Abstract
While dopamine-agonists are the first-line approach in treating prolactinomas, surgery can be considered in selected cases besides non-responders or patients with dopamine-agonist intolerance. The aim of the present study was to compare the long-term outcome in women with prolactinomas treated primarily either surgically or medically who had not had prior dopamine-agonist treatment. Retrospective case-note study of all consecutive women with prolactinomas primarily managed with medical therapy or surgery in a tertiary referral centre. The clinical, biochemical, and radiological responses to first-line treatment at early and long-term follow-up were analysed. The primary therapeutic strategy was dopamine-agonists for 36 (34 %) and surgery for 71 (66 %) of the women. Baseline clinical and biochemical characteristics were not significantly different between the primary surgical and medical cohort. Median follow-up time was 90 months (range 13–408). Following primary treatment, prolactin level significantly decreased in both cohorts, on average to 13.5 µg/L (IQR 7–21; p < 0.001), and was within the normal range in 82 % of all patients. No women in the surgical cohort demonstrated permanent sequelae and morbidity was low. At final follow-up, control of hyperprolactinaemia required dopamine-agonist therapy in 64 % of women who had undergone primary medical therapy vs. 32 % of those who had primary surgical therapy (p = 0.003). Logistic regression revealed that the primary therapeutic strategy, but not adenoma size, was an independent risk factor for long-term dependence on dopamine-agonists. The present data indicate that in a dedicated tertiary referral centre, long-term control of hyperprolactinaemia in women with prolactinomas is high. In selected cases, a primary neurosurgical approach might at least be interdisciplinarily discussed with the primary goal of minimizing long-term dependence on dopamine-agonists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long-term studies comparing a primary medical with a primary surgical approach in women with prolactinomas are scarce, given that dopamine (DA)-agonists are considered the first-line therapeutic approach and have a well-documented high efficiency. Whereas transsphenoidal surgery is mainly recommended for non-responders, patients with DA-agonist intolerance, and those with cystic tumours, intratumoural haemorrhage, or progressive visual impairment while on medication therapy [1–3], surgery can be considered as an alternative to DA-agonists in selected cases, particularly patients with microprolactinomas where there is no evidence of cavernous sinus infiltration [4–8]. Nevertheless, with a few exceptions, patients are mostly treated with DA-agonists prior to surgery [9], even when a primary surgical approach might be considered.
In the present audit of practice in a dedicated tertiary referral centre, we compared our 10-year follow-up data on women with prolactinomas treated primarily either surgically or medically without prior DA-agonist treatment. We specifically aimed to investigate whether: (1) long-term control of hyperprolactinaemia is similar in both cohorts, (2) long-term dependence on DA-agonist therapy is different between the two cohorts, and (3) tumour size influences the long-term outcome in both groups.
Patients and methods
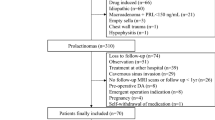
This was a single-centre retrospective study of prospectively collected data from 1997 to 2010 including all consecutive women managed with primary medical therapy or surgery. Patients who met the inclusion criteria had not been treated with DA-agonists prior to study entry and had documented long-term follow-up data (>12 months). All women fulfilled the diagnostic criteria of a prolactin (PRL)-secreting pituitary adenoma [i.e., PRL levels > 30 µg/L without evidence of pituitary stalk compression, primary hypothyroidism or drug-induced hyperprolactinaemia, and positive pituitary magnetic resonance imaging (MRI) scan].
The indication for first-line pituitary surgery was local complications of the adenomas such as haemorrhage with subsequent visual impairment or the patient’s preference to undergo surgery rather than long-term DA-agonist therapy. The decision to perform surgery was discussed for each patient individually at the pituitary tumour board, with interdisciplinary consensus-finding tailored to prevent patients from becoming dependent on long-term DA-agonist therapy and/or on pituitary hormone replacement therapy. Therefore, macroadenoma or cavernous sinus infiltration were not a priori exclusion criteria for surgery, but were primarily considered in cases of local complications such as adenoma bleeding. It is important to note that health insurance in Switzerland covers medical and/or surgical treatment of all persons residing in Switzerland and therefore does not influence decision-making in favour of either treatment option.
The observation period extended from the time of proven prolactinoma (i.e. baseline) to the last follow-up. A conventional 1.5-T pituitary MRI with proton density/T2-weighted whole-brain scan using a 5-mm slice thickness and both unenhanced and contrast-enhanced overlapping 3-mm scans in the sagittal and coronal planes over the sellar region was performed [10]. A tumour diameter of 1–10 mm was defined as a microadenoma and of >10 mm as a macroadenoma. Invasion of the cavernous sinus by the adenoma was recorded as described in the literature [11, 12].
Partial hypopituitarism was defined as impaired secretion of one or more pituitary hormones. Secondary adrenal insufficiency was noted in the presence of low cortisol levels in the serum or in cases of normal cortisol but inadequate responses to the adrenocorticotropin (ACTH) stimulation test or insulin tolerance test. The diagnosis of hypothyroidism was made in cases of normal-low thyreotropine (TSH) level and low free thyroxin (FT4) level. A gonadotropin deficiency or central hypogonadism was considered in the case of normal-low levels of gonadotropins in parallel with low estradiol levels.
Types of DA-agonists and maximal doses were noted (e.g., bromocriptine, quinagolide, cabergoline). Operations were performed by the senior neurosurgeon (RWS) using a transseptal, transsphenoidal microsurgical approach [4, 10, 13]. Body weight, fluid intake and output, serum electrolytes and osmolality, and urine osmolality were checked daily postoperatively. Antibiotics were administered immediately before surgery and were stopped 24 h afterwards. The duration of the operation was between 60 and 150 min, and patients were generally discharged on postoperative day 3 or 4. In the rare cases where radiation therapy was delivered, stereotactic radiosurgery (i.e., gamma knife or stereotactic linear accelerator) was applied.
Early follow-up took place 3 months after surgery or after initiating DA-agonists. In the medical cohort, the dose of DA-agonist was increased if PRL levels were still elevated (>30 µg/L). In the surgical cohort, remission was defined as PRL ≤ 30 µg/L at the 3-month follow-up evaluation. In patients whose PRL remained elevated at pathological levels, DA-agonist therapy was initiated.
A standardised protocol was used for the withdrawal of DA-agonists in all patients. In the medical cohort, DA-agonists were tapered 24 months after initiation of the medical therapy if PRL levels had normalised and tumour reduction of >50 % was attained at the time of radiological follow-up, as defined previously [14, 15]. In patients treated according to the surgical protocol, DA-agonists were tapered 12 months postoperatively if the PRL levels were normalised. Recurrence was defined as an increase in PRL levels above the normal range during the last follow-up period after a previous remission irrespective of radiological findings [8, 16, 17].
Statistics
Data were analysed using IBM SPSS statistical software Version 21.0 (IBM Corp., New York, NY, USA) and GraphPad Prism (V6.03 Software, San Diego, CA, USA). Continuous variables were examined for homogeneity of variance and are expressed as mean ± standard deviation (SD) unless otherwise noted. PRL levels are presented as median values with interquartile range. For comparisons of means between the two groups, Student’s t-test was used for normally distributed data, and the Mann–Whitney test for nonparametric data. The Wilcoxon signed-rank test was used to evaluate paired differences in PRL levels before and after treatment. Categorical variables were compared using Pearson’s χ 2 test or Fisher’s exact test, as appropriate. Odds ratios (ORs) and 95 % confidence intervals (CIs) of independent factors associated with persistent hyperprolactinaemia and dependence on DA-agonists at the last follow-up were analysed by univariate and multivariate logistic regression. The variables tested were: age at diagnosis, initial presentation (headache, amenorrhoea, galactorrhoea, hypopituitarism), patient’s body mass index (BMI; kg/m2) at diagnosis, latency to diagnosis, initial PRL levels, adenoma size and cavernous sinus invasion, as well as primary therapeutic strategy. The multivariable logistic regression analysis included all dependent risk factors in the univariable regression with a p-value ≤ 0.3. Differences were considered significant when p < 0.05 for reported two-sided p-values.
Results
Patients’ characteristics at baseline
Baseline characteristics of the patients in each of the primary treatment cohorts are summarised in Table 1. Patients in the surgical and medical cohorts were matched for age, chief complaints, pituitary axes affected, median prolactin levels, body mass index (BMI), and follow-up time. Neither the number of microprolactinomas or macroprolactinomas, nor the number of tumours infiltrating the cavernous sinus was significantly different between the medically and surgically treated cohorts. Mean age at diagnosis was 34 years (range 14–73 years). BMIs in patients with a macroadenoma or microadenoma were 28.2 ± 5.7 and 24.3 ± 5.4 kg/m2, respectively (p = 0.01). Median PRL levels in women in the medical cohort were 105 μg/L [interquartile range (IQR) 84–653] vs. 182 μg/L (IQR 89–251) in the surgical cohort (p = 0.6). Baseline median PRL levels in all patients were significantly higher in those with a macroprolactinoma than those with a microadenoma [303 µg/L (IQR, 203–1000) versus 105 µg/L (IQR, 70–160), respectively; p < 0.001]. For both cohorts, the most common symptom at the time of diagnosis was secondary amenorrhoea (77 %). Headache was reported by 24 % of women. Radiation therapy was applied in 2.9 % of patients who underwent primary medical therapy and 3 % of patients treated with primary surgical therapy.
Early follow-up
PRL levels decreased significantly in both groups: from 105 μg/L (IQR 84–653) to 20 μg/L (IQR 10–38) (p < 0.001) in the medical cohort, and from 182 μg/L (IQR 89–251) to 13 μg/L (IQR; 7–38) (p < 0.001) in the surgical cohort. Similarly, PRL levels decreased significantly in patients with a macroadenoma or microadenoma: from 303 µg/L (IQR 203–1000) to 15 µg/L (IQR 8–61) (p < 0.001) in patients with a macroadenoma, and from 105 µg/L (IQR 70–161) to 15 µg/L (IQR 7–36) (p < 0.001) in patients with a microadenoma.
Overall, there was no significant difference in the treatment outcome between the two groups at early follow-up. Hyperprolactinaemia was controlled in 61 % of all patients (56 % in the medical vs. 63 % in the surgical cohort; p = 0.53). There was no significant difference in the remission rate in patients with microprolactinomas (73 % in the medical vs. 67 % in the surgical cohort; p = 0.99) or with macroprolactinomas (48 % in the medical vs. 61 % in the surgical cohort; p = 0.32).
Long-term outcome
Median follow-up time was 90 months (range 13–408 months), with no difference between the two cohorts (p = 0.62). Serum PRL levels significantly decreased in both cohorts compared to baseline values: from 105 µg/L (IQR 84–653) to 14 µg/L (IQR 6–25) (p < 0.001) in the medical cohort, and from 182 µg/L (IQR 89–251) to 13 µg/L (IQR 8–20) (p < 0.001) in the surgical cohort. For neither cohort was there a significant decrease in serum PRL values from early to long-term follow-up: i.e., from 20 µg/L (IQR 10–38) to 14 µg/L (IQR 6–25); (p = 0.06) in the medical cohort, and from 13 µg/L (IQR 7–38) to 13 µg/L (IQR 8–20); (p = 0.16) in the surgical cohort. Hyperprolactinaemia was controlled in 78 % of patients in the medical cohort compared to 85 % of patients in the surgical cohort (p = 0.43). We noted hyperprolcatinaemia at last follow-up in 8 (22 %) women in the medical cohort. DA-agonists were administered in 5 (63 %) of them; namely, 2.5 mg bromocriptine in one patient, 37.5 µg quinagolid in three patients, and 0.5 mg cabergoline in one patient. In the three others, no DA-agonists were administered—although the cut-off was <30 µg/L—given that they were asymptomatic with concentration of PRL (median ± SD) of 33.2 ± 3.1 µg/L and normal gonadotropin concentrations. Hyperprolactinaemia was controlled in 79 % of patients with macroprolactinomas compared to 88 % of those with microprolactinomas (p = 0.30). Overall, there was no difference in the remission rate in patients with microprolactinomas (i.e., 82 % in the medical vs. 90 % in the surgical cohort; p = 0.59) or with macroprolactinomas (i.e., 76 % in the medical vs. 81 % in the surgical cohort; p = 0.76). All but one patient with invasive tumours who underwent surgery attained remission at long-term follow-up (67 %).
Control of hyperprolactinaemia required DA-agonist therapy in 32 % (bromocriptine 10 %; cabergoline 22 %) of patients who underwent primary surgical therapy vs. 64 % (bromocriptine 11 %; cabergoline 53 %) of patients who had primary medical therapy (p = 0.003; Fig. 1a). DA-agonist therapy was required by 36 % of patients with a microadenoma compared to 54 % of patients with a macroadenoma (p = 0.12; Fig. 1b). About half (52 %) of patients with a microadenoma in the medical cohort required DA-agonist therapy at the long-term follow-up compared to 27 % of patients in the surgical cohort (p = 0.06). In contrast, 91 % of patients with a macroadenoma in the medical cohort required DA-agonist therapy compared to 40 % in the surgical cohort (p = 0.01; Fig. 1c). In the medical cohort, 2 out of 36 patients (6 %) had surgery within 150 ± 36 months due to DA-agonist intolerance. Prolactinoma recurred in 34 % of patients in the medical cohort and in 25 % of patients in the surgical cohort (p = 0.35).
Long-term control of hyperprolactinaemia. a Control of hyperprolactinaemia was not statistically different between the two cohorts (p = 0.35), yet DA-agonists were required in 32 % of patients with primary surgical therapy compared to 64 % of patients with primary medical therapy (p = 0.003). b Normalisation of hyperprolactinaemia and long-term dependence on DA-agonists was similar in patients with a micro- or macroadenoma. c For control of hyperprolactinaemia in microadenomas, 52 % of patients in the medical group required DA-agonist therapy at long-term follow-up compared to 27 % in the surgical group (p = 0.06). For macroadenomas, 91 % of patients in the medical group required DA-agonists compared to 40 % in the surgical group (p = 0.01). **p < 0.01; *p < 0 .05; ns, not significant
Logistic regression revealed that the primary therapeutic strategy (i.e., surgical treatment; OR 0.2, 95 % CI 0.1–0.6; p = 0.004) was an independent risk factor for long-term dependence on DA-agonists, but not adenoma size (i.e., macroadenoma; OR 2.1, 95 % CI 0.7–6.5; p = 0.18) (Table 2). No independent predictors for persistent hyperprolactinaemia were noted (Table 3).
The main clinical symptoms improved in both groups; occurrence of amenorrhoea dropped from 77 % to 13 % (p < 0.001), galactorrhoea from 49 % to 6 % (p < 0.001), and headache from 24 % to 4 % (p < 0.001). The number of women who did not require any DA-agonists for the long-term control of hyperprolactinaemia (PRL < 30) was 13 (36 %) patients who had primary medical therapy vs. 48 (68 %) patients who had primary surgical therapy (p = 0.003).
Characteristics of patients’ BMI and the respective changes are depicted in Supplementary Table 1 and Table 2. At baseline, mean BMIs in patients with a macroadenoma and microadenoma were 28.2 ± 5.7 and 24.3 ± 5.4 kg/m2, respectively (p = 0.01). Mean BMI in patients with a macroadenoma was significantly higher at last follow-up than for patients with a microadenoma (28.0 ± 5.2 kg/m2 vs. 24.8 ± 5.5 kg/m2; p = 0.01). In patients with primary medical therapy, mean BMI had significantly decreased at the long-term follow-up in patients with macroadenomas (p = 0.04), but not in patients with microadenomas. Patients being treated with bromocriptine at last follow-up had significantly higher BMI values compared to those with bromocriptine at baseline (30.9 ± 7.2 kg/m2 vs. 24.7 ± 4.3 kg/m2, p = 0.03). Furthermore, there was a significant positive correlation between PRL levels and BMI at last follow-up (r = 0.30; p = 0.04), but not at baseline (r = 0.06, p = 0.65). Furthermore, patients with tumour recurrence at last follow-up presented with significantly higher BMI values than patients without a tumour recurrence (p = 0.01).
Daily doses of DA-agonists at baseline were as follows (mean ± SD): bromocriptine 7.5 ± 2.1 mg, quinagolid 75 ± 0 µg, and cabergoline 0.46 ± 0.11 mg. Daily doses at last follow-up were 5.2 ± 3.1 mg for bromocriptine, 61.5 ± 19.5 µg for quinagolid, and 0.45 ± 0.11 mg for cabergoline.
At baseline, hypogonadism was noted in 21 (84 %) patients in the medical and in 45 (86 %) patients in the surgical group (p = 0.71). There was no significant difference in the prevalence of hypoganodism at long-term follow-up between the two treatment groups; namely, 7 (39 %) in the medical and 11 (32 %) in the surgical cohort (p = 0.77). Accordingly, there was a significant decrease in the prevalence of hypogonadism following either treatment (medical, p = 0.004; surgical, p = 0.001). None of the women 7 (14 %) without central hypogonadism at baseline suffered from postoperative central hypogonadism after surgery. At last follow-up, partial hypopituitarism was noted in 24 % of all patients, with no significant differences between the two treatment groups (p = 0.34). Secondary hypothyroidism was noted in 7 (10 %) patients following surgical therapy, 5 of them without prior deficits. A secondary adrenal insufficiency was noted in 3 (8 %) patients in the medical cohort and in 2 (3 %) patients in the surgical cohort (p = 0.11).
Complications of therapeutic intervention
Neither cohort showed mortality or permanent morbidity. In the surgical cohort, transient diabetes insipidus (15 %), transient syndrome of inappropriate secretion of antidiuretic hormone (10 %) and rhinoliquorrhoea (3 %) were noted in the perioperative period, requiring transsphenoidal revision by autologous fat graft and dural patching in one patient. Transient side effects of DA-agonist therapy were present in 38 % of the patients, including prolonged nausea in 7 patients (19 %) with bromocriptine and 2 (6 %) patients with cabergoline, vertigo/orthostatic dysregulation in 3 patients (8 %) and Raynaud’s phenomenon in one patient receiving bromocriptine (3 %).
Discussion
The main findings of this study can be summarised as follows: (1) long-term control of hyperprolactinaemia was achieved in 82 % of patients and was independent of the primary treatment strategy, (2) patients who underwent primary surgical treatment needed significantly less DA-agonists than the primary medically treated patients at long-term follow-up, and (3) the primary therapeutic strategy, but not the adenoma size, was an independent predictor for long-term dependence on DA-agonist therapy.
After a mean follow-up period of 10 years, hyperprolactinaemia was controlled in the majority of patients (82 %) independent of the primary therapeutic strategy and independent of the adenoma size (i.e., microadenoma vs. macroadenoma). DA-agonists have mostly replaced surgery as the first-line therapy for both microprolactinomas and macroprolactinomas, although no prospective randomised trial comparing the two therapeutic strategies has been reported [18, 19]. Indications for DA-agonists also include the presence of macroprolactinomas that are symptomatic due to mass effect and compression of the optic chiasm [20, 21]. Surgical resection techniques continue to improve, and comparable long-term control of hyperprolactinaemia after surgical and medical therapy has been demonstrated, most notably in patients with microprolactinomas [4, 5, 8], but also independent of tumour size [16], corroborating our results. Furthermore, we noted no difference in the long-term recurrences of prolactinomas between the two primary treatment strategies. These results are consistent with data from studies of cohorts of patients with prolactinomas treated both medically and surgically [2, 5, 7, 8, 21–28]. During a mean follow-up period of 50 months, Losa et al. noted that after surgical treatment, patients with intrasellar macroadenoma had higher remission rates than those with cavernous sinus infiltration [16], and fewer patients experienced recurrence of hyperprolactinaemia. Since prolactinomas are slow-growing tumours, the longer mean follow-up period of 118 months in our cohort may well explain the divergent results.
While serum PRL levels were within the normal range in 82 % of patients, surgery averted the need for long-term DA-agonist therapy in around half of the cases, with primary treatment strategy being an independent predictor for long-term dependence on medical therapy. Nevertheless, 32 % of patients in the surgical group were also still dependent on DA therapy at the last follow-up. Interestingly, dependence on DA-agonist therapy at long-term follow-up was not statistically different between the surgical and the medical cohorts for patients with microadenomas, but was significantly different between the cohorts for patients with macroadenomas. Pretreatment of prolactinomas with DA-agonists, particularly with bromocriptine, has been shown to result in perivascular tumour fibrosis, possibly compromising complete surgical resection and lowering the probability of biochemical remission compared to non-fibrous tumours [29]. Therefore, as in the present study, no prior DA-agonist treatment was initiated before surgery, possibly contributing to the higher chance of averting the need for medical therapy in the surgical group at long-term follow-up. Long-term adverse effects of DA-agonists have been described when administered over a prolonged period. [30–34] Therefore, in selected patients with adenomas not infiltrating the cavernous sinus, a primary neurosurgical approach might at least be interdisciplinarily discussed with the goal of minimizing long-term dependence on DA-agonists.
We noted a general but non-significant increase in patients’ BMI over the study period after both treatment modalities. Most likely, this is related to the normal age-associated increase in body weight. However, patients presenting with a macroadenoma had significantly higher BMI at long-term follow-up than patients with a microadenoma. This is an intriguing finding with an unclear underlying mechanism [24]. It may be related to the fact that the macroadenoma patients have longer exposure to increased PRL levels, given that BMIs in patients with macroprolactinomas were noted to be significantly higher than in patients with inactive adenomas [35]. We further noted that patients with tumour recurrence at last follow-up presented with significantly higher BMI values than patients without tumour recurrence, corroborating the observation that hyperprolactinaemia may influence patients’ BMI. Although the mechanism is not fully understood and there are conflicting data in the literature [36], a relationship between the presence of a PRL-secreting pituitary tumour and weight gain in patients has previously been described [37, 38]. Accordingly, we noted a significant correlation between PRL levels and mean BMI at long-term follow-up. In addition, Ciresi et al. recently showed that the effect of cabergoline might be dose dependent; patients receiving higher doses (>0.50 mg/week) showed significantly lower BMI levels than those on a lower dose [39]. In women with a persisting need for DA-agonists, at long-term follow-up a decrease in the BMI was noted only in those treated with quinagolid and cabergoline, but not in women treated with bromocriptine. Pala et al. noted a significant decrease of BMI at both 3 and 6 months (p < 0.001) following cabergoline in patients with prolactinomas. [7] However, the short follow-up might interfere with patients’ normal age-associated increase in body weight as noted above.
Conclusion
The present data indicate that in a dedicated tertiary referral centre, long-term control of hyperprolactinaemia in women with prolactinomas is high. In selected cases, a primary neurosurgical approach might at least be interdisciplinarily discussed with the primary goal of minimizing long-term dependence on DA-agonists.
Study limitations
This is an audit of practice at a tertiary referral centre that suffers from the limitations of any retrospective study. Patients were not randomised, and therefore a selection bias for either therapy might have minimised the long-term dependence on DA-agonists in the surgical treatment arm, given that more patients with macroadenomas infiltrating the cavernous sinus were treated in the medical cohort, although their allocation to either group was not statistically significant. Furthermore, not all patients were systematically screened for growth hormone deficiency using validated dynamic testing. A shorter follow-up (<24 months) might interfere with the long-term dependency on DA-agonists, as our treatment regime consisted of tapering medications 24 months after initiation of the medical therapy if PRL levels had normalized and tumour reduction of >50 % was attained.
References
M.C. Oh, M.K. Aghi, Dopamine agonist-resistant prolactinomas. J. Neurosurg. 114, 1369–1379 (2011)
M. Ono, N. Miki, T. Kawamata et al., Prospective study of high-dose cabergoline treatment of prolactinomas in 150 patients. J. Clin. Endocrinol. Metab. 93, 4721–4727 (2008)
A.I. Green, M. Sherlock, P.M. Stewart, N.J. Gittoes, A.A. Toogood, Extensive experience in the management of macroprolactinomas. Clin. Endocrinol. 81, 85–92 (2014)
M. Babey, R. Sahli, I. Vajtai, R.H. Andres, R.W. Seiler, Pituitary surgery for small prolactinomas as an alternative to treatment with dopamine agonists. Pituitary 14, 222–230 (2011)
J. Kreutzer, R. Buslei, H. Wallaschofski et al., Operative treatment of prolactinomas: indications and results in a current consecutive series of 212 patients. Eur. J. Endocrinol. 158, 11–18 (2008)
M. Loyo-Varela, T. Herrada-Pineda, F. Revilla-Pacheco, S. Manrique-Guzman, Pituitary tumor surgery: review of 3004 cases. World Neurosurg. 79, 331–336 (2013)
V. Primeau, C. Raftopoulos, D. Maiter, Outcomes of transsphenoidal surgery in prolactinomas: improvement of hormonal control in dopamine agonist-resistant patients. Eur. J. Endocrinol. 166, 779–786 (2012)
X. Qu, M. Wang, G. Wang et al., Surgical outcomes and prognostic factors of transsphenoidal surgery for prolactinoma in men: a single-center experience with 87 consecutive cases. Eur. J. Endocrinol. 164, 499–504 (2011)
T.R. Smith, M.M. Hulou, K.T. Huang et al., Current indications for the surgical treatment of prolactinomas. J. Clin. Neurosci. 22, 1785–1791 (2015)
L. Andereggen, G. Schroth, J. Gralla et al., Selective inferior petrosal sinus sampling without venous outflow diversion in the detection of a pituitary adenoma in Cushing’s syndrome. Neuroradiology 54, 495–503 (2012)
J.P. Cottier, C. Destrieux, L. Brunereau et al., Cavernous sinus invasion by pituitary adenoma: MR imaging. Radiology 215, 463–469 (2000)
Z.B. Wu, Z.P. Su, J.S. Wu, W.M. Zheng, Q.C. Zhuge, M. Zhong, Five years follow-up of invasive prolactinomas with special reference to the control of cavernous sinus invasion. Pituitary 11, 63–70 (2008)
R.W. Seiler, L. Mariani, Sellar reconstruction with resorbable vicryl patches, gelatin foam, and fibrin glue in transsphenoidal surgery: a 10-year experience with 376 patients. J. Neurosurg. 93, 762–765 (2000)
J.A. Wass, When to discontinue treatment of prolactinoma? Nat. Clin. Pract. Endocrinol. Metab. 2, 298–299 (2006)
A. Colao, A. Di Sarno, P. Cappabianca, C. Di Somma, R. Pivonello, G. Lombardi, Withdrawal of long-term cabergoline therapy for tumoral and nontumoral hyperprolactinemia. N. Engl. J. Med. 349, 2023–2033 (2003)
M. Losa, P. Mortini, R. Barzaghi, L. Gioia, M. Giovanelli, Surgical treatment of prolactin-secreting pituitary adenomas: early results and long-term outcome. J. Clin. Endocrinol. Metab. 87, 3180–3186 (2002)
G. Raverot, A. Wierinckx, E. Dantony et al., Prognostic factors in prolactin pituitary tumors: clinical, histological, and molecular data from a series of 94 patients with a long postoperative follow-up. J. Clin. Endocrinol. Metab. 95, 1708–1716 (2010)
Bloomgarden E., Molitch M.E. Surgical treatment of prolactinomas: cons. Endocrine 47, 730–733 (2014)
M.E. Molitch, Management of medically refractory prolactinoma. J. Neuro-oncol. 117, 421–428 (2014)
M.E. Molitch, R.L. Elton, R.E. Blackwell et al., Bromocriptine as primary therapy for prolactin-secreting macroadenomas: results of a prospective multicenter study. J. Clin. Endocrinol. Metab. 60, 698–705 (1985)
J. Webster, G. Piscitelli, A. Polli, C.I. Ferrari, I. Ismail, M.F. Scanlon, A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. Cabergoline Comparative Study Group. N. Engl. J. Med. 331, 904–909 (1994)
I. Bancos, M.R. Nannenga, J.M. Bostwick, M.H. Silber, D. Erickson, T.B. Nippoldt, Impulse control disorders in patients with dopamine agonist-treated prolactinomas and nonfunctioning pituitary adenomas: a case-control study. Clin. Endocrinol. 80, 863–868 (2014)
W.M. Drake, C.E. Stiles, T.A. Howlett, A.A. Toogood, J.S. Bevan, R.P. Steeds, A cross-sectional study of the prevalence of cardiac valvular abnormalities in hyperprolactinemic patients treated with ergot-derived dopamine agonists. J. Clin. Endocrinol. Metab. 99, 90–96 (2014)
F. Galluzzi, R. Salti, S. Stagi, F. La Cauza, F. Chiarelli, Reversible weight gain and prolactin levels--long-term follow-up in childhood. J. Pediatr. Endocrinol. Metab. 18, 921–924 (2005)
B.L. Roth, Drugs and valvular heart disease. N. Engl. J. Med. 356, 6–9 (2007)
J. Verhelst, R. Abs, D. Maiter et al., Cabergoline in the treatment of hyperprolactinemia: a study in 455 patients. J. Clin. Endocrinol. Metab. 84, 2518–2522 (1999)
J. Kharlip, R. Salvatori, G. Yenokyan, G.S. Wand, Recurrence of hyperprolactinemia after withdrawal of long-term cabergoline therapy. J. Clin. Endocrinol. Metab. 94, 2428–2436 (2009)
O. Cohen-Inbar, Z. Xu, D. Schlesinger, M.L. Vance, J.P. Sheehan, Gamma knife radiosurgery for medically and surgically refractory prolactinomas: long-term results. Pituitary 18, 820–830 (2015)
M. Menucci, A. Quinones-Hinojosa, P. Burger, R. Salvatori, Effect of dopaminergic drug treatment on surgical findings in prolactinomas. Pituitary 14, 68–74 (2011)
C. Caputo, D. Prior, W.J. Inder, The need for annual echocardiography to detect cabergoline-associated valvulopathy in patients with prolactinoma: a systematic review and additional clinical data. Lancet Diabetes Endocrinol. 3, 906–913 (2015)
A. Elenkova, R. Shabani, K. Kalinov, S. Zacharieva, Increased prevalence of subclinical cardiac valve fibrosis in patients with prolactinomas on long-term bromocriptine and cabergoline treatment. Eur. J. Endocrinol. 167, 17–25 (2012)
V. Delgado, N.R. Biermasz, S.W. van Thiel et al., Changes in heart valve structure and function in patients treated with dopamine agonists for prolactinomas, a 2-year follow-up study. Clin. Endocrinol. 77, 99–105 (2012)
R. Schade, F. Andersohn, S. Suissa, W. Haverkamp, E. Garbe, Dopamine agonists and the risk of cardiac-valve regurgitation. N. Engl. J. Med. 356, 29–38 (2007)
P. Anagnostis, F. Adamidou, S.A. Polyzos, Z. Efstathiadou, E. Karathanassi, M. Kita, Long term follow-up of patients with prolactinomas and outcome of dopamine agonist withdrawal: a single center experience. Pituitary 15, 25–29 (2012)
C. Schmid, D.L. Goede, R.S. Hauser, M. Brandle, Increased prevalence of high Body Mass Index in patients presenting with pituitary tumours: severe obesity in patients with macroprolactinoma. Swiss Med. Wkly. 136, 254–258 (2006)
C.M. dos Santos Silva, F.R. Barbosa, G.A. Lima et al., BMI and metabolic profile in patients with prolactinoma before and after treatment with dopamine agonists. Obesity 19, 800–805 (2011)
R. Yermus, S. Ezzat, Does normalization of prolactin levels result in weight loss in patients with prolactin secreting pituitary adenomas? Clin. Endocrinol. 56, 562 (2002)
Y. Greenman, K. Tordjman, N. Stern, Increased body weight associated with prolactin secreting pituitary adenomas: weight loss with normalization of prolactin levels. Clin. Endocrinol. 48, 547–553 (1998)
A. Ciresi, M.C. Amato, V. Guarnotta, F. Lo Castro, C. Giordano, Higher doses of cabergoline further improve metabolic parameters in patients with prolactinoma regardless of the degree of reduction in prolactin levels. Clin. Endocrinol. 79, 845–852 (2013)
Acknowledgments
We are grateful for the support of the Swiss National Science Foundation (PBBEB-146099, and -155299 to L.A.). We wish to thank Dr. Edward R. Laws, Jr., MD, FACS, Director, Pituitary and Neuroendocrine Centre, Brigham & Women’s Hospital, Boston, for his insightful suggestions. The assistance of Ms. Susan Kaplan in editing the manuscript is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
This work or part of this work has not been previously published and/or is not under consideration for publication anywhere else. The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Ethical standards and patient consent
This study was approved by the Ethical Committee of Bern (Kantonale Ethikkommision, KEK, Bern, Switzerland), the Swiss Ethics Committee on research involving humans. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Additional information
Lukas Andereggen and Janine Frey contributed equally to this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Andereggen, L., Frey, J., Andres, R.H. et al. 10-year follow-up study comparing primary medical vs. surgical therapy in women with prolactinomas. Endocrine 55, 223–230 (2017). https://doi.org/10.1007/s12020-016-1115-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-1115-2