Abstract
The lingual thyroid is the most common form of thyroid ectopy. The ectopic tissue may display any disease affecting the thyroid, including malignancies, which have an estimated incidence of less than 1 %. To date only 51 cases of lingual thyroid cancer were reported. Analogously to what observed in orthotopic thyroid, papillary carcinoma is the predominant histotype in lingual thyroid carcinoma. The higher frequency of lingual follicular thyroid carcinoma previously reported is possibly related to histological misclassification in some early reports, prior to the standardization of histological typing of differentiated thyroid carcinomas. Nonetheless, the frequency of the follicular histotype is not negligible, accounting for about one-third of the reported cases. Both natural history and prognosis of lingual thyroid carcinoma are poorly known, likely because of the rarity of the disease and the heterogeneity in the therapeutic approach. However, among the cases more recently reported, surgical excision of the mass, either alone or followed by radioiodine ablation, is the first-line approach, with only two cases treated by radioiodine alone. The nonsignificant rate of neoplastic transformation in lingual thyroid should encourage efforts to obtain a widely accepted consensus for the management of this rare condition, along with standardization of either diagnostic or therapeutic handling of malignancies arising in ectopic thyroid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The thyroid gland is defined as being ectopic when it is not located in the anterior portion of the neck, between the second and the fourth tracheal cartilages [1]. Ectopic thyroid tissue is most frequently found along the midline from the base of the tongue to the mediastinum, the former representing the predominant aberrant location [2]. Although lingual thyroid (LT) is in most cases asymptomatic, any disease affecting the thyroid may involve the ectopic tissue, including malignancies [3–5]. Since 1910 [6, 7], only 51 cases of thyroid cancer arising from LT have been reported, thus indicating this condition to be an extremely rare entity. The infrequency of LT cancer (LTC) makes characterization of the natural history and biological behavior of this condition rather difficult. Presently available data would indicate the prognosis for LTC to be worse than that reported for patients with differentiated thyroid cancer arising in orthotopic thyroid, possibly because of a slightly higher prevalence of the follicular histotype in the former [5]. This article is aimed at shortly reviewing the literature on this specific topic and reporting a new case of follicular thyroid cancer arising in LT.
Data source
The terms “ectopic thyroid” and “lingual thyroid” were used both separately and in conjunction with the terms “cancer,” “carcinoma,” “malignancy(ies),” and “tongue” to search MEDLINE for articles published between 1910 and 2014. Studies reporting cases of lingual thyroid cancer were then reviewed to identify details of interest for the purposes of this review. Reported cases of thyroid malignancies of doubtful lingual origin or cancers arising from lingual tissue and coexisting with and/or infiltrating normal lingual thyroid tissue were excluded.
Clinical case
A 63-year-old female was first admitted to our Unit in April 2009 with a swelling at the base of her tongue. Her personal medical history included a diagnosis of “angioma of the tongue base” made when she was 17, on the basis of an otorhinolaryngological evaluation. Over the years there was a gradual increase in the size of the swelling and the woman experienced dysphagia when eating and drinking. In July 2008, the patient underwent oropharingoscopy, which revealed a walnut-size lesion resembling a fibroangioma at the base of the tongue. A contrast-enhanced computed tomography (CT) scan showed a mass extending to the posterior 2/3 of the tongue and displaying intense contrastografic enhancement in both the arterial and the venous phases (Fig. 1). In August 2008, the patient underwent partial CO2 laser excision of the lingual lesion. Histological examination revealed the lesion to be an invasive follicular thyroid carcinoma with insular-like areas. Thyroid function tests revealed normal serum thyrotropin (TSH) (1.9 mU/L, normal range 0.4–4.0) and free thyroxine (FT4) (14.4 pmol/L, normal range 10.3–24.4) levels. Post-operative neck ultrasonography (US) did not detect orthotopic thyroid tissue, and a 99m−Tc scintiscan showed specific and intense radioisotope uptake in two delineated areas in the inferior half of the tongue, with no uptake in the anterior region of the neck (Fig. 2). A CT scan of the neck revealed partial excision of the cranial portion of the lesion. In March 2009, our patient underwent partial suprahyoid glossectomy of a massive (43 × 33 × 8 mm) lingual lesion. Histology revealed an infiltrating adenocarcinoma displaying the cytological and architectural features of a follicular thyroid carcinoma (Fig. 3a). Morphological characteristics were confirmed by immunohistochemical evidence of extensive thyroglobulin (Tg) and thyroid transcription factor 1 (TTF-1) positivity in the cancer cells (Fig. 3b, c). Electron microscopy also revealed atypical thyrocytes exhibiting thin and discontinuous basement membranes as well as inversion of polarity (Fig. 3d, e). The final diagnosis was of infiltrating follicular carcinoma in lingual thyroid (pT3NxMx).
Widespread mass showing intense contrastografic enhancement and occupying the posterior 2/3 portion of the tongue. The lesion consisted of two components (red arrows), the first (35 × 25 mm) extending from the median part of the base to the right side of the anterior 2/3 of the tongue, and the second (30 × 25 mm) occupying the deep lingual base up to the hyoid bone, thus inducing minimal reduction in the oropharyngeal lumen
One month later, Tg levels as high as 1440 ng/mL and radioiodine lingual uptake of 10 % at 24 h (TSH 94.2 mU/L; TgAb negative) were detected. Furthermore, US revealed multiple cervical lymph nodes at levels II, III, and V.
In May 2009, a further local intervention, along with the removal of 50 lymph nodes from the II, III, and V left levels, revealed several areas of neoplastic infiltration of follicular carcinoma at the basis of the tongue and metastasis of the follicular carcinoma in 1/50 lymph nodes examined. Subsequent oropharingoscopic evaluation showed complete removal of the lingual lesion (Fig. 4).
Because of the follicular histotype, the extension of the lesion, and the lymph node metastasis, in July 2009, a radiometabolic treatment with 3.7 GBq of 131I, following levothyroxine withdrawal (TSH 201 mU/L; anti-thyroglobulin antibodies (TgAb) 1.18 IU/mL; Tg 497 ng/mL), was performed. The 24/h cervical uptake was 10 %.
Post-treatment whole-body scintigraphy (pT-WBS), performed 5 days following radioiodine administration, documented multiple accumulations of the radioisotope in the hyoid and submandibular left region (Fig. 5a, b). Neck ultrasound was negative for suspicious lymph nodes. In February 2010, the contrast-enhanced CT scan of the neck and chest revealed the presence of a 12-mm lymph node with a small central hypodense area in the left paratracheal region. In July 2010, an additional activity of 131I (4.6 GBq) on levothyroxine withdrawal (TSH 176.2 mU/L, TgAb 5.14 IU/mL; Tg 18 ng/mL) was administered to the patient. The second pT-WBS showed a slight and focal radioiodine uptake, corresponding to the lymph node lesion detected on CT scan (Fig. 5c, d). Over a 5-year follow-up period, either US evaluation of the neck or recombinant human TSH (rh-TSH) stimulated Tg values proved the disease to be persistently cured.
Anterior (a) and posterior (b) views of pT-WBS (July 2009). Two well-defined and intense radioiodine uptake were detectable in the hyoid region. Slight radioiodine uptake was also evident in submandibular left region (black arrow). Anterior (c) and posterior (d) views of pT-WBS (July 2010). A slight and focal radioiodine uptake was evident in the cervical anterior region (black arrow). pT-WBS scans were obtained 5 days after radioiodine administration using a double-headed gamma camera (Millennium VG, GE Medical System) equipped with high-energy low-resolution parallel hole collimators (HEHRPAR). Whole-body images were obtained from head to proximal thighs (anterior and posterior views, matrix 256 × 256, magnification: 1, acquisition time: 10 cm/min)
Thyroid ectopy: genetic factors, epidemiology, and clinical features
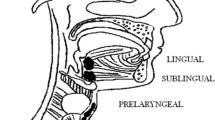
During embryonic life, the developing thyroid migrates from the thyroid anlage region to its definitive location in front of the trachea, leaving behind the foramen cecum. Occasionally, the thyroid primordium (or a portion of it) fails to descend throughout the normal pathway leading to disturbances in thyroid organogenesis, finally resulting in a gland located in an unusual position (thyroid ectopy), hypoplastic (thyroid hypoplasia), or absent (thyroid agenesis) [1]. All this conditions are grouped under the term “thyroid dysgenesis” (TD), and represent 80–85 % of the cases of congenital hypothyroidism [8]. The most common ectopic location is the tongue (90 % of reported cases), followed by the submandibular area, larynx, trachea, esophagus, mediastinum, diaphragm, and heart [2].
Although TD is generally assumed to be a sporadic disease, there is evidence that in about 5 % of cases genetic factors are involved in its pathogenesis. Molecular studies demonstrated that the transcription factors TITF1/NKX2-1, FOXE1, PAX8, and HHEX are expressed in both mature thyroid cells and their precursors, and that all of them are co-expressed in the thyroid anlage only. This co-expression, at the very beginning of thyroid morphogenesis, suggests that these genes might actually play an important role in the organogenesis of the thyroid gland. Gene-targeting experiments also demonstrated that FOXE1 is required for thyroid migration and that mice homozygous for Foxe1 mutations show a sublingual thyroid [9, 10].
So far, no mutation in known genes has been associated with the human ectopic thyroid, which represents more than 50 % of TD.
The prevalence of LT is estimated between 1:100,000 and 1:300,000, with a female/male ratio of 3:1–8:1. However, the reported prevalence of this dysembriogenetic defect increases to 1:4000–8000 when patients with known thyroid diseases are considered [11]. Also, the presence of thyroid-like inclusions was found at histological examination of cadaver tongues in almost 10 % of the examined specimens, with no significant differences in sex distribution [12].
An overall figure of less than 500 symptomatic cases has been described to date [2, 5, 13]. In 70 % of cases, the LT is the only functionally active thyroid tissue and most patients display mild thyroid insufficiency [5], which often becomes clinically manifest during periods of physiological increased demand of thyroid hormone. In accordance, although symptoms may occur at any age, most LT cases are detected during puberty and pregnancy [11, 13, 14]. Symptoms are mostly related to the growth of the lingual mass, and include sensation of foreign body, dysphagia, dysphonia, cough, snoring, and, in more severe cases, respiratory obstruction and bleeding [2].
LT may be clinically suspected when examination reveals the presence of a median lingual mass. The most sensitive diagnostic tool to confirm the diagnosis of LT is radionuclide imaging [11, 15, 16]. It is interesting to note that the LT tissue shows a rapid turnover of iodine, with a half-life much shorter than normal thyroid tissue [17], thus explaining the resistance of LT tissue to radioiodine ablation occasionally reported in the literature [18]. When LT uptake is confirmed, cervical activity is absent in most cases, and US may either confirm the absence of a normal thyroid gland in the pre-tracheal site or visualizing the ectopic thyroid tissue at the base of the tongue. Both color doppler US and B-flow imaging have been proposed as useful tools for assessing ectopic thyroid vascularity [11, 19]. Finally, CT or magnetic resonance imaging (MRI) scans are usually performed to delineate the extension of the mass, mainly pre-operatively. Initial diagnostic investigations should also include thyroid function tests.
Lingual thyroid cancer: epidemiology, clinical presentation, and management
Carcinoma arising from LT is rare. Its incidence is about 1 %, with a female/male ratio of approximately 2:1 and a higher prevalence in the third decade of life [5, 20]. Follicular carcinoma is usually reported as being the predominating histopathology in LTC [10, 20–24]. However, among the 51 anecdotal cases of LTC described so far (Table 1), histological findings range from equivocal descriptions of malignancies [6, 7, 25–44], ultimately dating back from the beginning of the last century to early 70s, to detailed histopathological diagnoses of differentiated thyroid carcinoma [20–23, 45–65]. Tightening our focus on LTC diagnosed following the release of standardized criteria of histological typing [66], the available data show that papillary thyroid carcinoma (PTC) is actually the prevailing type of LTC, accounting for about 65 % of the reported cases. Nonetheless, the papillary to follicular ratio still remains higher than that observed in eutopic thyroid (approximately 2:1 in LT versus 4:1 in orthotopic gland [67]). Some authors suggested this difference to be related to the long history of hypothyroidism and compensatory hyperplasia due to the absence of the orthotopic thyroid [24], thus resembling the higher occurrence of more aggressive cancers reported in iodine-deficient areas [68, 69]. Also, the hypothesis of histological misclassification in some early reports, at a time when the follicular variant of papillary thyroid carcinoma was classified as follicular, has been advocated [24, 55].
So far, no cases of anaplastic LTC have been reported. Interestingly, one case of medullary carcinoma has been described, and its pathogenesis explained by an aberrant migration to the tongue tissue of occasionally parafollicular C-cells [61].
No specific risk factors for LTC are described in the literature, and the same factors as are responsible for neoplastic transformation in the cervical thyroid are likely involved in LTC tumorigenesis [5].
The natural history of LTC remains largely unknown due to the rarity of the condition. Like papillary tumors arising from orthotopic thyroid tissue, lingual PTC shows an indolent clinical course and a tendency to lymphatic and especially cervical diffusion [2, 5, 20, 70]. In contrast, hematogenous metastases are more common in follicular carcinoma. In most of the cases reported, the neoplasia was confined to the tongue, and locoregional lymph node metastases and distant metastases (usually confined to the lung or mediastinum) accounted for 20 and 14 % of cases, respectively [5]. The coexistence of LTC with a squamous cell carcinoma of the tongue was reported in one patient whose LTC histotype was a PTC [71].
Early diagnosis of LTC is rather difficult as, if any, symptoms are aspecific and may include hoarseness, dyspnea, or the perception that a foreign body is present due to pressure from the mass. Less frequently, the neoplasm may cause dysphagia and/or hemoptysis. Clinical signs include the presence of an oral mass, with or without bleeding. Differential diagnosis includes symptomatic LT, thyroglossal duct cysts, lipoma, lymphangioma, hemangioma, fibroma, lingual tonsil hypertrophy, mucous and dermoid cysts, squamous cell carcinoma and lymphoma [2].
A biopsy of the lesion is required to distinguish the LTC from normal LT tissue [5], even though cytological diagnosis may not be easy. Indeed, detailed histological studies have shown that the LT tissue characteristically has an incomplete or poorly defined capsule. Thus, normal lingual thyroid tissue may erroneously be considered malignant due to the muscle invasion that may occur as a result of a defect in the capsule, which leads, in turn, to a fusion of muscle and glandular elements [11, 72]. When the biopsy is suggestive of lingual thyroid malignancy, the further management is basically the same as for carcinoma arising in orthotopic thyroid gland. Accordingly, preoperative neck US is recommended to identify suspicious cervical masses, and US-guided fine-needle aspiration of sonographically suspicious lymph nodes should be performed to confirm malignancy and plan the most appropriate therapeutic approach. Differently from cancer arising in orthotopic thyroid, routine preoperative use of other noninvasive imaging technique (CT or MRI) is recommended. Although MRI is more expensive and technically demanding compared to CT, it offers less radiation exposure and is particularly helpful in differentiating thyroid tissue from tongue muscle [20].
Because of the rarity of the condition there is no consensus in the literature regarding the most appropriate therapeutic approach [2, 5, 14, 20]. Surgical excision with wide margins is usually recommended as a first-line therapy, whereas a neck dissection is indicated only in the presence of additional suspected lesions or metastatic disease [73]. The surgical procedure can be performed by a number of techniques, including lateral pharyngotomy, trans-hyoid incision or trans-oral intervention [11], the last-named being the more frequently used approach reported in the literature. In addition to the poor visualization of large tumor masses that this access entails, the increased risk of bleeding from the lingual arteries should also be taken into account [74]. In a comparison of different surgical options, Prasad and Bhat [75] argued the trans-oral approach to be the easiest and to offer the best cost-benefit ratio. However, in the case of large masses that extend deep into the tongue, an external approach is indicated. Recently, some authors described a minimally invasive trans-oral approach that includes harmonic technology and high-resolution endoscopy, which can be accomplished on an outpatient stay [76]. Post-operative edema was frequently reported, in many cases requiring a temporary tracheotomy. Finally, the use of both trans-oral robotic surgery (TORS) and CO2 laser for excision of ectopic lingual thyroid tissue has been recently proposed, as they offer advantages over traditional surgical techniques in terms of lesser bleeding and post-operative morbidity [77–80].
To date, few reports have been published of cases that allowed complete surgical removal of the LTC and high-dose 131I ablation treatment should be considered where there is involvement of surgical margins by tumor cells or more advanced disease [5, 52]. Treatment with 131I alone should be limited to large cancers in which surgery would be invalidating and to those patients who refuse surgery. In literature two cases of LTC are described in which 131I ablation was used as a first-line treatment and resulted in reduction of tumor size, resolution of symptoms, and no further evidence of disease progression in one of them [20, 44].
In addition to their diagnostic usefulness in detecting the extension of the neoplasia, both CT and MRI are suitable tools in early postsurgical/radioiodine follow-up, as they provide complementary information to radionuclide imaging scans on actual completeness of surgical resection or reduction in tumor size [20].
As stated above, about 30 % of patients with lingual thyroid have also an orthotopic gland. In such circumstances, when LTC is diagnosed, total removal of the orthotopic gland should be performed in order to permit the further follow-up of thyroid cancer by means of either suppressed or stimulated Tg levels and diagnostic/therapeutic use of 131I.
Concerning the long-term management, the same recommendations pertaining to thyroid cancer arising from orthotopic gland [81] are reasonably suitable for lingual thyroid cancer. Accordingly, post-operative staging is crucial to tailor appropriate management, including RAI therapy and TSH suppression, as well as surveillance for recurrent and metastatic disease.
Conclusions
The lingual thyroid is the most common form of thyroid ectopy and, although rarely, it can harbor differentiated thyroid cancer. As in orthotopic thyroid, papillary carcinoma is the predominating histopathology in LTC. Nonetheless, the frequency of follicular carcinoma is not negligible, accounting for about one-third of the reported cases. A combination of radiological assessments, including scintiscan, ultrasound scan, magnetic resonance imaging, and bioptic evaluation, should be performed in the presence of all suspicious lingual masses, especially in hypothyroid patients. When a suspicious lesion is detected, surgery should be offered as a first-line treatment in order to confirm the diagnosis of malignancy. In the lack of evidence-based recommendations, we believe that procedures pertaining to follow-up of thyroid cancer arising from orthotopic gland (levothyroxine therapy, imaging, and periodic Tg assessment) may also be reasonably applied to patients diagnosed with LTC. The nonsignificant rate of neoplastic transformation in this dysembriogenetic thyroid lesion should probably encourage efforts to obtain a widely accepted consensus for the management of this rare condition.
References
J.E. Pintar, Normal development of the hypothalamic-pituitary-thyroid axis, in Werner and Ingbar’s the thyroid: a fundamental and clinical text, 8th edn., ed. by L.E. Braverman, R.D. Utiger (Lippincott William and Wilkins, Philadelphia, 2000), pp. 7–19
G. Noussios, P. Anagnostis, D.G. Goulis, D. Lappas, K. Natsis, Ectopic thyroid tissue: anatomical, clinical, and surgical implications of a rare entity. Eur. J. Endocrinol. 3, 375–382 (2011)
R.J. Paresi Jr, D. Saha, Hashimoto’s thyroiditis presenting as an enlarging submandibular mass in a patient with lingual thyroid. Otolaryngol. Head Neck Surg. 132, 806–808 (2005)
M.J. Jacob, M. Ravina, A rare case of lingual thyroid with hyperthyroidism: a case report and review of the literature. Indian J. Endocrinol. Metab. 3, 441–443 (2012)
J. Klubo-Gwiezdinska, R.P. Manes, H.S. Chia, K.D. Burman, N.A. Stathatos, Z.E. Deeb, L. Wartofsky, Ectopic cervical thyroid carcinoma: review of the literature with illustrative case series. J. Clin. Endocrinol. Metab. 96, 2684–2691 (2011)
L.G. Gunn, Thyroid tumour. Trans. R. Acad. Med. Ire. 28, 381 (1910)
M. Rutgers, Tongstruma. Ned. Tijdschr. Geeneeskd. 1b, 1505–1506 (1910)
D.A. Fisher, A. Klein, Thyroid development and disorders of thyroid function in the newborn. N. Engl. J. Med. 304, 702–712 (1981)
M. De Felice, R. Di Lauro, Thyroid development and its disorders: genetic and molecular mechanism. Endocr. Rev. 25, 722–746 (2004)
I.C. Nettore, V. Cacace, C. De Fusco, A. Colao, P.E. Macchia, The molecular causes of thyroid dysgenesis: a systematic review. J. Endocrinol. Invest. 36, 654–664 (2013)
G. Guerra, M. Cinelli, M. Mesolella, D. Tafuri, A. Rocca, B. Amato, S. Rengo, D. Testa, Morphological, diagnostic and surgical features of ectopic thyroid gland: a review of literature. Int. J. Surg. 12, S3–S11 (2014)
R.A. Baughman, Lingual thyroid and lingual thyroglossal tract remnants: a clinical and histopathological study with review of the literature. Oral Surg. 34, 781–798 (1972)
J.S. Yoon, K.C. Won, I.H. Cho, J.T. Lee, H.W. Lee, Clinical characteristics of ectopic thyroid in Korea. Thyroid 17, 1117–1121 (2007)
N.A. Ibrahim, I.O. Fadeyibi, Ectopic thyroid: etiology, pathology and management. Hormones (Athens) 10, 261–269 (2011)
K.A. Warnakulasuriya, K.B. Herath, Investigating a lingual thyroid. Int. J. Oral Maxillofac. Surg. 21, 227 (1992)
C. Aktolum, H. Demir, F. Berk, K. Metin Kir, Diagnosis of complete ectopic lingual thyroid with Tc-99 pertechnetate scintigraphy. Clin. Nucl. Med. 26, 933–935 (2001)
A. Ramos-Gabatin, H.T. Pretorius, Radionuclide turnover studies on ectopic thyroid glands-case report and survey of the literature. J. Nucl. Med. 26, 258–262 (1985)
S. Basaria, H.W. Westra, D.S. Cooper, Ectopic lingual thyroid masquerading as thyroid cancer metastases. J. Clin. Endocrinol. Metab. 86, 392–395 (2001)
H. Ohnishi, H. Sato, H. Noda, H. Inomata, N. Sasaki, Color Doppler ultrasonography: diagnosis of ectopic thyroid gland in patients with congenital hypothyroidism caused by thyroid dysgenesis. J. Clin. Endocrinol. Metab. 88, 5145–5149 (2003)
R.E. Massine, S.J. Durning, T.M. Koroscil, Lingual thyroid carcinoma: a case report and review of literature. Thyroid 12, 1191–1196 (2001)
C.K. Hari, M. Kumar, M.M. Abo-Khatwa, J. Adams-Williams, H. Zeitoun, Follicular variant of papillary carcinoma arising from lingual thyroid. Ear Nose Throat J. 88, E7 (2009)
P. Gooder, Follicular carcinoma in a lingual thyroid. J. Laryngol. Otol. 94, 437–439 (1980)
A.A. Diaz-Arias, J.T. Bickel, T.S. Loy, G.H. Croll, C.L. Puckett, A.D. Havey, Follicular carcinoma with clear cell change arising in lingual thyroid. Oral Surg. Oral Med. Oral Pathol. 74, 206–211 (1992)
J.M. Seoane, J. Cameselle-Teijeiro, M.A. Romero, Poorly differentiated oxyphilic (Hürthle cell) carcinoma arising in lingual thyroid: a case report and review of the literature. Endocr. Pathol. 13, 353–360 (2002)
H. Brentano, Struma Aberrata Linguae mit Drusen metastasen. Deutsche Med. Wchnschr. 37, 665–666 (1911)
W.K. Seeyle, Thyroglosal tumor (aberrant thyroid): report of case. Northwest Med. 15, 334–335 (1916)
H.M. Ray, Carcinomatous transformation of a lingual goiter. Proc. NY Pathol. Soc. 18, 12–14 (1918)
G. Hofer, Tumor am Zungengrund. Wien Med. Wochenschr 70, 476 (1920)
A.F. Tyler, Carcinoma of lingual thyroid with metastasis to the lungs. J. Radiol. 4, 381–384 (1923)
A.P.C. Ashurst, C.Y. White, Carcinoma in an aberrant thyroid at base of tongue. JAMA 85, 1219 (1925)
G.C. Peracchia, Struma maligno. Clin. Chir. 30, 209–224 (1927)
L. Moulonguet, Deux cas de tumeur thyroidienne rares. Ann. Mal. Oreille Larynx 49, 1005–1009 (1930)
J. Caderas, Les tumeurs malignes du tractus thyreoglosse. Ann. Otolaringol. 3, 309–319 (1931)
G. Marchal, P. Soulie, C. Grupper, A. Roy, Adenocancer d’un vestige thyroidien de la langue. Metastases multiples avec aneurisme arterio-vieneux de l’humerale. Bull. Mem. Soc. Med. Hop. Paris. 51, 953–958 (1935)
L.M. Levi, F.D. Hankins, Carcinoma of lingual thyroid. Am. J. Cancer 23, 328–333 (1935)
W.L. Watson, J.L. Pool, Cancer of the thyroid. Surg. Gynec. Obstet. 70, 1037–1050 (1940)
W.J. Cromartie, O.G. Nelson, Adenocarcinoma of tongue arising from vestige of median anlage of thyroid gland. Arch. Surg. 43, 599–608 (1941)
H. Wapshaw, Lingual thyroid. A report of a case with unusual histology. Br. J. Surg. 30, 160–165 (1942)
J.M. Graham, R. McWhirter, Carcinoma of the thyroid. Proc. R. Soc. Med. 40, 669–680 (1947)
R.A. Willis, Pathology of tumours (Butterworth and Company, London, 1948)
H.J. Denecke, Zur malignen Zungengrundstruma. Arch. Ohr. Nas. Kehlkheilk. 157, 117–120 (1950)
D. Canciullo, G. Motta, I carcinomi tiroidei della base della lingua. Otorinolar. Ital. 23, 185–211 (1955)
U. Rubbiani, Adenocarcinoma linguale da tessuto tiroideo ectopico. Arch. Ital. Patol. Clin. Tum. 2, 1368–1377 (1958)
W. Mill, N.F.C. Growing, B. Reeves, D.W. Smithers, Carcinoma of the lingual thyroid treated with radioactive iodine. Lancet 1, 76–79 (1959)
G.G. Potdar, P.B. Desai, Carcinoma of the lingual thyroid. Laryngoscope 81, 427–429 (1971)
M.R. Kamat, J.N. Kulkarni, P.B. Desai, D.J. Jussawalla, Lingual thyroid: a review of 12 cases. Br. J. Surg. 66, 537–539 (1979)
H.B. Singh, H.C. Joshi, M. Chakravarty, Carcinoma of the lingual thyroid. Review and case report. J. Laryngol. Otol. 93, 839–844 (1979)
R. Hoffmann, B. Bubeck, F. Raue, Papillary thyroid carcinoma in a median ectopic thyroid associated with intra-abdominal thyroid tissue. Dtsch. Med. Wochenschr. 116, 654–658 (1991)
A. Betkowski, K. Gotkowska, M. Rzepka, Follicular carcinoma of the ectopic lingual thyroid. Otolaryngol. Pol. 47, 368–373 (1993)
G. Jayaram, A. Kakar, R. Prakash, Papillary carcinoma arising in sublingual ectopic thyroid concentrating both Tc-99m pertechnetate and I-131. Diagnosis by fine needle aspiration cytology. Clin. Nucl. Med. 20, 381–383 (1995)
I. Polo Tomás, O. Alemán López, J.J. LópezRico, J. Talavera Sánchez, C. Córdoba, Follicular carcinoma originating in the lingual thyroid: a case report. Acta Otorrinolaringol. Esp. 47, 407–410 (1996)
G. Bigotti, A. Coli, Follicular carcinoma in lingual thyroid presenting as a latero-cervical mass. Case report and review of the literature. J. Oral Pathol. Med. 26, 142–146 (1997)
C.P. Winslow, E.C. Weisberger, Lingual thyroid and neoplastic change: a review of the literature and description of a case. Otolaryngol. Head Neck Surg. 117, S100–S102 (1997)
A. Casella, R. Pisano, C. Navarro Cuellar, P. Llopis, R. Mallagray, G. Lavorgna, Papillary carcinoma of the base of the tongue. Case clinic. Minerva Stomatol. 48, 535–538 (1999)
S.Y. Kao, H. Tu, R.C. Chang, A.H. Yang, K.W. Chang, C.H. Lee, Primary ectopic thyroid papillary carcinoma in the floor of the mouth and tongue: a case report Br. J. Oral Maxillofac. Surg. 40, 213–215 (2002)
B. Goldstein, W.H. Westra, J. Califano, Multifocal papillary thyroid carcinoma arising in a lingual thyroid: a case report. Arch. Otolaryngol. Head Neck Surg. 128, 1198–1200 (2002)
J.S. Pérez, M. Muñoz, L. Naval, A. Blasco, F.J. Diaz, Papillary carcinoma arising in lingual thyroid. J. Craniomaxillofac. Surg. 31, 179–182 (2003)
L. Falvo, A. Berni, A. Catania, V. D’Andrea, S. Palermo, C. Giustiniani, E. De Antoni, Sclerosing papillary carcinoma arising in a lingual thyroid: report of a case. Surg. Today 35, 304–308 (2005)
A.M. Chaabouni, S. Intidhar Labidi, T. Kraiem, A. Gammoudi, A. Ladgham, F. Ben Slimane, Papillary-follicular carcinoma arising in a lingual thyroid. Ann. Otolaryngol. Chir. Cervicofac. 123, 199–202 (2006)
T.L. Kennedy, W.L. Riefkohl, Lingual thyroid carcinoma with nodal metastasis. Laryngoscope 117, 1969–1973 (2007)
J. Addams-Williams, M. Abo-Khatwa, H. Zeitoun, Papillary carcinoma arising in the lingual thyroid: a case report and review of the literature. Int. J Head Neck Surg. (2007). https://ispub.com/IJHNS/2/2/13603
S. Yaday, I. Singh, J. Singh, N. Aggarwal, Medullary carcinoma in a lingual thyroid. Singap. Med. J. 49, 251–253 (2008)
G. Raju, M. Kameswaran, Unusual presentations of thyroid malignancies: a case series. Indian J. Otolaryngol. Head Neck Surg. 61, 230–234 (2009)
K.M. Bhojwani, M.C. Hegde, A. Alva, K.V. Vishwas, Papillary carcinoma in a lingual thyroid: an unusual presentation. Ear Nose Throat J. 91, 289–291 (2012)
X.Q. Chen, R.H. Shi, J.T. Huang, Y.F. Zhao, Follicular variant of papillary carcinoma arising from lingual thyroid with orthotopic hypoplasia of thyroid lobes. J. Oral Maxillofac. Surg. 71, 644–648 (2013)
C. Hedinger, L.H. Sobin, Histological typing of thyroid tumours, in International Histological Classification of Tumours, World Health Organization, Geneva, 1974
E.L. Mazzaferri, R.T. Kloos, Current approaches to primary therapy for papillary and follicular thyroid cancer. J. Clin. Endocrinol. Metab. 86, 1447–1463 (2001)
A. Belfiore, G. La Rosa, G.A. La Porta, D. Giuffrida, G. Milazzo, L. Lupo, C. Regalbuto, R. Vigneri, Cancer risk in patients with cold thyroid nodules: relevance of iodine intake; sex; age; and multinodularity. Am. J. Med. 93, 363–369 (1992)
J.P. Monson, The epidemiology of endocrine tumours. Endocr. Rel. Cancer. 7, 29–36 (2000)
V.A. LiVolsi, K.H. Perzin, L. Savetsky, Carcinoma arising in a median ectopic thyroid tissue. Cancer 34, 1303–1315 (1974)
R.P. Bukachevsky, J.D. Casler, J. Oliver, J. Conley, Squamos cell carcinoma and lingual thyroid. Ear Nose Throat J. 70, 505–507 (1991)
C.Y. Wang, T.C. Chang, Preoperative thyroid ultrasonography and fine-needle aspiration cytology in ectopic thyroid. Am. Surg. 61, 1029–1031 (1995)
S.D. Weiss, C.C. Orlich, Primary papillary carcinoma of a thyroglossal duct cyst: report of a case and literature review. Br. J. Surg. 78, 87–89 (1991)
B.S. Atiyeh, A. Abdelnour, F.F. Haddad, Lingual thyroid: tongue-splitting incision for transoral excision. J. Laringol. Otol. 109, 520–524 (1995)
K.C. Prasad, V. Bhat, Surgical Management of lingual thyroid: a report of four cases. J. Oral Maxillofac. Surg. 58, 227–232 (2000)
D.J. Terris, M.W. Seybt, R.B. Vaughters, A new minimally invasive lingual thyroidectomy technique. Thyroid 20, 1367–1369 (2010)
M.A. Hafidh, P. Sheahan, N.A. Khan, M. Colreavy, C. Timon, Role of CO2 laser in the management of obstructive ectopic lingual thyroids. J. Laryngol. Otol. 118, 807–809 (2004)
R. Pellini, G. Mercante, P. Ruscito, G. Cristalli, G. Spriano, Ectopic lingual goiter treated by transoral robotic surgery. Acta Otorhinolaryngol. Ital. 33, 343–346 (2013)
E. Brittany, B.E. Howard, E.J. Moore, M.L. Hinni, Lingual thyroidectomy: the Mayo clinic experience with transoral laser microsurgery and transoral robotic surgery. Ann. Otol. Rhinol. Laryngol. 123, 183–187 (2014)
E. Prisman, A. Patsias, E.M. Genden, Transoral robotic excision of ectopic lingual thyroid: case series and literature review. Head Neck (2014). doi:10.1002/hed.23757
D.S. Cooper, G.M. Doherty, B.R. Haugen, R.T. Kloos, S.L. Lee, S.J. Mandel, E.L. Mazzaferri, B. McIver, F. Pacini, M. Schlumberger, S.I. Sherman, D.L. Steward, R.M. Tuttle, Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 19, 1167–1214 (2009)
Acknowledgments
The authors gratefully acknowledge Prof. Vittorio Cavallari, pathologist, for providing histological diagnosis and images, and Prof. Francesco Trimarchi for his erudite and critical review of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sturniolo, G., Violi, M.A., Galletti, B. et al. Differentiated thyroid carcinoma in lingual thyroid. Endocrine 51, 189–198 (2016). https://doi.org/10.1007/s12020-015-0593-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-015-0593-y