Abstract
Cerebral ischemic stroke is one of the foremost global causes of death and disability. Due to inadequate knowledge in its sequential disease mechanisms, therapeutic efforts to mitigate acute ischemia-induced brain injury are limited. Recent studies have implicated epigenetic mechanisms, mostly histone lysine acetylation/deacetylation, in ischemia-induced neural damage and death. However, the role of lysine methylation/demethylation, another prevalent epigenetic mechanism in cerebral ischemia has not undergone comprehensive investigation, except a few recent reports, including those from our research cohort. Considering the impact of sex on post-stroke outcomes, we studied both male and female mice to elucidate molecular details using our recently developed Internal Carotid Artery Occlusion (ICAO) model, which induces mild to moderate cerebral ischemia, primarily affecting the striatum and ventral hippocampus. Here, we demonstrate for the first time that female mice exhibit faster recovery than male mice following ICAO, evaluated through neurological deficit score and motor coordination assessment. Furthermore, our investigation unveiled that dysregulated histone lysine demethylases (KDMs), particularly kdm4b/jmjd2b are responsible for the sex-specific variance in the modulation of inflammatory genes. Building upon our prior reportage blocking KDMs by DMOG (Dimethyloxalylglycine) and thus preventing the attenuation in H3k9me2 reduced the post-ICAO transcript levels of the inflammatory molecules and neural damage, our present study delved into investigating the differential role of H3k9me2 in the regulation of pro-inflammatory genes in female vis-à-vis male mice underlying ICAO-induced neural damage and recovery. Overall, our results reveal the important role of epigenetic mark H3k9me2 in mediating sex-specific sequential events in inflammatory response, elicited post-ICAO.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral ischemic stroke is one of the leading causes of death and disability under the non-communicable diseases category globally, and the alarming rise in cases in recent years appears to be associated with lifestyle changes. Yet, there are limited therapeutic interventions to mitigate neural damage, and the main reason is our lack of insight into the underlying molecular mechanism. The majority of survivors experience persistent neurological abnormalities and severe disability (Donnan et al., 2008; Jorgenson et al., 1999; Moskowitz, 2010). To reveal the pathophysiology, cellular, and molecular processes involved in post-stroke cerebral infarction and to assess the efficiency of potentially therapeutic molecules, numerous credible models for inducing cerebral ischemic stroke in rodents have been established (Stenzel et al., 2007; McAuley et al., 1995; Leng et al., 2016).Another lacuna in the field is that stroke-related studies have failed to consider sex as a critical variable until now, despite the fact that gender differences clearly exist in stroke risk factors, particularly risks conferred by atrial fibrillation, metabolic syndromes, and age-related changes in sex hormones. Recent studies have even shown the importance of sex chromosomes in stroke pathophysiology (Roy et al., 2018). Contrary, studies in MCAO model suggest that the sensitivity to ischemic stroke differs between sexes, and intriguingly, this divergence is attributed to the influence of gonadal hormones rather than the sex chromosome complement (Manwani et al., 2015), whereas in a recent report, the estrogen was represented as an immunomodulator in behind the cause of immune reactions to bring the sex difference in post-stroke changes (Zhong et al., 2023). This challenges traditional assumptions and highlights the complex interplay of biological factors in understanding sex differences in ischemic stroke susceptibility. Despite recent substantial progress in exploring sex-specific factors in stroke pathophysiology, significant research gaps still persist. Moreover, in females with a history of adverse pregnancy outcomes like hypertensive disorders, pre-term delivery, fetal growth retardation, specific treatments, or guidelines to reduce the risk are lacking. Hence, it is of paramount importance to comprehend and unveil whether females articulate stroke symptoms in a distinct manner.
The middle cerebral artery occlusion (MCAO) model in rodents (Aspey et al., 2000; Shingeno et al., 1985; Sundt et al., 1969; Tamura et al., 1981) simulates stroke conditions in humans and has therefore been used in the majority of molecular studies over the past several decades. However, occlusion in the internal carotid artery, a cerebral ischemic condition that ranges from asymptomatic to symptomatic, comprises 13–25% of all stroke cases (Dyken et al., 1974; Flaberty et al., 2004; Fisher et al., 1954; Mead et al., 1998; Thanvi et al., 2007; Thiele et al., 1980). Hence, we have established a novel Internal Carotid Artery Occlusion (ICAO) model in CD1 mice of age 8–10 weeks (early adult), inducing mild to moderate levels of neural damage primarily within the striatal region and to some extent in the hippocampal region (Chakravarty et al., 2017; Jhelum et al., 2022; Jhelum et al., 2017). Here, in this study we have used 7-month-old CD1 male and female mice, as clinically stroke incident showed clear sex difference in mortality and recovery rate in middle-aged human population (Abdu et al., 2022; Klöting et al., 2003). In our experiments, we have used CD1 strain mice to study transient ischemic condition through ICAO model. According to previous literatures, mammals have intra-specific differences in responding to acute hypoxia. There exists a clear cut strain difference in response to acute hypoxic conditions in mouse, as hypoxia, inbred (C57) strain, the most popular mouse model maximizes oxygen intake efficiency to markedly extend hypoxic tolerance. Following the maximized oxygen usage eventually inbred strain (C57) becomes more tolerant to hypoxia but outbred strain (CD1) gets more vulnerable. Hence, we have preferred CD1 strain over C57 strain. Additionally, using outbred strains (CDI) is supposedly more accurate in mimicking what one would find in human stroke because of obvious heterogeneity in humans (Zwemer et al., 2007).
The molecular investigations in recent years using several rodent stroke models have suggested that ischemia-induced damage and recovery are mediated by epigenetic alterations, such as DNA methylation (Doerfler et al., 2008; Narlikar et al., 2002, Dell et al., 2012) and histone lysine (K) acetylation and deacetylation (Marks et al., 2010; Hebbes et al., 1988). It has been demonstrated that the administration of potential compounds which reduce DNA methyltransferases (DNMTs) and histone deacetylases (HDACs) (Endres et al., 2000; Faraco et al., 2006; Kim et al., 2007; Ren et al., 2004) slow down the ischemia-induced brain damage. However, extensive research is yet to be conducted to encompass the involvement of other epigenetic mechanisms, such as H3 and H4 methylation and demethylation, in particular, H3k9, H3k27, and H4k20 methylations, which are transcriptionally repressive epigenetic modifications, recently implicated in the etiopathology of most neurodegenerative and neuropsychiatric disorders (Bouet et al., 2007; Covington et al., 2011; Kumar et al., 2005; Wilkinson et al., 2009; Tsankova et al., 2004, 2006, 2007). Another study by Schweizer et al. (Formisano et al., 2007) employing an in vitro OGD (oxygen glucose deprivation) model, linked H3k9me2 or me3 and histone lysine methyltransferases (KMTs) to hypoxia-induced oxidative stress, neuronal survival, and death. The lists of histone lysine methyltransferases (KMTs) and histone lysine demethylases (KDMs) are growing as new members are discovered, but their roles in stroke-induced pathophysiology and recovery remain elusive (Scheweizer et al., 2015). Excluding a handful of studies that mention H3k4me3 and the specific lysine demethylase LSD1 (Lysine-specific demethylase 1A) (Zhao et al., 2016; Zhang et al., 2010), only a few studies delve into the significance of histone lysine methylation and demethylation in the context of the stroke model. Recent research has demonstrated of diminished H3k4me3 activity in astrocytes following stroke in the middle-aged female rats when compared with adult females. Similar to LSD1, the KDMs operate on transcriptionally repressive H3K9me2/3 residues. Transcriptionally activating epigenetic modifications on H3k4me3 have also been shown to be regulated at the mRNA level in a spatial and temporal manner after transient global ischemia and reperfusion and have been suggested to aid neural regeneration in the affected rats in one of the studies (Chisholm et al., 2015).
Our previous investigations in this direction indicated attenuation in the transcriptionally repressive epigenetic mark H3k9me2 in the affected striatal region of male CD1 mouse and was associated with changes in several KMTs and KDMs that control the methylation of H3k9. Interestingly, blocking KDMs by DMOG (Dimethyloxaloylglycine) and thus preventing the attenuation in H3k9me2 reduced the post-ICAO transcript levels of the inflammatory molecules and neural damage. We also identified the mRNA levels of KMTs and KDMs that regulate H3 K9, K27, and H4 K20 di/trimethylations since these epigenetic modifications are regulated by two distinct classes/families of enzymes: histone lysine methyltransferases (KMTs) and histone lysine demethylases (KDMs) (Chakravarty et al., 2017). In light of our previous findings on the epigenetic regulatory mechanisms in regulating the inflammatory response in striatum, as it was the most affected region post-ICAO (Chakravarty et al., 2017), and the fact that the females have a lower incidence of stroke and are more protected from the acute effects of cerebral ischemia (Sudlow et al., 1997; Harder et al., 1998), in the present study we developed and characterized ICAO mouse model in both sexes. We uncovered the underlying molecular and inflammatory pathways of sex disparity in neural response to cerebral stroke. Furthermore, our results unveiled the sex-specific role of kdm4b/jmjd2b and H3k9me2-mediated epigenetic regulatory mechanism in differential regulation of the neuroinflammatory response post-ICAO and in the recovery mechanism. The study suggests that the occlusion of the internal carotid artery (ICAO) leads to gender-specific variations in the way neural responses and recovery unfold after an ischemic stroke. We posit that these distinctions arise from complex interactions among molecular processes, inflammatory pathways, and epigenetic alterations. More specifically, our hypothesis posits that females demonstrate a swifter recuperation post-ICAO, attributed to early inflammatory responses, unique vulnerabilities in brain regions related to mitochondria, and distinct regulation of processes, like autophagy and epigenetics, notably involving H3k9me2. The exploration of sex-specific elements influencing stroke recovery has the potential to reveal new therapeutic targets, with H3k9me2 emerging as a plausible regulator in the context of neural damage induced by stroke.
Materials and Methods
Animals and Housing
Adult CD1 male and female mice bred and maintained in CCMB Animal House were used throughout the study. All animal procedures were approved by the Institutional Animal Ethics Committee, 20/GO/RBi/99/CPCSEA at the Centre for Cellular and Molecular Biology, Hyderabad, India. Adult male and female CD1 mice aged 7 months were used in the experiments. Animals were acclimatized with standard experimental room conditions maintained at 24 ± 2 °C and 12/12-h light/dark schedule in individually ventilated cages (IVC) system. Food and water were available ad libitum to the animals. After the habituation period, the animals were grouped as sham (control) and ICAO (experimental). In the study, five distinct experimental groups, designated as Group 1, Group 2, Group 3, Group 4, and Group 5, were established to investigate the effects of varying time intervals following internal carotid artery occlusion (ICAO) in both male and female subjects. These time intervals encompassed 6-h, 1-day, 3-day, 5-day, and 7-day post-ICAO. Each of these experimental groups consisted of a cohort of 12 to 15 animals, ensuring an adequate sample size to facilitate robust statistical analysis and accurate assessment of the neurobiological responses to ICAO within the specified time frames.
Surgical Procedure
Internal carotid artery occlusion (ICAO) was performed as previously established (Chakravarty et al., 2017). Briefly, adult CD1 mice of both the sexes were anesthetized with a cocktail of ketamine (100 mg/Kg) and xylazine (10 mg/kg), intraperitoneally (i.p). The left common carotid artery was carefully identified and separated from vagus nerve. The internal carotid artery (ICA) was traced and occluded for 90 min with a cotton suture and then reperfused by removing the occlusion. Mice in the sham-operated group were subjected to the same surgical procedure, without occluding the internal carotid artery, to serve as a control group.
Laser Doppler Perfusion Imaging
ICAO-induced changes in blood perfusion rate were analyzed and measured in both male and female using Laser Doppler imaging (LDI) technique using Moor LDI2-HR laser Doppler, as described previously (Jhelum et al., 2022). Briefly, the blood flow rate was analyzed at three different time points, i.e., before occlusion, after occlusion, and after reperfusion, in different region of interests, such as left common carotid (ROI 1), internal (ROI 2), and external (ROI 3) carotid arteries.
Behavioral Assays
The behavioral tasks like Neurodeficit assessment, Rotarod, Grip Strength Measurement (GSM), and Open field Test (OFT) were performed at different time points from 1-day post-ICAO till 7d -day post-ICAO. The behavioral tracking was performed with the help of video tracking software Ethovision 3.1 (Noldus Information Technology, Leesburg, VA) and then analyzed. After the completion of behavioral assays, microdissection of specific brain regions was performed. The striatum, hippocampus, and hypothalamus were carefully microdissected from each mouse and collected for further molecular studies, including quantitative polymerase chain reaction (qPCR), Western blot analysis, and chromatin immunoprecipitation followed by quantitative PCR (ChIP-qPCR). These brain tissues were collected to assess the molecular changes in response to ICAO and reperfusion.
Neurobehavioral Assessment
Animals subjected to ICAO/reperfusion were evaluated on Neurological Deficit Score (NDS) at different time periods following ICAO. Neurological deficit scoring was carried out at 6-h, 1-D, 3-D, 5-D, and 7-D post-ICAO based of different phenotypical symptoms, as described previously (Chakravarty et al., 2017; Jhelum et al., 2022) with minor modifications for better understanding [please check Fig. 2A for details].
Grip Strength Measurement
Grip Strength Test (GST) is a tool to assess grip strength function of rodents. Grip strength function test were performed at 1-D, 3-D, 5-D, and 7-D post-ICAO by grip strength meter (Ugo Basile, Italy) as per the standard procedure reported earlier (Chakravarty et al., 2017; Jhelum et al., 2022). In brief, animals were kept in inclined position on a mesh which was attached to the transducer. A slight force was applied to pull the animal away from the mesh. The force applied by animal to resist this force was recorded and measured in terms of newton (N).
Rotarod Performance Test
Rotarod performance test is a behavioral experiment to assess the motor coordination function of rodents. Here, we have used rotarod instrument procured from Ugo Basile, Italy. The animals were placed on rotating rotarod for acclimatization followed by training for 3–4 consecutive days of 2–3 trials each day (4–40 rpm for 5 min) on an accelerating rotarod and recorded the latency to fall from rotating rotarod at 1-D, 3-D, 5-D, and 7-D post-ICAO (Chakravarty et al., 2017; Jhelum et al., 2022).
Open Field Test
Open field test is used to evaluate basal locomotor activity of animals (Chakravarty et al., 2017; Jhelum et al., 2022) at 1-D, 3-D, 5-D, and 7-D post-ICAO. Locomotion and exploration behavior were calculated and analyzed using animal tracking software Ethovision 3.1.
Tissue Collection and Fixation
Mice were euthanized by cervical dislocation at time points of 6 h, 1D, 3D, 5D, and 7D following internal carotid artery occlusion (ICAO) for subsequent neurobiological investigations. In the pursuit of molecular studies, animals were humanely terminated and their cerebral tissue was meticulously extracted. Specific brain regions, including the striatum, hippocampus, and hypothalamus, were meticulously microdissected following established protocols as previously described by Wahul et al. (2018). In summary, within each targeted region, precise areas were identified and extracted under the guidance of a stereomicroscope. These samples were promptly frozen in a liquid nitrogen container and preserved at − 80 °C to facilitate subsequent quantitative qPCR and western blot experiments. For histological investigations, animals under anesthesia were subject to transcardial perfusion with cold phosphate-buffered saline (1 × PBS), followed by fixation with 4% paraformaldehyde, as previously outlined by Chakravarty et al. (2017). Subsequently, the fixed brains were sectioned in a serial manner, with each section having a thickness of 25 μm, utilizing a cryostat from Leica, Germany, and were further processed according to the specific needs of the experiments.
Mitochondrial Enzyme Activity Assay
Hypoxic-ischemic neural damage induced by ICAO was evaluated by TTC (2, 3, 5 Triphenyl tetrazolium chloride, Sigma-Aldrich) colorimetric assay on specific affected regions after 24 h of ICAO. For quantifying the infarct, formazan produced from TTC were measured as per published protocols (Das et al., 2019).
Histological Staining
Brain sections from ICAO and sham group from both males and females post-1D of surgery were processed for hematoxylin and eosin (H&E) staining. Briefly, sections were hydrated and dehydrated in graded alcohol series, followed by staining with Hematoxylin and Eosin (Chakravarty et al., 2017). The stained slides were observed under a microscope to visualize vacuolization, a hallmark of damaged tissue, and results were analyzed using ImageJ software.
Quantitative Polymerase Chain Reaction
Total RNA was isolated from affected brain tissue (striatum) using TRIzol Reagent (Invitrogen, USA) according to the manufacturer’s protocol. Briefly, cDNA was synthesized using Thermo fisher First-Strand cDNA Synthesis kit following the manufacturer’s instructions followed by Quantitative real-time PCR (qPCR) (BioradCFX 96). Primer sequences used are listed in Supplementary Table 1. Relative gene expression analysis was performed with RPL32 as housekeeping gene (Chakravarty et al., 2017; Jhelum et al., 2022, Wahul et al., 2018).
Western Blot Analysis
Micro-dissected striatal tissue samples were homogenized in 8-M urea buffer and further the tissue samples were sonicated for a period of 1 min (Pulse -30 Sec ON/OFF, Amplitude-40%). Later, the homogenized and sonicated sample was centrifuged for 20 min at 12,000 rpm at 4 °C and the supernatant that contained proteins was carefully removed and placed in a fresh tube. The concentration of protein was estimated using Bradford Assay (Chakravarty et al., 2017; Jhelum et al., 2022; Wahul et al., 2018; Karisetty et al., 2017) and performed SDS-PAGE gel electrophoresis as per the standard protocol. Densitometry analysis for western blotting was performed using ImageJ software and intensity values are represented as bar graphs. The antibodies used in this study are Hif-1α (ab2185), Il-1β (P420B), Nlrp3 (ab214185), and H3k9me2 (ab176882).
ChIP-qPCR
Chromatin immunoprecipitation assay (ChIP) was performed as described previously (Chakravarty et al., 2017). In brief, ChIP cross-linked samples were pooled from ICAO and sham groups. Prior to being incubated with an anti-rabbit H3K9me2 antibody (ab176882), 30 ug of chromatin from each sample was pre-cleared with SureBeads Protein G Magnetic beads (161-4023, Bio-Rad), utilizing non-immune mouse IgG antibody as a negative control. Further, the unique primers (Supplementary Table 1) targeting the gene-specific 5′ region upstream of the transcription start site were employed to measure the enrichment of the histone mark H3K9me2 with IgG as a negative control.
Statistical Analysis
We employed varying sample sizes for distinct experimental techniques to ensure statistical rigor and the robustness of our findings. Specifically, for Western blot analysis, we utilized a sample size (n) of 4, whereas for quantitative polymerase chain reaction (qPCR) experiments, our sample size was set at n = 7. For behavioral analysis, we included a cohort of 12 to 15 animals in our study to obtain sufficient statistical power. Additionally, in the context of chromatin immunoprecipitation followed by quantitative PCR (ChIP-qPCR), our sample size was maintained at n = 4. Two-way ANOVA was used for analyzing the statistical significance of the data and calculation of p-values. Data are represented as mean + standard error of mean (SEM) (in error bars) wherever applicable. P values less than 0.05 were considered to be significant and the level of significance was assigned as follows: *, p < 0.05; **, p < 0.01; ***p < 0.001; and ****, p < 0. 0001. The behavioral results were analyzed by two-way ANOVA. Post hoc comparisons were performed by Tukey’s test. Statistical significance was set at p value < 0.05.
Results
Laser Doppler Imaging Shows Differential Blood Flux Rate in Males and Females Post-ICAO
To verify the effectiveness of internal carotid artery occlusion in inducing an ischemic state followed by reperfusion, we conducted perfusion rate analysis using the Moor LDI2-HR laser Doppler before occlusion, after occlusion, and after reperfusion in both male and female subjects (Fig. 1A). Interestingly, we observed a significant difference in baseline blood flux rates between males and females prior to occlusion, as illustrated in (Fig. 1B). However, regardless of sex, we found that occlusion caused a significant reduction in blood flux rates in the left common carotid (ROI 1) and internal carotid artery (ROI 2), which were subsequently restored upon reperfusion.
Assessment of ICAO-induced changes in blood flux using Moor LDI2-HR laser Doppler imaging. A Representative heatmap image showing ICAO-induced changes in blood flux rate in three different regions of interests before occlusion, after occlusion, and reperfusion in different region of interests, namely left common carotid (ROI 1), internal (ROI 2), and external (ROI 3) carotid arteries in both males and females. B Evaluation of the blood flux rate post-ICAO in left common carotid (ROI 1), internal carotid artery (ROI 2), and external carotid artery n = 4, *p < 0.05 and **p < 0.01 compared to blood flux rate to corresponding arteries before occlusion. (C) Representative image showing timepoints at which behavioural assays were performed
Behavioral Functional Analysis Shows Faster Recovery Rate in Females than Males at Different Timepoints Post-ICAO
Behavioral functional assessment was determined by Neurodeficit score (NDS) and grip strength measurement. Further behavioral tests, Open field test and rotarod test provided insights on motor coordination at different timepoints (Fig. 1C). The Neurodeficit score (Fig. 2A, B) was evaluated at different time points, 6-h, 1-d, 3-d, 5-d, and 7-d post-ICAO, by evaluating different phenotypic symptoms exhibited post-ICAO and the results indicated maximum deficit in both male and female mice at 6-h post-ICAO. Interestingly, neurodeficit score persisted in males throughout the post-ICAO 1D, while females exhibited a significant decrement in the score by 1-D post-ICAO. Forelimb grip strength (Fig. 2B) was measured at 1-D, 3-D, 5-D, and 7-D post-ICAO, which revealed a significant reduction in grip strength in both male and female mice at 1-D post-ICAO as compared to the Sham group. It was observed that females showed a faster recovery rate at 3-D post-ICAO, whereas males showed significant recovery at 5-D post-ICAO compared to the 1-d post-ICAO group. In the Rotarod test (Fig. 2C), ICAO significantly impaired the ability of both male and female mice to coordinate their motor activity as compared to the Sham. Females exhibited greater latency to fall at 3-D post-ICAO, whereas males showed similar changes at 5-D post-ICAO compared to the 1D group. Females also recovered completely from ICAO-induced impairment by 7-D post-ICAO, whereas males could not exhibit full recovery. Finally, the assessment of velocity and distance moved post-ICAO indicated a similar trend in recovery rate post-ICAO in both male and female mice (Fig. 2D, E). Overall, our findings demonstrate that females recover faster than males from ICAO-induced neural damage. We conducted an assessment of post-surgical mortality rates among male and female mice. For the male mice, the observed mortality rates at 6-h, 1-D, 3-D, 5-D, and 7D post-surgery groups were 0%, 60%, 23%, 18%, and 10%, respectively. In the case of female mice, the corresponding mortality rates within respective groups were 0%, 25%, 7%, 5%, and 0%. The mortality post-ICAO surgery differed significantly between males and females over time, with females exhibiting higher survival rates, which correlates to the accelerated behavioral functional recovery rate in females (Fig. 2F).
Analysis of behavioral functional outcomes and percentage of survival in both males and females post-ICAO. A Neurological deficit scoring (NDS) was performed at B 6-h, 1-D, 3-D, 5-D, and 7-D post-ICAO, n = 17 in each group (*p < 0.05, **p < 0.01 compared to 6-h post-ICAO) C Grip strength test performed at 1-D, 3-D, 5-D, and 7-D post-ICAO, n = 13, **p < 0.01compared to sham, #p < 0.01 compared to 1D. D Rotarod performance test performed at 1-D, 3-D, 5-D, and 7-D post-ICAO n = 13, **p < 0.01 compared to sham, #p < 0.05 compared to 1d. E, F Mortality at 1-D, 3-D, 5-D, and 7-D post-ICAO
Mitochondrial Enzyme Activity Assay Shows Sex-Specific and Region-Specific Neural Damage in Different Brain Regions 1 day Post-ICAO
The study aimed to evaluate the region-specific neural damage caused by ICAO using TTC (2, 3, 5 Triphenyl tetrazolium chloride, Sigma-Aldrich) by quantifying mitochondrial enzyme, succinate dehydrogenase, in live cells in the affected regions, striatum, hippocampus, and hypothalamus. The findings revealed sex-specific differences in mitochondrial dysfunction within different brain regions. The ICAO-induced damage was significantly evident in the striatum of both sexes. However, the hippocampus remained unaffected in males, whereas significant mitochondrial dysfunction was found in females (Fig. 3A, B). Notably, the hypothalamus, a noted sexually dimorphic region, was found to be intact without any significant mitochondrial damage in females, unlike males post-1D-ICAO (Fig. 3C).
Assessment of ICAO-induced neural damage. 1. Estimation of mitochondrial enzyme activity by TTC (2, 3, 5 Triphenyl tetrazolium chloride, Sigma-Aldrich) at 1-D post-ICAO. Mitochondrial enzyme activity showing sex-specific and region-specific differential mitochondrial damage post-1D ICAO in A Striatum, B Hippocampus, and C Hypothalamus. Statistical analyses were carried out using One-way Anova, n = 4 mice in each group. *p < 0.05 and **p < 0.01 v/s Sham group. D H&E staining showed enormous vacuolarization in males and females post-ICAO when compared with sham group and ICAO group. Arrows represent vacoular area E graphically represented % of vacuolar area in males and females (n = 5, ***p < 0.05 compared to sham)
Hematoxylin and Eosin Staining Showed ICAO-Induced Cellular Damage Localized to Striatum in Males and Females
Hematoxylin and Eosin (H&E) staining shows broad range of cytoplasmic, nuclear, and extracellular matrix features. Hematoxylin has a deep blue–purple color and stains nucleic acids and Eosin is pink and stains proteins. Ischemia triggers destruction of cytoplasmic nuclear and extracellular matrix and forms irregular spaces within the damaged cells in the form of vacuoles. Hematoxylin and Eosin staining showed larger vacuolization, a hallmark of damaged tissue, in striatal tissue of ICAO group as compared to the sham group 1-D post-ICAO in both males and females (Fig. 3D). Quantitative analysis showed that ICAO group encompassed about 6.9% of vacuolar area in males and 8% in females, indicating remarkable damage in the striatum, whereas, 1.7 and 2.5% of vacuolar area in sham male and female group, respectively (n = 4–5).
Hif-1α and Inflammatory Cytokines are Differentially Regulated in Males and Females Post-ICAO in Striatum
To investigate the differential expression pattern of Hif-1α, both the transcriptional and protein level studies were performed at various time points (6 h, 1D, 3D, 5D, and 7D) post-ICAO (internal carotid artery occlusion). At the transcriptional level, hif-1α was significantly upregulated in both male and female mice 1-D post-ICAO. In males, hif-1α remained elevated until 3-D post-ICAO, returning to basal levels by 5-D post-ICAO. However, in females, the striatal hif-1α level returned to basal level by 3-D post-ICAO. Surprisingly, we did not observe an increase in Hif-1α protein levels in females, unlike in males at 1-d post-ICAO. These findings prompted us to investigate whether there was an early elevation of Hif-1α protein levels in females. Interestingly, we observed high levels of hif-1α at mRNA as well as protein level in females at 6-h post-ICAO, unlike males (Fig. 4A–C). Also, to examine whether there were any sex-specific inflammatory response post-ICAO we checked for the transcriptional levels of pro-inflammatory cytokines, such as tnf-α and il-1α. At 1-d post-ICAO, we found a significant upregulation of tnf-α and il-1α at the transcriptional level in males, followed by a gradual decrease in expression over time. A similar gene expression pattern was observed in females at 6 h along with a sudden boost in tnf-α and il-1α expression at 7-D post-ICAO (Fig. 5A, B). We did not observe any noticeable change in Il-1β and inflammasome Nlrp3 in female striatum 1-d post-ICAO. Therefore, we probed the protein expression level of Il-1β and Nlrp3 in females along with we checked for the transcriptional levels of pro-inflammatory cytokines such as tnf-α and il-1α at 6-h post-ICAO. Surprisingly, we observed a significant upregulation of Il-1β and Nlrp3 in females in protein level and tnf-α and il-1α transcript levels at 6-h post-ICAO (Fig. 5C–F).
The levels of Hypoxia-inducing factor (HIF-1α) in ICAO-induced neural damage in male and females. A mRNA expression of Hif-1α at different timepoints from 6-h to 7-D post-ICAO. B Protein level expression pattern revealed upregulation of Hif-1α expression at an early timepoint in females, i.e., 6-h post-ICAO, unlike males. C Representative western blot images showing differential expression pattern of HIF-1α in males and females at different timepoints. Statistical analyses were carried out using One-way Anova, n = 6 to 8 mice in each group. *p < 0.05, and **p < 0.01 v/s Sham group
Inflammatory response post-ICAO in males and females at different timepoints post-ICAO. A, B Differential mRNA expression pattern of tnf-α and il-1α at different timepoints in males and females post-ICAO. C–F Densitometric analysis of protein level expression and representative images of western blots of IL-1β and NLRP3 in males and females analyzed at different timepoints post-ICAO compared with SHAM group. Statistical analyses were carried out using One-way Anova, n = 6 to 8 mice in each group. *p < 0.05 and **p < 0.01 v/s Sham group
H3k9me2 Plays Sex-Specific Differential Role in Regulating Transcriptional Activation of Neuroinflammatory Genes in Striatum Post-ICAO
According to previous reports on ICAO, the activation of neuroinflammatory genes was associated with histone-based epigenetic regulators such as histone lysine methyltransferases (KMTs) and histone lysine demethylases (KDMs) in males, with very low occupancy of H3k9me2 at the promoters of different inflammatory genes (Chakravarty et al., 2017), we investigated whether there are any changes in transcription regulatory mechanisms across the sexes.
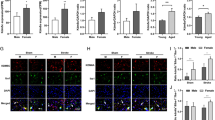
Upon examining the immediate post-ischemia–reperfusion (ICAO) response at 1D, a striking gender disparity emerged. While males exhibited noticeable changes in the expression of H3k9me2, females did not display such alterations. This intriguing finding prompted us to explore whether the heightened inflammatory response observed in females at this early time point, i.e., at 6-h post-ICAO correlated with H3k9me2 dynamics. We analyzed the expression levels of H3k9me2 and kdm4b/jmjd2b, a transcription repressor involved in the demethylation of H3k9me2. Interestingly, we could not find any noticeable changes in the expression of H3k9me2 at 1-D post-ICAO in females, unlike in males. To investigate whether the elevation of the inflammatory response at an early time point in females is correlated with H3k9me2 data, we assessed the expression of H3k9me2 in protein level and kdm4b/jmjd2b in mRNA level at 6-h post-ICAO. Surprisingly, there was a significant downregulation of H3k9me2 in females at 6-h post-ICAO and an upregulation in kdm4b/jmjd2b, unlike in males. To strengthen our findings, we also examined the expression pattern of H3k9me2 and kdm4b/jmjd2b at 3-D, 5-D, and 7-D post-ICAO, in addition to 6 h and 1D. We observed a gradual increase in the expression of H3k9me2 and a decrease in expression of kdm4b/jmjd2b, which diminished the inflammatory response in males until 7-D post-ICAO. Conversely, in females, the inflammatory response showed an early surge at 6 h, marked by the upregulation of kdm4b/jmjd2b, challenging the conventional trajectory observed in males. (Fig. 6A–C).
Sex-specific comparison of KDM4b/jmjd2b and H3k9me2 expression at different timepoints along with quantifying occupancy of H3k9me2 in the promoters of different inflammatory genes through ChIP-qPCR at 1-D post-ICAO. A Gene expression study shows sex-specific differential dysregulation of JMJD2b at various time periods post-ICAO compared to the sham in both males and females. B, C Densitometric analysis on protein level expression of H3k9me2 in males and females analyzed at different timepoints post-ICAO compared with SHAM group and representative image of western blot of H3k9me2. Statistical analyses were carried out using One-way Anova, n = 6 to 8 mice in each group. *p < 0.05 and ***p < 0.01 v/s Sham group. D, E Chromatin immunoprecipitation (ChIP) assay on pooled striatal tissue (n = 4) at 1-D post-ICAO in both males and females. ChIP–qPCR data show a decrease in H3K9me2 level at inflammatory gene promoters TNF-α and IL-1α in male ICAO group as compared to its sham group, unlike females. Statistical analyses were carried out using One-way Anova, n = 4 mice in each group. *p < 0.05 and ***p < 0.01 v/s Sham group
Transcriptional Activation of tnf-α and il-1α in Striatum is Differentially Regulated in Males and Females Through Altered H3k9me2 Occupancy in Gene Promoters
At 1-D post-ICAO, males exhibited a significant decrease in the transcriptionally repressive epigenetic marker, H3k9me2 occupancy in the promoters of inflammatory markers, in striatum. In contrast, females showed a significant increase in H3k9me2 occupancy in the promoter region. To quantify the H3k9 methylation levels at the promoter regions of the neuroinflammatory genes, tnf-α and il-1α, we performed a chromatin immunoprecipitation (ChIP) assay using an H3k9me2-specific antibody in pooled striatal tissue samples (Fig. 6D, E) from both males and females at 1-day post-ICAO and sham controls.
Discussion
Stroke is a major cause of morbidity and mortality worldwide, with significant clinical and socioeconomic implications. The most frequently used in vivo model of ischemic stroke is the intraluminal suture middle cerebral artery occlusion (MCAO) model, which has been fundamental in revealing various aspects of stroke pathology. However, the MCAO model produces lesion volumes which mimics severe cerebral stroke conditions with high mortality rate (Trotman et al., 2021). There was a need to refine the MCAO model to establish a model which translates transient cerebral ischemic condition in humans as internal carotid artery occlusion (ICAO) accounts for approximately 25% of all cerebral ischemic incidents (Song et al., 2020). However, one of the major disadvantages of ICAO model is that the thrombolytic medicines or medications made to target the reperfusion phase after ischemia cannot be studied since it causes permanent localized ischemia. ICAO-induced infarct size can be highly variable and may result in mild to moderate ischemic damage, limiting the ability to precisely control and reproduce the extent of injury seen in other models. The results presented in this study provide intriguing insights into the sex-specific differences in neural response and recovery following internal carotid artery occlusion (ICAO), shedding light on the intricate interplay between molecular mechanisms, inflammatory pathways, and epigenetic modifications in response to ischemic stroke. We established an ICAO mouse model that mimics clinical presentations in patients with unilateral ICAO (Chakravarty et al., 2017). The significant differences in baseline blood flux rates between males and females prior to occlusion, as noted in the study, may indeed have relevance to the subsequent research and the conclusions drawn. While the primary goal of using laser Doppler imaging in this study was to confirm the proper occlusion in the ICAO model, the observation of baseline differences between sexes can provide valuable insights into the physiological variations between male and female subjects. The observed differences in baseline blood flux rates suggest that there are inherent sex-related disparities in vascular physiology, even in the absence of induced ischemia. This finding aligns with known differences between male and female vascular systems, including variations in vessel size, endothelial function, and hormonal influences (Alisch et al., 2021, Szadvári, et al., 2023, Aanerud et al., 2017).
The behavioral functional analysis, including neurodeficit score, grip strength, and motor coordination tests, revealed a striking sex-specific difference in recovery rates. Females exhibited a faster recovery compared to males, as evident from improvement in neurodeficit scores, grip strength, and rotarod performance. This aligns with previous studies that have highlighted the neuroprotective effects of female sex hormones especially, estrogen and their impact on recovery after stroke; however, the outcome was mostly described in light of estrogenic role in immunomodulation (Zhong et al., 2023). The observed baseline differences align with known disparities between male and female vascular systems, including variations in vessel size, endothelial function, hormonal influences, brain organization, and function with respect to the underlying biological mechanisms has been recently reported (Szadvári et al., 2023). Additionally, the observed sex-specific differences in mortality rates post-ICAO further emphasize the need to investigate the underlying molecular and cellular mechanisms contributing to these disparities. Post-ICAO surgery, we observed non-uniform deficits, with some mice remaining asymptomatic, thereby highlighting the similarity of ICAO model to clinical cases. The examination of mitochondrial enzyme activity using TTC staining offered insights into the region-specific neural damage induced by ICAO. Reactive oxygen species (ROS), which are overproduced by mitochondria after brain ischemia, have been carefully investigated using superoxide dismutase transgenic or mutant rats. Lipids, proteins, and nucleic acids within the cell are all directly harmed by ROS. Additionally, ROS stimulate a number of molecular signaling pathways. Returning apoptosis-related signals to mitochondria causes mitochondria to release pro-apoptotic proteins such cytochrome c or apoptosis-inducing factor, which cause cell death (Ten et al., 2019). Notably, the striatum emerged as a vulnerable region for both sexes, consistent with its high sensitivity to ischemic insult. However, the hippocampus displayed sex-specific vulnerability, with mitochondrial dysfunction observed only in females. These findings suggest that distinct brain regions respond differently to ischemic insult based on sex, implicating complex interactions between hormonal, molecular, and metabolic factors. The absence of significant mitochondrial damage in the hypothalamus, a sexually dimorphic brain region, in females warrants further investigation into the protective mechanisms at play.
Inflammation is a crucial factor in the pathological progression of ischemic stroke (Jayaraj et al., 2019). Following an acute stroke, the majority of which are ischemic, there occurs secondary neuroinflammation, which both encourages injury and cell death while also acting favorably by fostering recovery. Immune mediators’ pro-inflammatory signals quickly activate local cells and influence the influx of a variety of inflammatory cells (neutrophils, monocytes/macrophages, several T-cell subtypes, and other inflammatory cells) into the ischemic region, aggravating brain injury (Jayaraj et al., 2019). Interestingly, inflammation can either be detrimental or beneficial post-ischemia, depending on the severity and stage of ischemia. Early inflammatory responses may exacerbate ischemic injury, while later responses may trigger recovery and repair mechanisms. Our findings suggest a time-dependent inflammatory response in both males and females post-ICAO (Lu et al., 2022). Initially, we assessed the expression levels of a group of pro-inflammatory cytokines and found that Tnf-α, Il-1β, Il-1α, and Nlrp3, an inflammasome marker in both males and females striatum at 1-D post-ICAO were showing sex-specific differential expression pattern. Surprisingly, we observed no inflammatory response in females at this time point compared to control, unlike males. To determine if there was an early inflammatory trigger in females, we assessed the same biomarkers at 6-h post-ICAO. Interestingly, we observed significant upregulation of inflammatory cytokine’s transcription in female brain at this time point, unlike in males. This finding suggests that in females, there is an early inflammatory response, while in males, inflammation is triggered late. This difference may explain the faster recovery rate in females post-ICAO compared to males, as inflammatory mechanisms can trigger many survival genes to recover from ischemic insults. Hypoxia-inducible factor-1 alpha (HIF-1 alpha) plays a role in a number of cell-protective pathways after ischemia. There are obvious sex-related differences in the remodeling process, and male hearts have a tendency to enlarge more than female hearts do in response to pathological loads and ischemia (Bertogliat et al., 2020, Zampino et al., 2006). Here, we observed that the dynamics of Hif-1α expression in response to ICAO demonstrated intricate sex-specific differences. While males exhibited an early and sustained increase in Hif-1α levels, females displayed an early peak followed by a rapid decline which may be one of the reasons for faster recovery rate in females. Our results indicate a differential neuroinflammatory response post-ICAO in males and females, which may be yet another possible reason for the faster recovery rate in females. Overall, these results confirm that the inflammatory response post-ICAO is triggered at an early time point in females compared to males, possibly triggering a faster recovery mechanism in females. This gender-specific modulation of histone modification and transcriptional regulation underscores the complexity of the molecular landscape governing ischemia–reperfusion injury, emphasizing the importance of considering gender-specific responses in future investigations.
Autophagy is known to play a critical role in promoting neuronal survival and its activation has been proposed as a potential therapeutic strategy for stroke. Following an ischemic stroke, autophagy is either activated or inhibited in an array of brain cell types, including neurons, astrocytes, microglia, and microvascular endothelial cells. This results in the activation of the immune system, modification of microglial phenotypes, and increased permeability of the blood–brain barrier (Lu et al., 2022; Chen et al., 2014). Significant progress has been made in understanding sex-specific differences in autophagic responses to experimental ischemic stroke, as evidenced by comprehensive studies conducted in the middle cerebral artery occlusion (MCAO) mice model. These findings not only enhance our comprehension of the intricate mechanisms underlying stroke pathology but also present a promising avenue for the development of sex-specific therapeutics in future, marking a crucial step toward more targeted and effective interventions for stroke cases (Patrizz et al., 2021). However, it is not yet clear how autophagy affects the rate of neuronal death. Our western blot results, led us to uncover differential regulation of lc3b2 at the protein level, a marker for autophagic regulation, in sex-specific manner. Interestingly, we observed an early autophagic response in female striatum compared to that in males (Supplementary Fig. S1). This may also be a contributing factor to the faster recovery in females than males, post-ICAO. Further studies are needed, to fully understand the role of autophagy in stroke recovery.
Epigenetic modifications play critical roles in the regulation of neuronal gene expression and neurodegenerative disorders including ischemia-induced neural damage (Bertogliat et al., 2020). Previous studies have investigated the role of DNA methylation in neurodeficit progression following ischemic stroke. As per reports, in ischemic stroke patients on clopidogrel, reduced methylation of cg03548645 inside the TRAF3 body was linked to higher platelet aggregation and vascular recurrence, according to a new epigenome-wide analysis (Krupinski et al., 2018). Based on our previous findings, we observed differential regulation of kdm4b/jmjd2b class histone demethylases and its target H3k9me2 in controlling inflammatory response 1-day post-ICAO in both males and females. To investigate the epigenetic regulatory mechanism of H3k9me2 in controlling inflammatory response post-ICAO, we profiled kdm4b/jmjd2b and its target H3k9me2 along with different inflammatory markers in both males and females. Our investigation sought to elucidate the relationship between H3k9me2 and the inflammatory response, building upon our prior findings outlined in our previous publication (Chakravarty et.al, 2017). The dynamic downregulation of H3k9me2 at various temporal junctures in both male and female subjects underscores the intricate temporal dynamics of this epigenetic modification in response to ischemia resulting from bilateral common carotid artery occlusion (ICAO). Specifically, our study reveals that the reinstatement of H3k9me2 marks on the promoters of inflammatory genes in the female striatum manifests at an earlier time point compared to the male brain following ICAO. This prompt restoration contributes to the mitigation of inflammatory cytokine expression, culminating in an expedited recovery in females. Significantly, the inflammatory trigger in females is discernible at the 6-h mark through the downregulation of H3k9me2, followed by a swift recuperative response in contrast to males. The discerned sex-specific divergence in the temporal epigenetic regulatory mechanism involving H3k9me2 within the striatum post-ICAO constitutes a pivotal discovery in our study, accentuating the gender-specific nuances in the epigenetic response to cerebral ischemia. We performed a ChIP-qPCR to study the mechanism behind the differential inflammatory response regulated by H3k9me2 in males. Our results showed significantly higher levels of H3k9me2 occupancy in the promoters of different inflammatory markers in females compared to males, supporting our hypothesis that H3k9me2 differentially regulates inflammatory response post-ICAO in a sex-specific manner.
In summary, our study demonstrates that the restoration of H3k9me2 marks on the promoters of inflammatory genes in female striatum was at an early time point compared to that in male brain following ICAO; this resulted in attenuation of the expression levels of various inflammatory cytokines leading to the accelerated recovery. Our findings suggest that epigenetic mechanisms, such as histone lysine methylation and demethylation, particularly the ones controlling H3k9me2, play an important role in stroke-induced neural damage and subsequent recovery in a sex-specific manner. Altogether, our results suggest that H3k9me2 could be a novel target for the development of new therapeutics, with sex as an important variable to consider.
Conclusion
The outcome of this study indicate that the ICAO-induced neurological deficits and subsequent recovery mechanism differentially in male and female CD1 mice by inducing mild to moderate ischemic damage in the striatum. Our novel findings highlighted the role of the H3k9me2 and kdm4b/jmjd2b epigenetic modifications, which harness the substrate H3k9, in the damage and regeneration processes that occur after stroke and reperfusion. Based on the findings from our molecular level study, we propose H3k9me2 as a novel target for the therapeutic development with sex or gender as a crucial factor to be taken into the consideration.
Data Availability
The data that support the findings are available with the corresponding author, may be available on reasonable request.
References
Aanerud, J., Borghammer, P., Rodell, A., Jonsdottir, K. Y., & Gjedde, A. (2017). Sex differences of human cortical blood flow and energy metabolism. Journal of Cerebral Blood Flow & Metabolism, 37(7), 2433–2440.
Abdu, H., & Seyoum, G. (2022). Sex differences in stroke risk factors, clinical profiles, and in-hospital outcomes among stroke patients admitted to the medical ward of dessie comprehensive specialized hospital, Northeast Ethiopia. Degenerative Neurological and Neuromuscular Disease, 12, 133–144.
Alisch, J. S. R., Khattar, N., Kim, R. W., Cortina, L. E., Rejimon, A. C., Qian, W., Ferrucci, L., Resnick, S. M., Spencer, R. G., & Bouhrara, M. (2021). Sex and age-related differences in cerebral blood flow investigated using pseudo-continuous arterial spin labeling magnetic resonance imaging. Aging (albany NY), 13(4), 4911.
Aspey, B. S., Taylor, F. L., Terruli, M., & Harrison, M. J. G. (2000). Temporary middle cerebral artery occlusion in the rat: Consistent protocol for a model of stroke and reperfusion. Neuropathology and Applied Neurobiology, 26(3), 232–242.
Bertogliat, M. J., Morris-Blanco, K. C., & Vemuganti, R. (2020). Epigenetic mechanisms of neurodegenerative diseases and acute brain injury. Neurochemistry International, 133, 104642.
Bouët, V., Freret, T., Toutain, J., Divoux, D., Boulouard, M., & Schumann-Bard, P. (2007). Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Experimental Neurology, 203(2), 555–567.
Chakravarty, S., Jhelum, P., Bhat, U. A., Rajan, W. D., Maitra, S., Pathak, S. S., Patel, A. B., & Kumar, A. (2017). Insights into the epigenetic mechanisms involving histone lysine methylation and demethylation in ischemia induced damage and repair has therapeutic implication. Biochimica Et Biophysica Acta Molecular Basis of Disease, 1863(1), 152–164.
Chen, W., Sun, Y., Liu, K., & Sun, X. (2014). Autophagy: A double-edged sword for neuronal survival after cerebral ischemia. Neural Regeneration Research, 9(12), 1210.
Chisholm, N. C., Henderson, M. L., Selvamani, A., Park, M. J., Dindot, S., Miranda, R. C., & Sohrabji, F. (2015). Histone methylation patterns in astrocytes are influenced by age following ischemia. Epigenetics, 10(2), 142–152.
Covington, H. E., Maze, I., Sun, H. S., Bomze, H. M., DeMaio, K. D., Wu, E. Y., Dietz, D. M., et al. (2011). A role for repressive histone methylation in cocaine-induced vulnerability to stress. Neuron, 71(4), 656–670.
Das, T., Soren, K., Yerasi, M., Kumar, A., & Chakravarty, S. (2019). Revealing sex-specific molecular changes in hypoxia-ischemia induced neural damage and subsequent recovery using Zebrafish model. Neuroscience Letters, 712, 134492.
Dell’Aversana, C., Lepore, I., & Altucci, L. (2012). HDAC modulation and cell death in the clinic. Experimental Cell Research, 318(11), 1229–1244.
Doerfler, W. (2008). In pursuit of the first recognized epigenetic signal––DNA methylation: A 1976 to 2008 synopsis. Epigenetics, 3(3), 125–133.
Donnan, G. A., Fisher, M., Macleod, M., & Davis, S. M. (2008). Stroke. Lancet, 371(9624), 1612–1623.
Dyken, M. L., Klatte, E., Kolar, O. J., & Spurgeon, C. (1974). Complete occlusion of common or internal carotid arteries: Clinical significance. Archives of Neurology, 30(5), 343–346.
Endres, M., Meisel, A., Biniszkiewicz, D., Namura, S., Prass, K., Ruscher, K., Lipski, A., Jaenisch, R., Moskowitz, M. A., & Dirnagl, U. (2000). DNA methyltransferase contributes to delayed ischemic brain injury. Journal of Neuroscience, 20(9), 3175–3181.
Faraco, G., Pancani, T., Formentini, L., Mascagni, P., Fossati, G., Leoni, F., Moroni, F., & Chiarugi, A. (2006). Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Molecular Pharmacology, 70(6), 1876–1884.
Fisher, M. (1954). Occlusion of the carotid arteries: Further experiences. AMA Archives of Neurology & Psychiatry, 72(2), 187–204.
Flaherty, M. L., Flemming, K. D., McClelland, R., Jorgensen, N. W., & Brown, R. D. (2004). Population-based study of symptomatic internal carotid artery occlusion: Incidence and long-term follow-up. Stroke, 35(8), e349–e352.
Formisano, L., Noh, K.-M., Miyawaki, T., Mashiko, T., Bennett, M. V. L., & Suzanne Zukin, R. (2007). Ischemic insults promote epigenetic reprogramming of μ opioid receptor expression in hippocampal neurons. Proceedings of the National Academy of Sciences United State of America, 104(10), 4170–4175.
Harder, D. R., Alkayed, N. J., Lange, A. R., Gebremedhin, D., & Roman, R. J. (1998). Functional hyperemia in the brain: Hypothesis for astrocyte-derived vasodilator metabolites. Stroke, 29(1), 229–234.
Hebbes, T. R., Thorne, A. W., & Crane-Robinson, C. (1988). A direct link between core histone acetylation and transcriptionally active chromatin. The EMBO Journal, 7(5), 1395–1402.
Jayaraj, R. L., Azimullah, S., Beiram, R., Jalal, F. Y., & Rosenberg, G. A. (2019). Neuroinflammation: Friend and foe for ischemic stroke. Journal of Neuroinflammation, 16(1), 1–24.
Jhelum, P., Mydhili Radhakrishnan, A. R., Paul, S., Dey, S. K., Kamle, A., Kumar, A., Sharma, A., & Chakravarty, S. (2022). Neuroprotective and proneurogenic effects of glucosamine in an internal carotid artery occlusion model of ischemia. NeuroMolecular Medicine. https://doi.org/10.1007/s12017-021-08697-5
Jhelum, P., Wahul, A. B., Kamle, A., Kumawat, S., Kumar, A., Bhutani, K. K., Tripathi, S. M., & Chakravarty, S. (2017). Sameerpannag ras mixture (SRM) improved neurobehavioral deficits following acute ischemic stroke by attenuating neuroinflammatory response. Journal of Ethnopharmacology, 197, 147–156.
Jørgensen, H. S., Nakayama, H., Raaschou, H. O., & Olsen, T. S. (1999). Stroke: Neurologic and functional recovery the Copenhagen stroke study. Physical Medicine and Rehabilitation Clinics of North America, 10(4), 887–906.
Karisetty, B. C., Joshi, P. C., Kumar, A., & Chakravarty, S. (2017). Sex differences in the effect of chronic mild stress on mouse prefrontal cortical BDNF levels: A role of major ovarian hormones. Neuroscience, 356, 89–101.
Kim, H. J., Rowe, M., Ren, M., Hong, J.-S., Chen, P.-S., & Chuang, D.-M. (2007). Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: Multiple mechanisms of action. Journal of Pharmacology and Experimental Therapeutics, 321(3), 892–901.
Klöting, I., Nitschke, C., & van den Brandt, J. (2003). Impact of genetic profiles on experimental studies: Outbred versus wild rats. Toxicology and Applied Pharmacology, 189(1), 68–71.
Krupinski, J., Carrera, C., Muiño, E., Torres, N., Al-Baradie, R., Cullell, N., & Fernandez-Cadenas, I. (2018). DNA methylation in stroke. Update of latest advances. Computational and Structural Biotechnology Journal, 16, 1–5.
Kumar, A., Choi, K.-H., Renthal, W., Tsankova, N. M., Theobald, D. E. H., Truong, H.-T., Russo, S. J., et al. (2005). Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron, 48(2), 303–314.
Leng, X., Fang, H., Leung, T. W. H., Mao, C., Miao, Z., Liu, L., Wong, K. S., & Liebeskind, D. S. (2016). Impact of collaterals on the efficacy and safety of endovascular treatment in acute ischaemic stroke: A systematic review and meta-analysis. Journal of Neurology, Neurosurgery & Psychiatry, 87(5), 537–544.
Lu, X., Jian Zhang, Y., Ding, J. W., & Chen, G. (2022). Novel therapeutic strategies for ischemic stroke: Recent insights into autophagy. Oxidative Medicine and Cellular Longevity, 2022, 1–15.
Manwani, B., Bentivegna, K., Benashski, S. E., Venna, V. R., Yan, X., Arnold, A. P., & McCullough, L. D. (2015). Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. Journal of Cerebral Blood Flow & Metabolism, 35(2), 221–229.
Marks, P. A. (2010). Histone deacetylase inhibitors: a chemical genetics approach to understanding cellular functions. Biochimica Et Biophysica Acta Gene Regulatory Mechanisms, 1799, 717–725.
McAuley, M. A. (1995). Rodent models of focal ischemia. Cerebrovascular and Brain Metabolism Reviews, 7(2), 153–180.
Mead, G. E., Shingler, H., Farrell, A., O’neill, P. A., & Mccollum, C. N. (1998). Carotid disease in acute stroke. Age and Ageing, 27(6), 677–682.
Mhairi, M. I. (1992). New models of focal cerebral ischaemia. British Journal of Clinical Pharmacology, 34(4), 302–308.
Moskowitz, M. A., Lo, E. H., & Iadecola, C. (2010). The science of stroke: Mechanisms in search of treatments. Neuron, 67(2), 181–198.
Narlikar, G. J., Fan, H.-Y., & Kingston, R. E. (2002). Cooperation between complexes that regulate chromatin structure and transcription. Cell, 108(4), 475–487.
Patrizz, A. N., Moruno-Manchon, J. F., O’Keefe, L. M., Doran, S. J., Patel, A. R., Venna, V. R., Tsvetkov, A. S., Li, J., & McCullough, L. D. (2021). Sex-specific differences in autophagic responses to experimental ischemic stroke. Cells, 10(7), 1825.
Ren, M., Leng, Y., Jeong, MiRa., Leeds, P. R., & Chuang, D.-M. (2004). Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats: Potential roles of histone deacetylase inhibition and heat shock protein induction. Journal of Neurochemistry, 89(6), 1358–1367.
Roy-O’Reilly, M., & McCullough, L. D. (2018). Age and sex are critical factors in ischemic stroke pathology. Endocrinology, 159(8), 3120–3131.
Schweizer, S., Harms, C., Lerch, H., Flynn, J., Hecht, J., Yildirim, F., Meisel, A., & Märschenz, S. (2015). Inhibition of histone methyltransferases SUV39H1 and G9a leads to neuroprotection in an in vitro model of cerebral ischemia. Journal of Cerebral Blood Flow & Metabolism, 35(10), 1640–1647.
Shigeno, T., Teasdale, G. M., McCulloch, J., & Graham, D. I. (1985). Recirculation model following MCA occlusion in rats: Cerebral blood flow, cerebrovascular permeability, and brain edema. Journal of Neurosurgery, 63(2), 272–277.
Song, T.-J., Jeong, Y., Park, J., & Gwak, H. S. (2020). Risk factors and prevention of stroke. Cerebrovascular Diseases, 49(1), 1–149.
Stenzel-Poore, M. P., Stevens, S. L., King, J. S., & Simon, R. P. (2007). Preconditioning reprograms the response to ischemic injury and primes the emergence of unique endogenous neuroprotective phenotypes: A speculative synthesis. Stroke, 38(2), 680–685.
Sudlow, C. L. M., & Warlow, C. P. (1997). Comparable studies of the incidence of stroke and its pathological types: Results from an international collaboration. Stroke, 28(3), 491–499.
Sundt, T. M., Grant, W. C., & Garcia, J. H. (1969). Restoration of middle cerebral artery flow in experimental infarction. Journal of Neurosurgery, 31(3), 311–322.
Szadvári, I., Ostatníková, D., & Durdiaková, J. B. (2023). Sex differences matter: Males and females are equal but not the same. Physiology & Behavior, 259, 114038.
Tamura, A., Graham, D. I., McCulloch, J., & Teasdale, G. M. (1981). Focal cerebral ischaemia in the rat: Description of technique and early neuropathological consequences following middle cerebral artery occlusion. Journal of Cerebral Blood Flow & Metabolism, 1(1), 53–60.
Ten, V., & Galkin, A. (2019). Mechanism of mitochondrial complex I damage in brain ischemia/reperfusion injury: A hypothesis. Molecular and Cellular Neuroscience, 100, 103408.
Thanvi, B., & Robinson, T. (2007). Complete occlusion of extracranial internal carotid artery: Clinical features, pathophysiology, diagnosis and management. Postgraduate Medical Journal, 83(976), 95–99.
Thiele, B. L., Young, J. V., Chikos, P. M., Hirsch, J. H., & Strandness, D. E. (1980). Correlation of arteriographic findings and symptoms in cerebrovascular disease. Neurology, 30(10), 1041–1041.
Trotman-Lucas, M., & Gibson, C. L. (2021). A review of experimental models of focal cerebral ischemia focusing on the middle cerebral artery occlusion model. F1000Research, 10, 242.
Tsankova, N. M., Berton, O., Renthal, W., Kumar, A., Neve, R. L., & Nestler, E. J. (2006). Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nature Neuroscience, 9(4), 519–525.
Tsankova, N. M., Kumar, A., & Nestler, E. J. (2004). Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. Journal of Neuroscience, 24(24), 5603–5610.
Tsankova, N., Renthal, W., Kumar, A., & Nestler, E. J. (2007). Epigenetic regulation in psychiatric disorders. Nature Reviews Neuroscience, 8(5), 355–367.
Wahul, A. B., Joshi, P. C., Kumar, A., & Chakravarty, S. (2018). Transient global cerebral ischemia differentially affects cortex, striatum and hippocampus in bilateral common carotid arterial occlusion (BCCAo) mouse model. Journal of Chemical Neuroanatomy, 92, 1–15.
Wilkinson, M. B., Xiao, G., Kumar, A., LaPlant, Q., Renthal, W., Sikder, D., Kodadek, T. J., & Nestler, E. J. (2009). Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. Journal of Neuroscience, 29(24), 7820–7832.
Zampino, M., Yuzhakova, M., Hansen, J., McKinney, R. D., Goldspink, P. H., Geenen, D. L., & Buttrick, P. M. (2006). Sex-related dimorphic response of HIF-1α expression in myocardial ischemia. American Journal of Physiology-Heart and Circulatory Physiology, 291(2), H957–H964.
Zhang, Y.-Z., Zhang, Q.-H., Ye, H., Zhang, Y., Luo, Y.-M., Ji, X.-M., & Ying-Ying, Su. (2010). Distribution of lysine-specific demethylase 1 in the brain of rat and its response in transient global cerebral ischemia. Neuroscience Research, 68(1), 66–72.
Zhao, H., Han, Z., Ji, X., & Luo, Y. (2016). Epigenetic regulation of oxidative stress in ischemic stroke. Aging and Disease, 7(3), 295.
Zhong, X., Sun, Y., Yajun, Lu., & Lei, Xu. (2023). Immunomodulatory role of estrogen in ischemic stroke: Neuroinflammation and effect of sex. Frontiers in Immunology, 14, 1164258.
Zwemer, C. F., Song, M. Y., Carello, K. A., & D’Alecy, L. G. (2007). Strain differences in response to acute hypoxia: CD-1 versus C57BL/6J mice. Journal of Applied Physiology, 102(1), 286–293.
Acknowledgements
This research was initiated by the Council of Scientific and Industrial Research (CSIR), India network projects [(BSC0103-UNDO) to SC and AK] and later supported by ICMR grant [5/4-5/3/17/Neuro/2022-NCD-1 to SC]. MR, VV, PBT, SP and KS wish to acknowledge CSIR India, Department of Biotechnology (DBT) and DST-INSPIRE for their doctoral fellowships, respectively. In addition, the authors would like to specially acknowledge B. Jyothilakshmi of the Centre for Cellular and Molecular Biology (CCMB), Hyderabad, for the maintenance and care of animals throughout the study period. KIM Department of CSIR-IICT is greatly acknowledged for generating institutional publication number IICT/Pubs/2023/186.
Author information
Authors and Affiliations
Contributions
SC and AK conceived the study. SC and AK designed and supervised all the experiments. The experiments performed by MR, VV, SA, PB, SP, KS, and SC, SC and AK arranged the resources and analyzed the results. MR, SC, and AK. wrote the original draft of the manuscript and made the edits to make the final version. All authors reviewed and edited the manuscript for their part.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Radhakrishnan, M., Vijay, V., Supraja Acharya, B. et al. Uncovering Sex-Specific Epigenetic Regulatory Mechanism Involving H3k9me2 in Neural Inflammation, Damage, and Recovery in the Internal Carotid Artery Occlusion Mouse Model. Neuromol Med 26, 3 (2024). https://doi.org/10.1007/s12017-023-08768-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12017-023-08768-9