Abstract
The variable presence of adrenal insufficiency (AI) due to hypocortisolemia (HC) in patients with thalassemia is well established; however, the prevalence of adrenocortical hypofunction (ACH) in the zona glomerulosa and zona reticularis of the adrenal cortex is unknown. To establish the prevalence of ACH, we examined the cortisol response to 1-µg and 250-µg ACTH tests, plasma aldosterone (A)/plasma renin activity (PRA) ratio, and serum dehydroepiandrosterone sulfate (DHEAS) levels in a large cohort of patients with thalassemia, and to investigate the impact of total body iron load (TBIL) on adrenocortical function. The setting used was University hospital and government-based tertiary care center. One hundred twenty-one (52 females) patients with β-thalassemia major (β-TM) and 72 healthy peers (38 females) were enrolled. The patients underwent a 250-µg cosyntropin test if their peak cortisol was <500 nmol/L in a 1-µg cosyntropin test. Magnetic resonance imaging (MRI) was performed to assess the MRI-based liver iron content and cardiac MRI T2* iron. The associations between ACH and TBIL were investigated. The patients with thalassemia had lower ACTH, cortisol, DHEAS, and A/PRA values compared with the controls (p < 0.001). Thirty-nine patients (32.2 %) had HC [primary (n = 1), central (n = 36), combined (n = 2)], and 47 (38.8 %) patients had reduced DHEAS levels; 29 (24.0 %) patients had reduced A/PRA ratios. Forty-six (38.0 %) patients had hypofunction in one of the adrenal zones, 26 (21.5 %) had hypofunction in two adrenal zones, and 9 (7.4 %) had hypofunction in all three zones. Patient age and TBIL surrogates were significant independent parameters associated with ACH. Cardiac MRI T2* iron was the only significant parameter that predicted the severity of ACH at a cut-off of 20.6 ms, with 81 % sensitivity and 78 % specificity. Patients with thalassemia have a high prevalence of AI due to HC and zona glomerulosa and zona reticularis hypofunction. TBIL surrogates can predict ACH, but cardiac iron was the only surrogate that was adequately sensitive to predict the severity of ACH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Excess iron is first deposited in the form of hemosiderin in patients with thalassemia who are regularly transfused, mainly in the mononuclear phagocyte system. It is eventually deposited in endocrine organs and the heart, which leads to target-organ damage [1]. In the past, serum ferritin was considered a reliable indicator of total body iron load (TBIL). However, magnetic resonance imaging (MRI) evaluation of the liver and cardiac iron overload is now considered the standard of care whenever available [1], because it provides low interscanner and interobserver variability in tissue iron assessments.
Recent advances in the medical management of thalassemia have greatly increased patient's lifespan. The most common causes of mortality in these patients are heart failure and fatal arrhythmias. Iron load-related endocrinopathies are the most important complications that affect quality of life in patients with thalassemia [2].
Adrenal insufficiency (AI) is a common complication in thalassemia. Adrenal androgen production declines with advancing puberty in adolescents with thalassemia and may explain the poor development of pubic and axillary hair associated with this condition [3]. It is thought that transfusion-related iron overload results in nontransferrin-bound iron (NTBI) deposition in the adrenal glands, hypothalamus, or pituitary, which in turn leads to the development of primary or central AI. Autopsy findings indicated that iron deposition in the adrenal glands occured more frequently in the zona glomerulosa, where mineralocorticoids are produced, than in the cortisol-producing zona fasciculata [4]. The incidence of β-thalassemia (β-TM) and the number of globin gene mutation carriers are very high in Southeast Turkey [5]. However, there are no data regarding the prevalence of AI in patients with β-TM in this region. To the best of our knowledge, studies regarding the evaluation of adrenal function in patients with thalassemia have primarily focused on the glucocorticoid axis [1–3]. However, studies evaluating the secretion dynamics of mineralocorticoids of the adrenal cortex in patients with transfusion-dependent β-TM have not been performed to date. In this preliminary study, we aimed to evaluate the secretion dynamics of glucocorticoid, mineralocorticoid, and adrenal androgens of the adrenal cortex in a large cohort of patients with transfusion-dependent β-TM. We also evaluated the relationship between adrenocortical function and TBIL surrogates such as serum ferritin, and hepatic and cardiac iron load, which was measured using hepatic and cardiac MRI. To evaluate the involvement of the adrenal cortex zones in patients with β-TM, the term adrenocortical hypofunction (ACH) was defined in this study as the presence of hypocortisolemia (HC), reduced DHEAS levels, or reduced plasma A/plasma renin activity (PRA) ratio.
Subjects and methods
Of 167 consecutive patients with β-TM aged 6–18 years, 121 were eligible for enrollment. The diagnosis of β-TM was based on family history, complete transfusion dependence, hemoglobin electrophoresis, and a genetic analysis. Patients were excluded from the study in the presence of any of the following: low body mass index (BMI), which was defined as a BMI below the 5th percentile (−1.65 standard deviation score [SDS]; n = 29) according to the CDC BMI chart for sex; refugee status from Syria, because of incomplete data regarding transfusion history (n = 3); chelation therapy other than deferasirox (n = 3); hepatitis C (n = 2); the use of any medication within 3 months of the study that could influence the assessed hormones (n = 2); pregnancy (n = 1); or an increased resistance index on a renal Doppler ultrasound (n = 6). At the time of enrollment, each patient had been followed up for at least 2 years at the Pediatric Hematology Department of Harran University School of Medicine and Children’s State Hospital of Sanliurfa, and each required regular red cell transfusions (12–15 mL/kg) every 3–4 weeks to maintain hemoglobin levels above 9 g/dL. In 2006, the Ministry of Health in Turkey approved the use of deferasirox. All patients were given oral deferasirox [5–30 mg/(kg/day)]—Exjade (DFX), Novartis Pharma AG, Basel, Switzerland.
A list of random healthy children and adolescents was obtained from the Well-Child and Adolescent Health Care Unit registry. Invitation letters were sent to the families of the subjects who were selected by order sampling in each age group to yield an age- and sex-matched control group. Approximately, 40 % of the invited control subjects agreed to participate. Seventy-two (38 F) healthy controls were selected who lived in the same area and matched the patients’ ages (mean age 11.4 years, range 5.8–17.8 years).
The study was approved by the ethics committee of Harran University and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. Written informed consent was obtained from all of the patients or their legal guardians.
Data collection
Transfusion history and transfusion-related laboratory data were retrieved from an electronic patient records system.
Height and weight measurements were performed using a portable Seca stadiometer (Birmingham, UK) and an electronic device (sensitivity = 0.1 kg), respectively. The SDs of these measurements and the BMI (weight divided by height squared) were calculated [6]. Tanner’s pubertal staging was performed based on breast/testicular development and Tanner’s staging for pubic/axillary hair. Clinical blood pressure (BP) of all subjects and their parents was measured three times at 1-min interval using a mercury sphygmomanometer after the subjects had rested for at least 10 min. The SDs of systolic and diastolic BP were calculated [7]. In addition to measuring BP, an adult was considered hypertensive if there was a history of hypertension or if he/she was on treatment for hypertension. Controls who were menstruating were assessed during the early follicular stage (days 5–10) of their menstrual cycles. Patients with thalassemia who were taking sex steroid replacement therapy were tested during the estrogen phase of treatment on days 5–10 of the treatment cycle.
Annualized median hemoglobin (Hb) and serum ferritin levels were calculated using the median of the recorded values over a period of 1 year prior to our evaluation. Serum ferritin levels were measured using an electrochemiluminescence immunoassay (ECLIA; Roche Diagnostics, IN, USA) before hemotransfusion and long before or after iron chelator administration.
In all patients, systolic and diastolic parameters were evaluated by the same pediatric cardiologist (AY) using an M-mode echocardiography (Sonosite Titan). The measurements were performed one week after transfusion in accordance with the recommendations of the American Society of Echocardiography [8]. Cardiac and liver MRIs (1.5 T scanner, GE Sigma/Excite HD, Milwaukee, WI, USA) were performed, and a T2* algorithm was used to estimate iron loads. A T2* analysis was performed using Thalassemia Tools (a plug-in from CMR Tools, Cardiovascular Imaging Solutions, London, UK) with curve truncation to account for background noise [9]. MRI-based liver iron concentration (LIC) was calculated [10, 11].
Hormonal assessments
Fasting blood samples were drawn from all subjects between 08.00 and 09.00 am to assess plasma ACTH, aldosterone (A), PRA, DHEAS, sodium, potassium, and creatinine levels. A 20-h urine collection was performed between 19.00 pm and 07.00 am to calculate the fractional excretion of sodium (FeNa12h = [urinary sodium × serum creatinine]/[serum sodium × urinary creatinine] × 100). All patients underwent a 1-μg ACTH (Synacthen; Novartis, Basel, Switzerland) stimulation test. Patients with a peak cortisol <500 nmol/L in the 1-µg ACTH test underwent a 250-µg ACTH test to differentiate primary and central AI. Patients with low/normal ACTH levels and a peak cortisol level <500 nmol/L in the 250-µg ACTH test were diagnosed as having combined AI, i.e., both primary and central AI. Those with reduced DHEAS levels (SDS < −2 SDS), which were measured using reference curves developed by fitting two separate half-normal distributions to the age- and sex-specific reference ranges provided by the assay manufacturer were diagnosed as having DHEAS [12]. A reduced plasma A/PRA ratio was diagnosed using a cut-off value of 1.1, which was the 2.5th percentile value of the A/PRA ratios of the controls.
Hormone assays
Corticotropin was measured using a commercial enzyme immunoassay (DPC Biermann Gmbh, Bad Nauheim, Germany) and an Immulite automated hormone analyzer. The interassay coefficients were 9.6 % with 24 ± 3 pg/mL, 6.1 % with 101 ± 6.2 pg/mL, and 3.1 % with 504 ± 15.6 pg/mL. Cortisol levels were measured using a radioimmune assay (RIA), and a recovery-corrected extraction was performed for cortisol. For the cortisol assay, the intra-assay CV was 5.2 %, and the interassay CV was 8.7 %. DHEAS levels were analyzed using a chemiluminescence immunoassay (Immulite; Diagnostic Products Corp., Los Angeles, CA). The lower and upper limits of detection for the assays were 3 and 1500 µg/dL, respectively. Plasma A and PRA levels were measured using a radioimmunoassay (Marseille, France). The interassay CV for the A assay was 11.1 % at a plasma A level of 214 ng/L. The normal ranges for PRA were 0.2–0.8 ng/(mL h) with a normal salt intake in a sitting position, and the intra- and interassay CVs were <10 %.
Statistical analyses
All analyses were performed using IBM SPSS version 22 (SPSS IL, USA) and GraphPad Prism 6 (San Diego, USA) software. The normality of variables was tested using a D’Agostino-Pearson omnibus normality test. The results are presented as means (95 % confidence interval [CI]) or medians [25–75 % interquartile ranges (IQR)] unless and otherwise stated. Categorical variables were compared using the χ 2 test and Fisher’s exact test. Student’s t test was used to compare variables between groups. A nonparametric test (Mann–Whitney U test) was used if the variables did not have Gaussian distribution. Serum cortisol levels were compared using a univariate analysis after adjustments for Tanner’s pubertal staging [13].
Independent associations of the following putatively influential covariates with the presence and severity of ACH were assessed using binary logistic regression analyses: age, duration of chelation therapy, duration of transfusion, Tanner’s pubertal staging for breast/testicular development, serum ferritin, LIC, and cardiac MRI T2* iron. The last three covariates were not included in the assessed models because they were intercorrelated (r = 0.21–0.44; p < 0.001 for all).
Receiver operating characteristics (ROC) curves were used to assess the ability of the TBIL surrogates to predict the presence of one or more ACHs. The optimal cut-off values were determined for serum ferritin, MRI-based LIC, and cardiac MRI T2* iron for diagnosing AHC, and the area under the ROC curves (AUC) for the sensitivity and specificity of these parameters. The “best fit” value of the curve (the threshold value for which {Sensitivity + Specificity − 100} is maximized) was determined using the Youden index. The AUCs for serum ferritin, LIC, and cardiac MRI T2* iron were compared using the method described by Hanley et al. [14]. The results are presented with 95 % CIs. Two-tailed significance was set at p < 0.05.
Results
Transfusion history and total body iron load
The patients’ transfusion history and data related to TBIL are shown in Table 1. The patients had regularly received red cell transfusion since a median age of 8 months. All patients began receiving chelation therapy at a median age of 4 years. Twenty-five (20.7 %) patients had undergone a splenectomy. Forty-eight (39.7 %) patients had mildly elevated ALT levels (ALT > 35 U/L). Forty-three patients (35.5 %) had annualized mean Hb levels lower than 9.0 g/dL. The mean annualized serum ferritin was 1679 ng/mL [IQR: 1169–2805]. Median LIC (assessed using MRI) was 2.9 mg/g tissue [IQR: 2.6–6.2]. Cardiac iron levels were assessed using MRI T2* and were found as 27.40 ms [IQR: 18.87–32.61]. Echocardiographic assessment of systolic and diastolic dysfunction revealed normal findings in all patients (data not shown).
Comparative analyses of the patients with thalassemia major vs. controls
The comparative results of the anthropometric- and laboratory-related data of the patients with thalassemia and controls are shown in Table 2. The number of patients with history of hypertension in at least 1 parent was compared to that of the controls (p = 0.50). Sixteen (13.2 %) patients were aged less than 10 years of age at the time of the study. Overall, the patients had lower BMI SDs, Tanner’s pubertal staging, and diastolic BP SDs compared with the controls. The patients also had lower ACTH, cortisol, DHEAS, and A/PRA values than the controls (Table 3).The difference in serum cortisol levels between the patients and controls persisted after adjustment for Tanner’s pubertal staging. No patients had reduced A and/or PRA levels.
Estimated prevalence of adrenal cortex hypofunction in the patients with thalassemia major
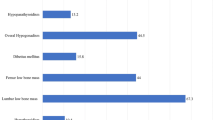
Thirty-nine (32.2 %) patients were diagnosed as having AI. Primary AI was diagnosed in one patient with a plasma ACTH level of 70 pg/mL and peak post-corticotropin (250 µg) cortisol level of 13 µg/dL. Central AI was diagnosed in 36 patients, and combined AI in two patients. Forty-seven (38.8 %) patients had reduced DHEAS levels, and 29 (24.0 %) patients had reduced A/PRA ratios. Forty-six patients had ACH in one zone of the adrenal cortex, 26 patients had ACHs in two zones, and 9 patients had hypofunction in three zones. Of the 16 patients aged less than 10 years, six (37.5 %) had ACHs, three had reduced A/PRA ratios, two had isolated HC, and one had both HC and a reduced DHEAS level. Forty (33.1 %) patients had normal adrenocortical functions.
Analyses of the putative influential factors on adrenal cortex functions and its severity in patients with thalassemia major
The comparison of TBIL in patients with ACH in at least one zone (n = 81) vs. those with normal adrenal cortex functions (n = 40) revealed higher median annualized serum ferritin, lower median LIC, and lower median cardiac MRI T2* iron in the former subgroup (1457 ng/ml [IQR: 1008–2096] and 896 ng/ml [IQR: 1399–2564], p = 0.004; 4.3 mg Fe/g [IQR: 2.8–9.3] and 2.8 mg Fe/g [IQR: 2.5–3.4], p = 0.007; 20.8 ms [IQR: 12.1–23.0] and 31.5 ms [IQR: 24.1–35.9], p < 0.001, respectively). When the patients with one ACH were compared with those with two or three ACHs, the median cardiac MRI T2* iron was significantly lower in the latter subgroup (21.4 ms [25–75 % IQR: 20.4–24.0] and 20.1 ms [25–75 % IQR: 9.9–20.7], respectively, p < 0.001). However, the serum ferritin and liver iron content were comparable between the subgroups (p > 0.05; data not shown).
The use of ROC curves for serum ferritin and MRI-based liver iron content and myocardial MRI T2* iron to predict the presence and severity of adrenocortical hypofunction in patients with thalassemia major
The ROC curve analysis revealed that the annualized serum ferritin, LIC, and cardiac MRI T2* iron levels predicted the presence of ACH in patients with thalassemia major (Fig. 1). An annualized serum ferritin cut-off of 1372 ng/mL predicted the presence of ACH with 78 % sensitivity and 48.9 % specificity. LIC cut-off of 3.2 mg Fe/g predicted the presence of ACH with 59 % sensitivity and 74 % specificity. A cardiac MRI T2* iron cut-off of 23.4 ms predicted ACH with 86 % sensitivity and 75 % specificity (Table 4). The comparison of the ROC curves of the TBIL surrogates revealed that cardiac MRI T2* iron was more sensitive than either serum ferritin or LIC for detecting ACHs (p < 0.01). The ROC curve analysis of cardiac MRI T2* iron levels at a cut-off of 20.6 ms predicted the presence of more than one ACH in patients with thalassemia major with 81 % sensitivity and 78 % specificity (Fig. 2).
Receiver operating characteristics (ROC) curves of annualized serum ferritin and MRI-based liver iron content (LIC) to predict the presence of adrenocortical hypofunction in patients with thalassemia. Area under the ROC curve (AUC) of cardiac MRI T2* iron (AUC: 0.83 [95 % CI 0.75–0.92], p < 0.0001) was significantly greater than those of annualized serum ferritin (AUC: 0.63[95 % CI 0.54–0.71], p = 0.0036) and LIC (AUC: 0.65 [95 % CI 0.54–0.75], p = 0.0041); p < 0.01
The logistic regression analysis revealed that the age and cut-off values of the TBIL surrogates derived from the ROC curve analyses (assessed in separate models) were independently associated with the presence of ACH in patients with thalassemia major odds ratio (OR)age = 1.5 (95 % CI 1.2–1.7); ORROC_ferritin = 3.7 (95 % CI 1.5–9.7); ORROC_LIC = 2.1 (95 % CI 1.2–5.6); and ORROC_cardiac MRI T2*iron = 18.6 (95 % CI 6.7–51.4). However, when the same covariates were investigated in relation to the presence of two or three ACHs, only cardiac MRI T2* iron was associated with the severity of ACH: ORROC_cardiacMRI T2*iron = 12.2 (95 % CI 4.0–37.5).
Discussion
To our knowledge, this is the first study to report the prevalence of subclinical mineralocorticoid deficiency in transfusion-dependent patients with thalassemia. Our large cohort of patients had a high prevalence of AI, which reflects the variable involvement of all three layers of the adrenal cortex. In our cohort, we found that serum ferritin, LIC, and cardiac MRI T2* iron were surrogates for the presence of ACH with either HC, reduced A/PRA, or reduced DHEAS levels, and that cardiac MRI T2* iron was a predictor of the severity of AI. Age was also an independent significant parameter that predicted ACH in our patients, which suggests that factors other than TBIL may influence the evolution of ACH in thalassemia.
Hypofunction in at least one adrenal cortex zone was diagnosed in 37 % of the patients aged less than 10 years, which suggests that ACH had developed in these patients and that the age limit for routine screening should possibly be lowered for patients with thalassemia in developing countries; suboptimal chelation therapy induces earlier onset endocrinopathies [1].
In the current study, we did not include patients with a BMI below the 5th percentile to eliminate malnutrition as a potential confounder for adrenal dysfunction. However, we cannot totally exclude the possibility of micronutrient deficiencies in our cohort because patients with thalassemia were reported to have decreased levels of several nutritional biomarkers, which could potentially affect endocrine function [15]. The patients with thalassemia in our study also had lower BMIs than the controls. Therefore, we included BMI with other covariates, such as anemia and splenectomy status [16], infection with hepatitis C, and other hepatotropic viruses [17] in our assessments to eliminate recognized sources of increased tendency toward endocrinopathy in the patients with thalassemia.
The prevalence of AI in our patients was similar to the results of previous studies of thalassemics with iron overload [18, 19], reviewed in [20]. The majority of the AI in the patients with thalassemia in our cohort was very likely the result of hemochromatosis of the hypothalamo-pituitary glands because of the much lower prevalence of primary AI. Using the 1-µg and 250-µg ACTH tests, it was possible that some patients with subtle primary AI could have been diagnosed as having central AI. Thus, we also measured baseline ACTH levels to discriminate between types of AI. In our cohort, combined involvement of the hypothalamo-pituitary and adrenal glands was identified in 2 patients who had normal or reduced ACTH levels and reduced peak cortisol responses to the 250-µg ACTH test. We recognize that the 1-µg ACTH test may have overestimated the prevalence of AI compared with the insulin tolerance test (ITT). However, we would rather avoid unwanted complications related to hypoglycemia in our patients. In addition, a recent study by Poomthavorn et al. [21] reported only a small discrepancy in the prevalence of AI using the 1-µg ACTH test vs. ITT in patients with thalassemia. Therefore, the prevalence of AI reported herein should be acceptable. Huang et al. [22] performed an ovine corticotropin-releasing hormone (oCRH) test in a small cohort of thalassemics and found hypothalamic AI in 91 % of their patients who had been diagnosed as having AI. However, the oCRH test was not standardized with pediatric reference values [23, 24].
Serum DHEAS levels were reduced in 39 % of the patients with thalassemia in our cohort, which suggests the involvement of the zona reticularis of the adrenal cortex. Our patients had significantly lower Tanner’s stages for pubarche than the healthy controls, which possibly represent a clinical delay of adrenarche in our patients. However, it should be noted that DHEAS is a weak androgen, and it is the peripheral metabolism of this weak androgen that causes clinical adrenarche [25]. DHEAS has pleiotropic effects related to well being in humans [26]. Treatment with DHEA improves mood and general well being in adult patients, children, and adolescents with adrenal insufficiency [27, 28]. However, DHEAS replacement in this population requires further study. Androstenedione is another adrenal androgen; however, we did not analyze androstenedione levels in our cohort because of its significant gonadal contribution during puberty, which could have interfered with our primary aim of assessing the adrenal cortex.
In vivo staining of the zona glomerulosa of the adrenal cortex has long been established [4]. However, the renin-angiotensin-aldosterone (RAS) system in patients with thalassemia has not yet been evaluated. We found subtle alterations in the RAS system, which were reflected by the reduced A/PRA ratio and possibly by the reduced diastolic BP SDS in the patients with thalassemia. However, the FeNa12h and serum electrolyte levels were similar to those of the controls, which suggests a compensated status. The association between RAS derangement and TBIL warrants further studies because this association was not found in an earlier study of patients with primary hemochromatosis [29]; however, this study was limited by the inclusion of very few patients with primary hemochromatosis, and only one patient was found to have hyporeninemic hypoaldosteronism [29]. None of the patients in our cohort had reduced A and/or PRA levels.
Our association analyses of TBIL surrogates also revealed novel findings. Annualized median ferritin and LIC values had significantly lower sensitivity and specificity for predicting the presence of ACH in patients with thalassemia than cardiac MRI T2* iron. In addition, cardiac MRI T2* iron was the only TBIL surrogate that predicted ACH severity with a sensitivity of 81 % and specificity of 78 % at a cut-off value of 20.6 ms. Serum ferritin and LIC were not reliable predictors of ACH in the current study. This finding is supported by ample evidence from recent studies [30–32]. Although serum ferritin trends remain an important monitoring tool, serum ferritin is a poor marker of iron balance because it varies with inflammation, ascorbate status, the intensity of transfusion therapy, other disorders, and liver damage [30]. Once elevated, serum ferritin levels are related to the degree of iron excess and especially to hepatic iron content. However, an absolute increase in serum ferritin is more important when iron deposits are located in the reticuloendothelial system than when they are located in parenchymal cells [31]. The liver is the dominant iron-storage depot for the body; it has high-capacity mechanisms for clearing both transferrin- and nontransferrin-bound iron (NTBI) species from the circulation. In contrast, the heart and endocrine tissues have tightly regulated transferrin uptakes and develop iron overload only in the presence of circulating NTBI. NTBI rebounds whenever iron chelators are not present because transferrin is nearly always saturated in TM. Although a retrospective study documented the association of liver iron with adrenal iron in a small number of patients [33], from a cardiac and endocrine perspective, no amounts of liver iron are currently considered safe and extrahepatic monitoring using MRI is essential [32]. The gold standard approach to screening tissue iron content is MRI T2* iron assessment of endocrine organs, but this procedure is costly and impractical. Herein, we suggest that cardiac MRI T2*iron, which must be evaluated to determine iron toxicity to the heart, would be a good surrogate of ACH.
Our study was not without limitations. We did not attempt to analyze the associations between biochemical alterations in adrenal cortex functions and symptoms of AI because of the high possibility that similar symptoms would be present (because of chronic anemia, for example). The possible effect of residual confounding factors on the findings of this study is an inherent weakness of any cross-sectional design. Nevertheless, this cross-sectional study was suitable for analyzing the prevalence of ACH in a large cohort of patients with thalassemia and for identifying associations between ACH status and TBIL surrogates after adjustment for potential confounders, even if it was not designed to establish causal relationships.
In conclusion, our study showed a high prevalence of subtle mineralocorticoid deficiency in transfusion-dependent patients with β-TM. Although TBIL surrogates are associated with ACH, cardiac MRI T2* iron is a better surrogate of the presence and severity of ACH in patients with thalassemia.
References
V. De Sanctis, N. Skordis, A. Soliman, Endocrine disease, in Guidelines for the Management of Transfusion Dependent Thalassemia, 3rd edn., ed. by M.D. Cappellini, A. Cohen, J. Porter, A. Taher, V. Viprakasit (Thalassemia International Federation, Nicosia, 2014), pp. 146–158
V. De Sanctis, C. Pintor, Italian Working Group on Endocrine Complications in Non-Endocrine Disease, Multicenter study on prevalence of endocrine complications in thalassemia major. Clin. Endocrinol. 42, 581–586 (1995)
H.H. Elsedfy, M. El Kholy, R. Tarif, A. Hamed, M. Elalfy, Adrenal function in thalassemia major adolescents. Pediatr. Endocrinol. Rev. 8(Suppl 2), 295–299 (2011)
A. Jacobs, Iron overload-clinic and pathologic aspects. Semin. Hematol. 14, 89–113 (1977)
A. Incebiyik, A. Genc, N.G. Hilali, A. Camuzcuoglu, H. Camuzcuoglu, A. Kilic, M. Vural, Prevalence of β-thalassemia trait and abnormal hemoglobins in Sanliurfa Province in Southeast Turkey. Hemoglobin 38, 402–404 (2014)
T.J. Cole, M.C. Bellizzi, K.M. Flegal, W.H. Dietz, Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320, 1240–1243 (2000)
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents Pediatrics 114, 555–576 (2004)
R.M. Lang, M. Bierig, R.B. Devereux, F.A. Flachskampf, E. Foster, P.A. Pellikka, M.H. Picard, M.J. Roman, J. Seward, J.S. Shanewise, S.D. Solomon, K.T. Spencer, M. St John Sutton, W.J. Stewart, Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 18, 1440–1463 (2005)
M. Westwood, L.J. Anderson, D.N. Firmin, P.D. Gatehouse, C.C. Charrier, B. Wonke, D.J. Pennell, A single breathold multiecho T2* cardiovascular magnetic resonance technique for diagnosis of myocardial iron overload. J. Magn. Reson. Imaging 18, 33–39 (2003)
J.C. Wood, C. Enriquez, N. Ghugre, J.M. Tyzka, S. Carson, M.D. Nelson, T.D. Coates, MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 106, 1460–1465 (2005)
J.S. Hankins, M.B. McCarville, R.B. Loeffler, M.P. Smeltzer, M. Onciu, F.A. Hoffer, C.S. Li, W.C. Wang, R.E. Ware, C.M. Hillenbrand, R2* magnetic resonance imaging of the liver in patients with iron overload. Blood 113(20), 4853–4855 (2009)
C.B. Barra, I.N. Silva, T.M. Rodrigues, J.L. Santos, E.A. Colosimo, Morning serum basal cortisol levels are affected by age and pubertal maturation in school-aged children and adolescents. Horm. Res. Paediatr. 83, 55–61 (2015)
B. Kirkwood, J. Sterne, Essential Medical Statistics, 2nd edn. (Blackwell Publishers, Oxford, 2003)
J.A. Hanley, B.J. McNeil, A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148, 839–843 (1983)
L.M. Sherief, S.M.A. El-Salam, N.M. Kamal, O. El safy, M.A.A. Almalky, S.F. Azab, H.M. Morsy, A.F. Gharieb, Nutritional biomarkers in Children and Adolescents with Beta-thalassemia major: an Egyptian center experience. Biomed. Res. Int. (2014). doi:10.1155/2014/261761
N. Skordis, M. Michaelidou, S.C. Savva, Y. Ioannou, A. Rousounides, M. Kleanthous, G. Skordos, S. Christou, The impact of genotype on endocrine complications in thalassaemia major. Eur. J. Haematol. 77, 150–156 (2006)
V. De Sanctis, A. Soliman, G. Candini, M. Yassin, G. Raiola, M.C. Galati, R. Elalaily, H. Elsedfy, N. Skordis, P. Garofalo, S. Anastasi, S. Campisi, M. Karimi, C. Kattamis, D. Canatan, Y. Kılınc, P. Sobti, B. Fiscina, M. El Kholy, Insulin like growth factor-1 (IGF-1): demographic, clinical and laboratory data in 120 consecutive adult patients with thalassemia Major. Mediterr J Hematol Infect Dis (2014). doi:10.4084/MJHID.2014.074
P. Nakavachara, V. Viprakasit, Adrenal insufficiency is prevalent in HbE/β-thalassaemia paediatric patients irrespective of their clinical severity and transfusion requirement. Clin. Endocrinol. 79, 776–783 (2013)
A. Soliman, M. Yassin, N.M.S.A. Majuid, A. Sabt, M. Abdulrahman, V. De Sanctis, Cortisol response to low dose versus standard dose (back-to-back) adrenocorticotrophic stimulation tests in children and young adults with thalassemia major. Indian J. Endocr. Metab. 17, 1046–1052 (2013)
S. Mohammadian, U.R. Bazrafshan, A. Sadeghi-Nejad, Endocrine gland abnormalities in thalassemia major: a brief review. J. Pediatr. Endocrinol. Metab. 16, 957–964 (2003)
P. Poomthavorn, B. Isaradisaikul, A. Chuansumrit, P. Khlairit, A. Sriphrapradang, P. Mahachoklertwattana, High prevalence of “biochemical” adrenal insufficiency in thalassemics: Is it a matter of different testings or decreased cortisol binding globulin? J. Clin. Endocrinol. Metab. 95, 4609–4615 (2010)
K.E. Huang, S.D. Mittelman, T.D. Coates, M.E. Geffner, J.C. Wood, A significant proportion of thalassemia major patients have adrenal insufficiency detectable on provocative testing. J. Pediatr. Hematol. Oncol. 37, 54–59 (2015)
M.G. Forest, Adrenal function tests, in Diagnostics of endocrine function in children and adolescents, 3rd edn., ed. by M.B. Ranke (Karger, Basel, 2003), pp. 372–426
N. Lytras, A. Grossman, L. Perry, S. Tomlin, J.A.H. Wass, D.H. Coy, A.V. Schally, L.H. Rees, G.M. Besser, Corticotrophin releasing factor: responses in normal subjects and patients with disorders of the hypothalamus and pituitary. Clin. Endocrinol. 20, 71–84 (1984)
A. Uçar, Age-related serum dehydroepiandrosterone sulphate (DHEAS) levels per se should not be considered a reliable surrogate parameter for clinical presentation of adrenarche. Clin. Endocrinol. 82, 912–913 (2015)
G.B. Gordon, D.E. Bush, H.F. Weisman, Reduction of atherosclerosis by administration of dehydroepiandrosterone. A study in the hypercholesterolemic New Zealand white rabbit with aortic intimal injury. J. Clin. Invest. 82, 712–720 (1988)
E.M. Gurnell, P.J. Hunt, S.E. Curran, C.L. Conway, E.M. Pullenayegum, F.A. Huppert, J.E. Compston, J. Herbert, V.K.K. Chatterjee, Long-term DHEA replacement in primary adrenal insuffi ciency: a randomized, controlled trial. J. Clin. Endocrinol. Metab. 93, 400–409 (2008)
P.J. Hunt, E.M. Gurnell, F.A. Huppert, C.A. Richards, T. Prevost, J.A.H. Wass, J. Herbert, V.K. Chatterjee, Improvement in mood and fatigue after dehydroepiandrosterone replacement in Addison’s disease in a randomized, double blind trial. J. Clin. Endocrinol. Metab. 85, 4650–4656 (2000)
L.M.C. Hempenius, P.S. Van Dam, J.J.M. Marx, H.P.F. Koppeschaar, Mineralocorticoid status and endocrine dysfunction in severe hemochromatosis. J. Endocrinol. Invest. 22, 369–376 (1999)
M.I. Argyropoulou, L. Astrakas, MRI evaluation of tissue iron burden in patients with β-thalassaemia major. Pediatr. Radiol. 37, 1191–1200 (2007)
J. Carpenter, T. He, P. Kirk, M. Roughton, L.J. Anderson, S.V. Noronha, M.N. Sheppard, J.B. Porter, J.M. Walker, J.C. Wood, R. Galanello, G. Forni, G. Catani, G. Matta, S. Fucharoen, A. Fleming, M.J. House, G. Black, D.N. Firmin, T.G. St. Pierre, On T2* magnetic resonance and cardiac iron. Circulation 123, 1519–1528 (2011)
J.C. Wood, Impact of iron assessment by MRI. Hematology 1, 443–450 (2011)
E. Drakonaki, O. Papakonstantinou, T. Maris, A. Vasiliadou, A. Papadakis, N. Gourtsoyiannis, Adrenal glands in beta-thalassemia major: magnetic resonance (MR) imaging features and correlation with iron stores. Eur. Radiol. 15, 2462–2468 (2005)
Acknowledgments
The authors would like to thank all of the patients and parents included in the study. The authors would also like to thank Dr. Hüseyin Demirbilek for his critical review of the manuscript and David Chapman for editing the manuscript. The authors also thank Omega Statistics, Ltd., and Mark Behar for the statistical analyses, the Sanliurfa Thalassemia Society for partial financial support, and the thalassemia nurses Ayfer Aşkın and Harun Cagan for their active involvement in recruiting patients for the study.
Author contributions
A.U. conceptualized and designed the study. A.U., N.O., G.O., A.Y., M.A., A.Y., Y.Y. M.G.C., and C.K. participated in the recruitment of the study subjects. A.U., H.C.E., N.O. and G.O. participated in the data interpretation, statistical analysis, writing, and critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors have nothing to disclose.
Rights and permissions
About this article
Cite this article
Uçar, A., Öner, N., Özek, G. et al. Evaluation of the glucocorticoid, mineralocorticoid, and adrenal androgen secretion dynamics in a large cohort of patients aged 6–18 years with transfusion-dependent β-thalassemia major, with an emphasis on the impact of cardiac iron load. Endocrine 53, 240–248 (2016). https://doi.org/10.1007/s12020-016-0872-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-0872-2