Abstract
Bilateral adrenal macronodular hyperplasia (BMAH) is a rare form of Cushing’s syndrome characterised by the presence of bilateral secretory adrenal nodules and hypercortisolism. Familial studies support a genetic basis for BMAH, and the disease has been linked to mutations in ARMC5, a gene shown to have a tumour suppressor-like action in the development of adrenal nodules. This study aimed to investigate whether ARMC5 mutations play a role in the development of incidentally discovered bilateral adrenal nodules. We investigated 39 patients with incidentally discovered bilateral adrenal nodules >0.8 cm in diameter who underwent extensive biochemical testing to look for signs of subclinical hypercortisolism. Genomic DNA was analysed by Sanger sequencing, using primers targeted to ARMC5 transcripts. Of the 39 patients included in our study, three were identified as having variants in ARMC5. Two of these are unlikely to be clinically significant, but there is evidence that the third mutation, Chr16:g.31476122;c.1778G>C (p.Arg593Pro), may be pathogenic. Another variant, affecting the same amino-acid residue c.1777C>T (p.Arg593Trp), has been identified previously in two studies of BMAH patients, where it has been shown to segregate with disease in one BMAH family. This patient had biochemical evidence of hypercortisolism in the absence of overt Cushing’s syndrome, and underwent bilateral adrenalectomy separated in time. The presence of a probably clinically significant mutation in ARMC5 in one patient with bilateral adrenal incidentalomas adds to the growing body of evidence in support of ARMC5 as a critical mediator of adrenal nodule development. In addition, the absence of significant ARMC5 mutations in 38 of our patients represents an important negative finding, demonstrating the degree of variability within the pathogenesis of adrenal nodule development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bilateral macronodular adrenal hyperplasia (BMAH) is a rare form of Cushing’s syndrome (CS) characterised by the presence of bilateral adrenocortical nodules which secrete small amounts of cortisol. It is thought to contribute to about 1 % of cases of CS [1], and has been classified as a form of adrenocorticotropic hormone (ACTH)-independent CS, since overproduction of cortisol is directly from the adrenals with no requirement from exogenous (pituitary) ACTH stimulation. Despite this, there is some evidence for local paracrine ACTH signalling in the secretion of cortisol within nodules [2]. It is also called primary macronodular hyperplasia (PMAH), but we will use BMAH here as it has to date been widely accepted. BMAH may have profound cardio-metabolic consequences for patients, since it evolves slowly and commonly manifests as subclinical hypercortisolism (SH) which can go undetected for many years [3]. There is thus an unmet need for the early detection of individuals at risk of, or possessing, BMAH and SH.
The familial distribution and bilateral nature of BMAH suggests that there may be a potential genetic basis to the disease. Specifically, recent work has provided evidence for the role of mutations in armadillo repeat containing 5 (ARMC5) gene [4, 5]. Analysis of gene expression in affected individuals suggests that ARMC5 may act similarly to a tumour suppressor gene, where BMAH patients have a single germline mutation in ARMC5, acting as the first ‘hit’, and subsequent somatic mutation of the gene within adrenal tissue causes the development of nodules and overproduction of cortisol. Variation in ARMC5 is thought to be responsible for 45–55 % of BMAH cases [4, 5] and its segregation to affected individuals within families suggests a direct role in disease pathogenesis [6].
Whilst BMAH is a well-recognised cause of subclinical hypercortisolism, such features may also be present in individuals with incidentally discovered bilateral adrenal nodules. These tumours, identified on imaging in the absence of any indication for investigating adrenal pathology, are a common finding with an estimated prevalence of up to 4.4 % [7]. Similarly to those found in BMAH, they have low-grade secretory capacity and therefore contribute to subclinical hypercortisolism [8], with significant clinical implications in terms of diabetes, hypertension and dyslipidaemia [9]. Again, the bilateral nature of such nodules implies a genetic predisposition to their development, and begs the question as to whether the same mutations in ARMC5 observed in BMAH may be present. The purpose of this study was therefore to investigate the extent to which ARMC5 mutations are responsible for bilateral adrenal disease, specifically asking whether the mutations in ARMC5 observed in some cases of BMAH with overt CS may also be detected in patients with incidentally detected bilateral adrenal incidentalomas.
Patients and methods
Study participants
We studied 39 patients with radiologically confirmed bilateral adrenal nodules. The study was approved by the Ethical Committee of the Evangelismos Hospital, and all participants gave written, informed consent. The age range of our study group was 39–77 years, the mean age 54.8 years, and the standard deviation was 10.3 years. Patients were recruited to the study after nodules were identified incidentally on high-resolution CT scanning performed for indications unrelated to adrenal disease. Only incidentalomas with features suggestive of benign adrenocortical adenomas were included: homogeneous, smoothly marginated lesions with average, unenhanced Hounsfield units of <10. In patients with higher values, we considered an absolute and/or relative contrast washout value of more than 60 % or a loss of signal intensity of 20 % or greater on out-of-phase MRI images as indicating their benign nature. The nodules were all >0.8 cm in diameter, with the majority >1 cm. In patients with nodules larger than 4 cm, a repeat scan was carried out after 6 months to exclude a primary or secondary malignancy. None of these patients exhibited any change in the size of adrenal nodules at this timepoint.
Biochemical analysis
Biochemical tests of blood and urine were carried out in all participants. The tests performed were late night serum cortisol, urinary free cortisol (UFC), overnight or low-dose dexamethasone suppression tests, basal ACTH, dehydroepiandrosterone sulphate (DHEAS), aldosterone, renin and 17-hydroxyprogesterone (17OH-PRG). For the assessment of aldosterone/renin ratio, anti-hypertensive medications were stopped 2–4 weeks prior to testing. When this was not possible, an alpha-adrenergic antagonist and/or calcium channel blocker were used in the place of any other medication for the same time period in order to minimise the effects of treatment on the renin–angiotensin axis. All reagents were supplied by Kyowa Medex Co., Ltd. on behalf of Siemens Healthcare Diagnostic. Serum cortisol was measured by chemiluminescence assay (ADVIA Centaur Immunoassay System), and UFC by the same assay after dichloromethane extraction. ACTH was measured by immunoradiometric assay (Cis Bio international, Gif-sur-Yvette, France). Tests were carried out prior to adrenalectomy, with the exception of three patients (Table 1 for details).
Histological analysis
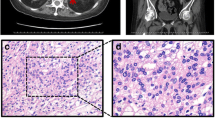
A total of 14 patients underwent surgery, such that tissue was available for histological analysis. Eight of these patients (G1, G6, G8, G25, G36, G37 and G38) had unilateral adrenalectomy of the largest lesion, and histology was compatible with an adrenocortical adenoma. The remaining six operated patients (G2, G3, G4, G7, G31 and G35) met the criteria for a diagnosis of BMAH. Due to the difficulty of diagnosing BMAH, we considered a confirmed diagnosis to be present in those patients who had undergone unilateral or bilateral adrenalectomy with a subsequent histological diagnosis of macronodular hyperplasia. The histological diagnosis of macronodular hyperplasia was established in the case of massively enlarged adrenals with multiple macronodules, distorting and completely obscuring the normal gland. Of the non-operated patients, the majority had discrete bilateral single adenomas on CT scanning.
Genetic sequencing
Genomic DNA was isolated from peripheral blood samples taken from the 39 patients, and ARMC5 analysis was carried out by Sanger sequencing. Primers were redesigned from those previously used to sequence ARMC5 [4] in order to meet clinical diagnostic standards (see Table 2 for a full list of primers used). The primers were designed to interrogate two known transcripts of ARMC5: transcript 1, NM_001105247.1, is the longer transcript containing six exons, and transcript 2, NM_024742.2, the shorter, containing four exons. Transcript 1 is thought to be most relevant to BMAH, as previous mutations in ARMC5 have all been found here [4, 6], but it is not known for certain whether this is the only relevant sequence. Mutation analysis was performed using Mutation Surveyor DNA variant analysis software [10]. For variant pathogenicity investigation, Alamut visual software (Alamut Visual v.2.7.1) was used [11].
Results
Clinical studies
Twenty of the 39 study participants (51 %) had subclinical hypercortisolism (see Table 1 for detailed clinical data). SH was diagnosed using previously published diagnostic criteria [8], viz. a post-dexamethasone suppression test cortisol level less than 1.8 μg/dl (50 nmol/l) and at least one ACTH level less than 10 pg/ml, a midnight serum cortisol less than 7 μg/dl (194 nmol/l) or urinary free cortisol >120 mg/24 h. All patients had normal aldosterone/renin ratios and 24-h fractionated urinary metanephrines. No participants had overt signs of Cushing’s syndrome.
Sanger sequencing
Genetic analysis of transcripts 1 and 2 of ARMC5 demonstrated no mutation in 36 of 39 participants. In three participants, variants in AMRC5 were found. In participant G10, there was a missense substitution Chr16:g.31475849;c.1505G>A (p.Arg502His) rs200054015. In silico modelling using Mutation Taster [11] and SIFT [12] did not predict pathogenicity for this variant. A second substitution in participant G38 was Chr16:g.31476200;c.1856G>A (p.Arg619Gln) rs377492831. Although in silico tools including MutationTaster suggest that this may be deleterious, the confidence of this prediction is low (p value = 0.519) [11]. Finally, patient G35 exhibited a Chr16:g.31476122; c.1778G>C (p.Arg593Pro) substitution: in silico modelling suggested that it may not be functionally significant although there is moderate physiochemical difference between Arg and Pro, and SIFT predicted a deleterious effect [12]. Variation at this location has been seen before (see “Discussion” section for details), and therefore this mutation is of interest and a further investigation into the background of this patient was performed.
Patient G35
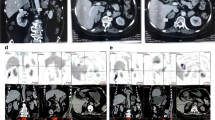
Patient G35, now a 77-year-old man, was incidentally found to have bilateral adrenal enlargement in 1999 after undergoing a CT scan for a lung infection. He was not overtly Cushingoid. Biochemical tests demonstrated a degree of subclinical hypercortisolism: basal 09:00 h serum cortisol was 18.2 μg/dl (502 nmol/l) with a concomitant plasma ACTH of 11 ng/l (October 2000). Subsequent MRI scanning in 2001 revealed bilateral adrenal hyperplasia, with both adrenals 2–3 cm in diameter. Based on this abnormal biochemistry, in June 2002 he underwent left adrenalectomy, which was histologically characterised as nodular adrenal hyperplasia. Three months later, there was no suppression of cortisol after the overnight 1 mg dexamethasone suppression test, with an 09:00 h serum cortisol of 11.8 μg/dl (326 nmol/l), so on February 2003 he underwent right adrenalectomy. Post-operative histology showed nodular hyperplasia. In 2005, CT scanning revealed a remnant of the left adrenal, which was 4.5 cm in diameter. The biochemical results from this remnant showed no suppression on the 48-h Liddle low-dose dexamethasone suppression test with a ‘2 + 48’ cortisol of 11.8 μg/dl (326 nmol/l, normal <1.8 μg/dl, 50 nmol/l), an elevated midnight serum cortisol of 10.5 and 9.5 μg/dl (290 and 262 nmol/l, normal <1.8 μg/dl, 50 nmol/l), a normal UFC of 96 μg/24 h (36–137 μg/24 h) and basal morning ACTH 11 ng/l (December 2007). Due to continuing subclinical hypercortisolism, the patient underwent removal of the left adrenal remnant (9 July 2008). The size of the remnant was 5 cm. Since the last surgery, he has been well on hydrocortisone and fludrocortisone replacement. At no point did this patient show any overt clinical signs of Cushing’s syndrome.
Discussion
Genetic sequencing of patients with incidentally discovered bilateral adrenal nodules revealed three mutations in ARMC5, a gene known to be involved in the pathogenesis of bilateral macronodular adrenal hyperplasia (BMAH). Two of these mutations, found in patients G10 and G38, are of uncertain clinical significance. In patient G10, a missense substitution Chr16:g.31475849;c.1505G>A (p.Arg502His) was found. In silico modelling (MutationTaster [11]/SIFT [12]) did not predict pathogenicity, and there is only a small physiochemical difference between Arg and His. The patient was not operated on as there was no evidence of SH, and therefore we are unable to say whether BMAH was present, or indeed to look for somatic mutations.
The second mutation, in patient G38, was a Chr16:g.31476200;c.1856G>A (p.Arg619Gln) substitution. In silico methods predict a deleterious effect, but with a low p value (0.519), and this variant has been reported on Exome Variant Server (NHLBI Exome Sequencing Project). Patient G38 underwent unilateral adrenalectomy due to SH, and histology was compatible with adrenocortical adenoma rather than BMAH. Beyond in silico prediction, there is no clear evidence at this time to suggest that either of these variants is pathogenic, and therefore the clinical significance remains unknown. Neither mutation has previously been reported in patients with SH or BMAH.
In patient G35, a Chr16:g.31476122;c.1778G>C (p.Arg593Pro) substitution was seen. Variation at this location was identified in BMAH patients by Faucz et al. [5], who noted a 593 arginine-to-tryptophan substitution that was considered very probably pathogenic. The same mutation was observed by Gagliardi et al. [6], where the variant clearly segregated with disease, being present in three affected siblings in one family and not in unaffected siblings. Neither the c.1777C>T or c.1778G>C variants are reported in population cohorts (ExAC or ESP). A substitution to proline rather than tryptophan, as seen in our study, is even more likely to be pathogenic due to structural differences, as proline has the additional potential to destabilise the secondary structure.
The patient in question had longstanding hypercortisolism in the absence of clinical signs of Cushing’s syndrome. His adrenal hyperplasia was diagnosed incidentally on CT scanning, and was subsequently confirmed histologically as nodular hyperplasia. The adrenal nodular hyperplasia was bilateral and persistent after surgery, with significant hypercortisolism from an adrenal remnant of 5 cm, and this suggests an important role for ARMC5 mutation in the pathogenesis of this patient’s adrenal incidentalomas. It is highly plausible that this mutation in ARMC5 removed the tumour suppressor function of the gene, allowing nodular hyperplasia and subclinical hypercortisolism to develop with a second somatic mutation. Unfortunately, no adrenal tissue is available from participants of the study for further analysis, and therefore we cannot confirm the presence of somatic mutations. In the future, it would be interesting to examine adrenal tissue of other patients for such mutations, which may form the second ‘hit’ in the proposed tumour suppressor role of ARMC5.
Whilst three mutations in ARMC5 were detected, the absence of any significant mutations in ARMC5 in the great majority (36/39) of our patients is an important negative finding. This is consistent with previous work: ARMC5 mutations have been identified in some cases of bilateral adrenal nodules, but by no means all. Assié et al found mutations in just over half of their cohort of BMAH patients [4], whilst in familial studies of BMAH an absence of genetic mutations in ARMC5 has been noted in some cohorts [6]. In one recent study of patients with primary hyperaldosteronism and bilateral adrenal nodules, no pathogenic mutations were found [13], although another series suggested that ARMC5 may be involved in patients with primary hyperaldosteronism of African-American origin [14]. It is clear, therefore, that whilst ARMC5 acts as a tumour suppressor-like gene in some cases of adrenal nodules, where the combination of a germline and a somatic mutation can lead to the proliferation of adrenal tissue and overproduction of cortisol, the genetic basis for adrenal nodules is not the same in all individuals. Indeed, the rate of ARMC5 mutations amongst BMAH individuals in this study, a single mutation in a group of six confirmed BMAH patients, is lower than the 45–55 % observed previously [4]. This is perhaps even more significant given that 25 of our study participants did not undergo surgery or histological analysis, and therefore amongst these may be some individuals with undiagnosed BMAH. However, the sample size of BMAH is small and it is difficult to make any firm conclusions.
A particularly relevant area for further work is in establishing the link between the ectopic expression of membrane-bound hormone receptors in adrenal tissue, and the over-production of cortisol. It seems that ectopic expression of a variety of receptors (GIP, β-adrenergic, vasopressin, luteinising hormone (LH), leptin, 5-hydroxytryptophan (5-HT)) mimics the downstream effects of the ACTH receptor, leading to the production of cortisol independent of ACTH, and instead controlled by receptor-specific triggers [1, 15, 16]. For example, with ectopic β-adrenergic receptor expression in adrenal nodules, hypercortisolism and CS are triggered by an increase in endogenous catecholamine production [17, 18]. The same is true for increases in endogenous levels of each hormone and its respective receptor. It is plausible that whatever the genetic predisposition to bilateral adrenal nodules, there is some effect of the mutation on ectopic receptor expression. This was investigated in the context of ARMC5 mutations [4], where metoclopramide or upright tests causing elevation of 5-HT and catecholamines, respectively, gave positive results (i.e. an increase in cortisol production) in three patients with ARMC5 mutations, implying that ARMC5 mutations are associated with hormone-dependent hypercortisolism in some instances. Although the precise mechanism by which ARMC5 mutations might lead to ectopic receptor expression is unknown, there is scope for investigating other genes which lead to aberrant hormone receptor function when further exploring the genetic basis of bilateral adrenal nodules.
Our study clearly identifies what is likely to be a clinically significant variant of ARMC5 in one patient amongst a cohort of patients with incidentally discovered adrenal nodules. This study lends further support to the growing body of evidence that suggests an important role for ARMC5 as a mediator of adrenal hyperplasia, not only in BMAH but in adrenal nodule development more widely. Bilateral incidentalomas are common, with a prevalence of up to 4.4 % [7], and therefore establishing their genetic basis is of great importance. It now seems that ARMC5, which is present in 45–55 % of BMAH patients [4, 5], may also play a role in incidentalomas. It is of note, however, that the majority of patients presenting with incidentally discovered bilateral adrenal nodules had no evidence of ARMC5 mutations, and thus at this stage the prevalence of mutations in bilateral incidentalomas seems too low to support any screening programme for ARMC5 mutations. Further characterisation of the extent to which ARMC5 is responsible for the development of BMAH and bilateral adrenal nodules will help sub-classify what is clearly a highly heterogeneous disease with a wide range of histological, biochemical and clinical findings [19, 20].
References
A. Lacroix, ACTH-independent macronodular adrenal hyperplasia. Best Pract Res: Clin Endocrinol Metab 23, 245–259 (2009)
E. Louiset, C. Duparc, J. Young et al., Intraadrenal corticotropin in bilateral macronodular adrenal hyperplasia. N. Engl. J. Med. 369, 2115–2125 (2013)
J.M. Swain, C.S. Grant, R.T. Schlinkert et al., Corticotropin-independent macronodular adrenal hyperplasia: a clinicopathologic correlation. Arch. Surg. 133, 541–546 (1998)
G. Assié, R. Libé, S. Espiard et al., ARMC5 mutations in macronodular adrenal hyperplasia with Cushing’s syndrome. N. Engl. J. Med. 369, 2105–2114 (2013)
F.R. Faucz, M. Zilbermint, M.B. Lodish et al., Macronodular adrenal hyperplasia due to mutations in an armadillo repeat containing 5 (ARMC5) gene: a clinical and genetic investigation. J. Clin. Endocrinol. Metab. 99, E1113–E1119 (2014)
L. Gagliardi, A.W. Schreiber, C.N. Hahn et al., ARMC5 mutations are common in familial bilateral macronodular adrenal hyperplasia. J. Clin. Endocrinol. Metab. 99, E1784–E1792 (2014)
R.T. Kloos, M.D. Gross, I.R. Francis, M. Korobkin, B. Shapiro, Incidentally discovered adrenal masses. Endocr. Rev. 16, 460–484 (1995)
D.A. Vassiliadi, G. Ntali, E. Vicha, S. Tsagarakis, High prevalence of subclinical hypercortisolism in patients with bilateral adrenal incidentalomas: a challenge to management. Clin. Endocrinol. 74, 438–444 (2011)
R. Rossi, L. Tauchmanova, A. Luciano et al., Subclinical Cushing’s syndrome in patients with adrenal incidentaloma: clinical and biochemical features. J. Clin. Endocrinol. Metab. 85, 1440–1448 (2000)
P. Mulatero, F. Schiavi, T.A. Williams et al., ARMC5 mutation analysis in patients with primary aldosteronism and bilateral adrenal lesions. J. Hum. Hypertens. 30, 374–378 (2015)
M. Zilbermint, P. Xekouki, F.R. Faucz et al., Primary aldosteronism and ARMC5 variants. J. Clin. Endocrinol. Metab. 100, E900–E909 (2015)
A. Lacroix, N. N’Diaye, J. Tremblay, P. Hamet, Ectopic and abnormal hormone receptors in adrenal Cushing’s syndrome. Endocr. Rev. 22, 75–110 (2001)
I. Bourdeau, P. D’Amour, P. Hamet, J.M. Boutin, A. Lacroix, Aberrant membrane hormone receptors in incidentally discovered bilateral macronodular adrenal hyperplasia with subclinical Cushing’s syndrome. J. Clin. Endocrinol. Metab. 86, 5534–5540 (2001)
N. Miyamura, T. Taguchi, Y. Murata et al., Inherited adrenocorticotropin-independent macronodular adrenal hyperplasia with abnormal cortisol secretion by vasopressin and catecholamines: detection of the aberrant hormone receptors on adrenal gland. Endocrine 19, 319–325 (2002)
D. Vezzosi, D. Carter, C. Régnier et al., Familial adrenocorticotropin-independent macronodular adrenal hyperplasia with aberrant serotonin and vasopressin adrenal receptors. Eur. J. Endocrinol. 156, 21–31 (2007)
D.A. Vassiliadi, M. Tzanela, V. Tsatlidis et al., Abnormal responsiveness to dexamethasone-suppressed CRH test in patients with bilateral adrenal incidentalomas. J. Clin. Endocrinol. Metab. 100, 3478–3485 (2015)
C.A. Stratakis, S.A. Boikos, Genetics of adrenal tumors associated with Cushing’s syndrome: a new classification for bilateral adrenocortical hyperplasias. Nat. Clin. Pract. Endocrinol. Metab. 3, 748–757 (2007)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Stylianos Tsagarakis and Ashley B. Grossman are to be considered as joint senior authors.
Rights and permissions
About this article
Cite this article
Emms, H., Tsirou, I., Cranston, T. et al. Do patients with incidentally discovered bilateral adrenal nodules represent an early form of ARMC5-mediated bilateral macronodular hyperplasia?. Endocrine 53, 801–808 (2016). https://doi.org/10.1007/s12020-016-0988-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-0988-4