chem. 275, 12701–12711 (2000)
I.C. Rulifson, S.K. Karnik, P.W. Heiser, D. ten Berge, H. Chen, X. Gu, M.M. Taketo, R. Nusse, M. Hebrok, S.K. Kim, Wnt signaling regulates pancreatic beta cell proliferation. Proc. Natl. Acad. Sci. U S A 104, 6247–6252 (2007)
T. Fujino, H. Asaba, M.J. Kang, Y. Ikeda, H. Sone, S. Takada, D.H. Kim, R.X. Ioka, M. Ono, H. Tomoyori, M. Okubo, T. Murase, A. Kamataki, J. Yamamoto, K. Magoori, S. Takahashi et al., Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc. Natl. Acad. Sci. U S A 100, 229–234 (2003)
S. Schinner, F. Ulgen, C. Papewalis, M. Schott, A. Woelk, A. Vidal-Puig, W.A. Scherbaum, Regulation of insulin secretion, glucokinase gene transcription and beta cell proliferation by adipocyte-derived Wnt signalling molecules. Diabetologia 51, 147–154 (2008)
C. Wilson, Diabetes: human beta-cell proliferation by promoting Wnt signalling. Nat Rev Endocrinol 9, 502 (2013)
M.L. Johnson, N. Rajamannan, Diseases of Wnt signaling. Rev. Endocr. Metab. Disord. 7, 41–49 (2006)
C. Heller, M.C. Kuhn, B. Mulders-Opgenoorth, M. Schott, H.S. Willenberg, W.A. Scherbaum, S. Schinner, Exendin-4 upregulates the expression of Wnt-4, a novel regulator of pancreatic beta-cell proliferation. Am. J. Physiol. Endocrinol. Metab. 301, E864–E872 (2011)
T.A. Matsuoka, L. Zhao, I. Artner, H.W. Jarrett, D. Friedman, A. Means, R. Stein, Members of the large Maf transcription family regulate insulin gene transcription in islet beta cells. Mol. Cell. Biol. 23, 6049–6062 (2003)
D.A. Stoffers, T.J. Kieffer, M.A. Hussain, D.J. Drucker, S. Bonner-Weir, J.F. Habener, J.M. Egan, Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes 49, 741–748 (2000)
R.S. Heller, T. Klein, Z. Ling, H. Heimberg, M. Katoh, O.D. Madsen, P. Serup, Expression of Wnt, Frizzled, sFRP, and DKK genes in adult human pancreas. Gene Expr. 11, 141–147 (2003)
Z. Liu, J.F. Habener, Glucagon-like peptide-1 activation of TCF7L2-dependent Wnt signaling enhances pancreatic beta cell proliferation. J. Biol. Chem. 283, 8723–8735 (2008)
W.J. Song, W.E. Schreiber, E. Zhong, F.F. Liu, B.D. Kornfeld, F.E. Wondisford, M.A. Hussain, Exendin-4 stimulation of cyclin A2 in beta-cell proliferation. Diabetes 57, 2371–2381 (2008)
L. Tian, J. Gao, G. Weng, H. Yi, B. Tian, T.D. O’Brien, Z. Guo, Comparison of exendin-4 on beta-cell replication in mouse and human islet grafts. Transpl. Int. 24, 856–864 (2011)
M. Arakawa, C. Ebato, T. Mita, T. Hirose, R. Kawamori, Y. Fujitani, H. Watada, Effects of exendin-4 on glucose tolerance, insulin secretion, and beta-cell proliferation depend on treatment dose, treatment duration and meal contents. Biochem. Biophys. Res. Commun. 390, 809–814 (2009)
J. Xie, N.M. El Sayed, C. Qi, X. Zhao, C.E. Moore, T.P. Herbert, Exendin-4 stimulates islet cell replication via the IGF1 receptor activation of mTORC1/S6K1. J. Mol. Endocrinol. 53, 105–115 (2014)
K. Kumawat, M.H. Menzen, R.M. Slegtenhorst, A.J. Halayko, M. Schmidt, R. Gosens, TGF-beta-activated kinase 1 (TAK1) signaling regulates TGF-beta-induced WNT-5A expression in airway smooth muscle cells via Sp1 and beta-catenin. PLoS ONE 9, e94801 (2014)
P. Michl, A.R. Ramjaun, O.E. Pardo, P.H. Warne, M. Wagner, R. Poulsom, C. D’Arrigo, K. Ryder, A. Menke, T. Gress, J. Downward, CUTL1 is a target of TGF(beta) signaling that enhances cancer cell motility and invasiveness. Cancer Cell 7, 521–532 (2005)
S. Ripka, A. Konig, M. Buchholz, M. Wagner, B. Sipos, G. Kloppel, J. Downward, T. Gress, P. Michl, WNT5A--target of CUTL1 and potent modulator of tumor cell migration and invasion in pancreatic cancer. Carcinogenesis 28, 1178–1187 (2007)
C.W. Park, H.W. Kim, S.H. Ko, J.H. Lim, G.R. Ryu, H.W. Chung, S.W. Han, S.J. Shin, B.K. Bang, M.D. Breyer, Y.S. Chang, Long-term treatment of glucagon-like peptide-1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J. Am. Soc. Nephrol. 18, 1227–1238 (2007)
W. Li, M. Cui, Y. Wei, X. Kong, L. Tang, D. Xu, Inhibition of the expression of TGF-beta1 and CTGF in human mesangial cells by exendin-4, a glucagon-like peptide-1 receptor agonist. Cell. Physiol. Biochem. 30, 749–757 (2012)
A. Shrivastava, C. Radziejewski, E. Campbell, L. Kovac, M. McGlynn, T.E. Ryan, S. Davis, M.P. Goldfarb, D.J. Glass, G. Lemke, G.D. Yancopoulos, An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol. Cell 1, 25–34 (1997)
R.J. Colbran, A.M. Brown, Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr. Opin. Neurobiol. 14, 318–327 (2004)
J.E. Lisman, A.M. Zhabotinsky, A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron 31, 191–201 (2001)
K.U. Bayer, H. Schulman, Regulation of signal transduction by protein targeting: the case for CaMKII. Biochem. Biophys. Res. Commun. 289, 917–923 (2001)
J. Lisman, H. Schulman, H. Cline, The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 3, 175–190 (2002)
C.C. Fink, T. Meyer, Molecular mechanisms of CaMKII activation in neuronal plasticity. Curr. Opin. Neurobiol. 12, 293–299 (2002)
Y.Y. Wang, R. Zhao, H. Zhe, The emerging role of CaMKII in cancer. Oncotarget 6, 11725–11734 (2015)
M.A. Torres, J.A. Yang-Snyder, S.M. Purcell, A.A. DeMarais, L.L. McGrew, R.T. Moon, Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J. Cell. Biol. 133, 1123–1137 (1996)
A. Koehler, J. Schlupf, M. Schneider, B. Kraft, C. Winter, J. Kashef, Loss of Xenopus cadherin-11 leads to increased Wnt/beta-catenin signaling and up-regulation of target genes c-myc and cyclin D1 in neural crest. Dev. Biol. 383, 132–145 (2013)
T. Ishitani, S. Kishida, J. Hyodo-Miura, N. Ueno, J. Yasuda, M. Waterman, H. Shibuya, R.T. Moon, J. Ninomiya-Tsuji, K. Matsumoto, The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol. Cell. Biol. 23, 131–139 (2003)
T.W. Austin, G.P. Solar, F.C. Ziegler, L. Liem, W. Matthews, A role for the Wnt gene family in hematopoiesis: expansion of multilineage progenitor cells. Blood 89, 3624–3635 (1997)
R.V. Iozzo, I. Eichstetter, K.G. Danielson, Aberrant expression of the growth factor Wnt-5A in human malignancy. Cancer. Res. 55, 3495–3499 (1995)
S. Lejeune, E.L. Huguet, A. Hamby, R. Poulsom, A.L. Harris, Wnt5a cloning, expression, and up-regulation in human primary breast cancers. Clin. Cancer. Res. 1, 215–222 (1995)
T. Saitoh, M. Katoh, Molecular cloning and characterization of human WNT5B on chromosome 12p13.3 region. Int. J. Oncol. 19, 347–351 (2001)
D.J. Van Den Berg, A.K. Sharma, E. Bruno, R. Hoffman, Role of members of the Wnt gene family in human hematopoiesis. Blood 92, 3189–3202 (1998)
D.C. Slusarski, J. Yang-Snyder, W.B. Busa, R.T. Moon, Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev. Biol. 182, 114–120 (1997)
A. Schambony, D. Wedlich, Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev. Cell 12, 779–792 (2007)
T. Ishitani, J. Ninomiya-Tsuji, K. Matsumoto, Regulation of lymphoid enhancer factor 1/T-cell factor by mitogen-activated protein kinase-related Nemo-like kinase-dependent phosphorylation in Wnt/beta-catenin signaling. Mol. Cell Biol. 23, 1379–1389 (2003)
A.J. Mikels, R. Nusse, Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol 4, e115 (2006)
Funding
This research was supported by grants from the Industrial Technology Research and Development funding projects, Guangdong Province (No. 2012A030400006); Guangzhou Municipal Science and Technology special fund (No. 1346000270); Medical and Health Major projects, Zhongshan (No.2016B1001); Doctoral Fund of Ministry of Education, China (No. 20130171110067); Sun Yat-sen University Clinical Research 5010 Program; Special Fund for Public Service of Ministry of Health, China (No. 201502007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Xinger Wu and Weiwei Liang contribute equally to this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wu, X., Liang, W., Guan, H. et al. Exendin-4 promotes pancreatic β-cell proliferation via inhibiting the expression of Wnt5a. Endocrine 55, 398–409 (2017). https://doi.org/10.1007/s12020-016-1160-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-1160-x