Abstract
Many population-based epidemiological studies have unveiled an inverse correlation between intake of herbal plants and incidence of stroke. C. nutans is a traditional herbal medicine widely used for snake bite, viral infection and cancer in Asian countries. However, its role in protecting stroke damage remains to be studied. Despite of growing evidence to support epigenetic regulation in the pathogenesis and recovery of stroke, a clear understanding of the underlying molecular mechanisms is still lacking. In the present study, primary cortical neurons were subjected to in vitro oxygen–glucose deprivation (OGD)–reoxygenation and hypoxic neuronal death was used to investigate the interaction between C. nutans and histone deacetylases (HDACs). Using pharmacological agents (HDAC inhibitor/activator), loss-of-function (HDAC siRNA) and gain-of-function (HDAC plasmid) approaches, we demonstrated an early induction of HDAC1/2/3/8 and HDAC6 in neurons after OGD insult. C. nutans extract selectively inhibited HDAC1 and HDAC6 expression and attenuated neuronal death. Results of reporter analysis further revealed that C. nutans suppressed HDAC1 and HDAC6 transcription. Besides ameliorating neuronal death, C. nutans also protected astrocytes and endothelial cells from hypoxic-induced cell death. In summary, results support ability for C. nutans to suppress post-hypoxic HDACs activation and mitigate against OGD-induced neuronal death. This study further opens a new avenue for the use of herbal medicines to regulate epigenetic control of brain injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke (cerebrovascular accident, CVA) is caused by either lacking of blood flow (ischemic) or leakage of blood (hemorrhage) in brain parenchyma, leading to rapid neuron cell death and loss of brain functions. Stroke is the third leading cause of death and the leading cause of adult disability worldwide. It is estimated that around 6.4 million people in USA suffer stroke with 85 % ischemic stroke and 15 % hemorrhagic stroke. Ischemic stroke (or cerebral ischemia) triggers a complex series of biochemical and molecular mechanisms involving excitotoxicity, calcium overload and oxidative stress leading to impaired neurologic functions, breakdown of cell integrity, cell death and development of infarction (Mehta et al. 2007).
Cerebral ischemia is known to alter the expression of a myriad of genes, suggesting that epigenetic modifications may play an important role in the pathogenesis and recovery of stroke (Fessler et al. 2013; Schweizer et al. 2013; Ziemka-Nalecz and Zalewska 2014).
Advances in the field of epigenetics have opened a new avenue for stroke treatment (Aune et al. 2015). Unlike simple genetics based on changes in DNA sequence (the genotype), epigenetic modifiers have been demonstrated to influence the expression of groups of genes through post-translational modification of histones or DNA. These modifications can include acetylation, methylation, phosphorylation, ubiquitination and citrullination. Among them, modulation of histone acetylation appears to be well studied. Histones are acetylated by histone acetyl transferase (HAT) enzymes, and histone deacetylases (HDACs) remove these modifications. Many compounds with known anti-inflammatory and neuroprotective functions, such as trichostatin (TSA) and valproic acid, have HDAC inhibitory activity. There is evidence that HDAC inhibitors possess anti-inflammatory as well as neuroprotective actions in experimental stroke models, suggesting that they may offer therapeutic potential for ameliorating CNS disorders (Fessler et al. 2013; Schweizer et al. 2013; Ziemka-Nalecz and Zalewska 2014;).
Multicenter clinical trials and large population epidemiological studies have focused on use of herbal medicine for treatment of stroke (Chen et al. 2012). Clinacanthus nutans Lindau (C. nutans), also known as Sabah snake grass, Belalai gajah (Malay) and 憂遁草 (Mandarin), is a herbal plant used for snake and insect bites, skin rashes and cancer due to its analgesic, anti-inflammatory, antiviral and antioxidant properties (Pongphasuk and Khunkitti 1996; Daduang et al. 2005; Sakdarat et al. 2006; Uawonggul et al. 2006; Yong et al. 2013). However, possible use of this herb to ameliorate stroke injury has not been investigated. A recent study by Tan et al. (2015) demonstrated regulation of cytosolic phospholipase A2 (cPLA2) mRNA expression by epigenetic factors and effects of C. nutans to modulate cPLA2 mRNA expression (Tan et al., submitted this issue). In the present study, we examined effects of C. nutans on the expression of HDAC isomers and their causal relationship on cell viability using primary cortical neurons subjected to transient hypoxic insult.
Materials and Methods
Plant Materials and Plant Extracts
The leaves extract of Clinacanthus nutans (CN) was provided by Prof. Ong Wei-Yi (National University of Singapore). In brief, the leaves (778 g) were rinsed with distilled water and soaked in 80 % ethanol (2 L) for 1 h. The leaves were blended using a homogenizer (Wiggen Hauser D-500), and the mixture was left to stand for 1 h. The ethanol extract was then filtered under vacuum (Gast USA DOA-PIO4-BN) using 90-mm glass microfiber filter membranes (Whatman, Little Chalfont, Buckinghamshire, UK). The filtrate was concentrated in a rotary evaporator at 50 °C (Buchi Labortechnik AG, Postfach, Switzerland). The resultant dark green condensate was subjected to freeze-drying (Christ Gamma 1-16 LSC) for 1–2 days. After drying, the resultant solid residue (27.02 g) appeared as a dark green powder and was stored for long-term usage at −80 °C. The extract yield (w/w) from 778 g of fresh SSG leaves was approximately 3.5 %. Prior to usage, the powder extract was dissolved in dimethyl sulfoxide (DMSO), and the final concentration of DMSO in each reaction is less than 0.1 %, which showed no toxic effect on primary cortical neurons (PNs).
Cell Cultures and Oxygen–Glucose Deprivation (OGD) Model
Primary cortical neurons (PNs) were prepared from E15.5 mouse embryos and cultured in neurobasal plus B27 medium (Gibco, Grand Island, NY) containing 2 mM l-glutamine, 10 μM glutamate, 1.6 % FBS, 0.4 % HS and penicillin/streptomycin. On days in vitro (DIV) 3, the cells were treated with 1 µM cytosine arabinoside (Ara-C) for 72 h to prevent glial proliferation and then maintained in serum-free neurobasal plus B27 medium at 37 °C in a humidified 5 % CO2 incubator. Experiments were conducted on DIV 8–10 (Huang et al. 2015). Cerebral astrocytes were obtained from newborn mice (C57BL6; postnatal day 1) following methods described previously with modifications (Yin et al. 2006). In brief, the dissected cerebral cortices were mechanically triturated in glia culture medium [DMEM/F12 (Invitrogen) with 10 % FBS, 100 U/ml penicillin and 100 μg/ml streptomycin], filtered through a 40-μm cell strainer, and the filtered cells were plated in 10-mm cell culture dishes coated with poly-l-lysine and grown in glia culture medium for 10 days followed by orbital shaking at 100 rpm in 37 °C for 6 h to remove the residual microglia and oligodendrocyte precursor cells. The final attached cells, mostly astrocytes, were used for experiments at day 3 after final seeding, and the purity was 95 %, as determined by GFAP immunostaining. Mouse cerebral endothelial cells (CECs) were prepared as described previously (Hu et al. 2006; Wu et al. 2014). CECs were grown to 70 % confluence in DMEM supplemented with 10 % FBS in a humidified 5 % CO2 atmosphere.
CN, trichostatin A (TSA; Sigma-aldrich, St. Louis, MO), MS275 (Sigma-aldrich), theophylline (Sigma-aldrich), MC1568 (Sigma-aldrich), NU9056 (Santa Cruz Bio, Santa Cruz, CA) and ITSA-1 (Sigma-aldrich) were added into cells either alone or in different combinations for 1 h prior to OGD treatment (Goldberg and Choi 1993; Huang et al. 2015). All chemicals were dissolved in DMSO (final concentration ≤0.1 %). In brief, OGD was conducted in a temperature-controlled (37 °C ± 1 °C) anaerobic chamber (Model 1025, Forma Scientific, Marietta, OH, USA) containing a gas mixture of 5 % CO2, 10 % H2, 85 % N2 and 0.02–0.1 % O2. Primary neurons on DIV 10 or cells grown to 70 % confluence were washed with deoxygenated glucose-free Hanks’ balanced salt solution (HBSS, GIBCO-BRL) and then transferred to an anaerobic chamber for 30 min. After OGD, cells then underwent reoxygenation by adding equal volume of oxygenated glucose-containing HBSS and returned to the normoxic 5 % CO2/95 % air incubator for various times. Cell viability was determined by use of the cell counting kit 8 (CCK-8) (Dojindo Molecular Technologies, Kumamoto, Japan).
RNA Isolation, Reverse Transcription (RT) and Polymerase Chain Reaction (PCR)
RNA isolation was performed as previously described (Wu et al. 2009). Total RNA (4 μg) was incubated with RevertAid™ H Minus First Strand cDNA Synthesis Kit (Fermentas, Vilnius, Lithuania). The reaction mixture was incubated at 65 °C for polydT oligomer annealing and then extension in buffer, dNTP, reverse transcriptase and RNase inhibitor in a final volume of 20 μl at 42 °C for 1 h and then 70 °C for 5 min to inactivate the enzyme. Finally, a total of 80 μl DEPC-treated water was added to the reaction mixture before storage at −80 °C. One to 2 μl of the RT reaction solution was used in the PCR. PCR was carried out in a 25 μl final volume containing 0.2 mM dNTP, 0.1 μM of each primer and 1 unit of Tag polymerase (NEB, Ipswich, MA). The mixture was subjected to PCR amplification for 25–30 cycles and incubated at 72 °C for 10 min then cooled to 4 °C (PE, Norwalk, CT). Primer sequences for HDAC1 to HDAC11 are: HDAC1: F 5′-gagacggcattgacgacgaatcctat-3′, R 5′-tgcgtttatcagaggagcagatggag-3′; HDAC2: F 5′-tacaacagatcgcgtgatgaccgt-3′, R 5′-agcaacattcctacgacctccttcac-3′; HDAC3: F 5′-caatgtgcccttacgagatggcattg-3′, R 5′-caccacagaggtgacaaggaactctt-3′; HDAC4: F 5′-agaggctgaatgtgagcaagatcctc-3′, R 5′-acgcaggagtgatacgggtaagtttc-3′; HDAC5: F 5′-gactgcattcaggtcaaggatgagga-3′, R 5′-ccatggtgaatatcccagtccacgat-3′; HDAC6: F 5′-gaagtggaagaagccgtgctagaaga-3′, R 5′-cataccggtgcagggacacgtataat-3′; HDAC7: F 5′-caagaaatccctggagagacgcaaga-3′, R 5′-actccctatgttccaggccatcattc-3′; HDAC8: F 5′-cctgggaatattacgattgcgacgga-3′, R 5′-aaccgcttgcatcaacacactgtc-3′; HDAC9: F 5′-aggagcacatcaaggaacttctagcc-3′, R 5′-acaccttgtctgagcatctgtgtctc-3′; HDAC10: F 5′-ggccagggcatccagtatatcttcaa-3′, R 5′-tcaagactgacccttcttgatggagc-3′; and HDAC11: F 5′-aaggaagaaggaagctgggattctcc-3′, R 5′-gaaggacactatgaaggctgtgggaa-3′ (Chen et al. 2012) by MDBIO, INC. (Taipei, Taiwan).
Western Blot analysis
Western blot analysis of proteins was performed as described (Wu et al. 2015) with HDAC1 (1:3000), HDAC6 (1:3000) and GAPDH (1:2000) antibodies from GeneTex Inc. (San Antonio, TX). Protein bands were visualized by enhanced chemiluminescence system (Merck Millipore; Billerica, MA, USA). Subcellular fractions of primary neurons were prepared using the ProteoJET Cytoplasmic and Nuclear Protein Extraction Kit (Fermentas, Burlington, ON, Canada).
Small Interference RNA (siRNA) Transient Transfection
SiRNA and scramble RNA (scRNA, control) were purchased MDBIO, INC (Taipei, Taiwan; HDAC1, scRNA). mRNA-In® Neuro (MTI-GlobalStem, Gaithersburg, MD, USA) was used as transfection carrier; in brief, 1.5 µl of mRNA-In® Neuro and 20 pmol siRNA was mixed in 50 µl of Optimedium and then added into 24-well dish containing 2.5 × 105 primary neurons/well. At 5 h after transfection, the medium was replaced by culture medium containing neurobasal plus B27 medium and cultured for an additional 19 h before OGD treatment. Transfection efficiency (~70 %) was evaluated by transfection of FAM-labeled siRNA (MDBio), and enhanced green fluorescent protein in primary neurons was measured by flow cytometry and fluorescence microscopy.
Plasmid Constructs, Transient Transfection and Reporter Assay
HDAC1 plasmid p181 pK7-HDAC1 (GFP) and p182 pK7-HDAC1 mut (GFP) were gift from Ramesh Shivdasani (Addgene plasmid # 11054 & 11055, Cambridge, MA; Tou et al. 2004). HDAC1 reporter constructs were prepared by cloning a mouse promoter sequence into the pGL4-Luc vector (Promega, Madison, WI, USA) as previously described (Wu et al. 2014).
For cloning HDAC1 (m) promoter, 5′-flanking region of mouse genomic sequence was synthesized by PCR using the following primers: (1) p1000-Luc (−1000 to 1), F: 5′-tttggtacccgtcccccccaaacaacaactta-3′, R: 5′-tttctcgagcctcccgtcagtctgtccg-3′; (2) p520-Luc (−520 to 1), F: 5′-tttggtaccagcacataaaggtcttgcccca-3′, R: 5′-tttctcgagcctcccgtcagtctgtccg-3′; and (3) p240-Luc (−240 to 1), F: 5′-tttggtaccttctctaagctgcccttgccc-3′, R: 5′-tttctcgagcctcccgtcagtctgtccg-3′. For cloning HDAC6 promoter, 5′-flanking region of mouse genomic sequence was synthesized by PCR using the following primers: (1) p1000-Luc (−1000 to 1), F: 5′-tttggtaccttttccttcctcggtccacc-3′, R: 5′-tttctcgagatctcccgcccagcca-3′. PCR products were cloned into pGL4 luciferase reporter. A minimal cytomegalovirus promoter pCMV-β-galactosidase (β-Gal) plasmid (Promega) was used as an internal control of transfection. Cells were lysed with reporter lysis buffer (Promega), and the luciferase activity was then determined by mixing 50 μL of the cell lysate with 50 μL of the luciferase assay reagent.
Transfection protocol for mouse primary cortical neurons was adopted from N2A neuroblastoma cells as previously described with modifications (Wu et al. 2015). In briefly, 1.25 μL of DNA-In® Neuro (MTI-GlobalStem) and 0.5ug of DNA were mixed in 30 μL of Opt medium and then added into 24-well dish containing 2.7 × 105 neuronal cells/well. Three hours after transfection, 0.5 ml medium was added to each well and incubated for an additional 21 h prior to OGD treatment. Transfection efficiency (~25 %) was evaluated by transfection of pENGF-N1, and measurement of enhanced green fluorescent protein in cells by flow cytometry, reporter activity/fluorescence microscopy, western blot and confocal microscope.
Statistical Analysis
ANOVA was used to compare the expression of proteins, mRNA or infarct volumes. Differences between groups were further analyzed by post hoc Fisher’s protected t test by use of GB-STAT 5.0.4 (Dynamic Microsystems, Silver Springs, MD). P < 0.05 was considered significant. We use at least n = 3 in triplicates for in vitro studies and n ≥ 6 for in vivo studies.
Results
-
A.
C. nutans (CN) protects mouse primary cortical neurons (PNs), astrocytes and endothelial cells (CECs) from hypoxia-induced cell death
In this study, PNs, astrocytes and CECs were subjected to a protocol involving 0.5-h OGD and 24-h reoxygenation (H0.5R24). All three cell types showed decrease in cell viability after OGD insult (Fig. 1). While only 37.3 ± 4.5 % of PNs remained viable at 24 h of reoxygenation, pretreatment of 5 μg/ml CN significantly increased PNs viability to 58 ± 5.6 % (Fig. 1). Similar degrees of protection were also noted in astrocytes (38.2–56.3 %) and CECs (40.9–57 %) (Fig. 1).
C. nutans (CN) protects brain neural cells from OGD-induced cell death. Primary cortical neurons (PNs), astrocytes and endothelial cells (CECs) are pretreated with 5 μg/ml CN for 1 h before subjected to 0.5-h OGD and 24-h reoxygenation (H0.5R24) and subsequently for cell viability analysis. H0 refers to no OGD normal control. Data are mean ± SD of at least three independent experiments performed in triplicate. **P < 0.01 versus vehicle control (Con)
-
B.
C. nutans (CN) protects mouse primary cortical neurons (PNs) from hypoxia-induced cell death via inhibiting HDAC1/6 transcription
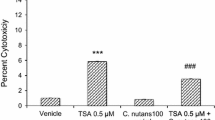
To investigate whether epigenetic regulation is involved in the protective function of CN, PNs were incubated with TSA (HDAC class I & II inhibitor; 10 μM), TSA + ITSA-1 (TSA inhibitor; 1 mM), MS275 (HDAC class I inhibitor; 1 μM), MC1568 (HDAC class II inhibitor; 20 μM), MU9056 (HAT inhibitor; 1.25 μM), CN (5 μg/ml), CN + TSA or CN + ITSA-1 for 1 h prior to subjecting to H0.5R24. Cell viability analysis indicated that PNs viability was decreased to ~37 % after H0.5R24. TSA and MS275 significantly increased cell viability to 52 and 50 %, respectively, while no visible changes in cell viability were noted in PNs treated with MC1568 or NU9056 (Fig. 2). Meanwhile, the protective effect of TSA was neutralized in the presence of its inhibitor ITSA-1 (39 % viability). CN significantly increased cell survival to 61 %. Intriguingly, TSA did not further enhance the protective effects of CN (59 % survival), whereas ITSA-1 abolished the beneficial effect of CN. These results suggest that CN and TSA may share same signaling mechanisms for their beneficial effect and likely to involve HDACs.
C. nutans (CN) attenuating OGD-induced neuronal death is mediated by HDACs. PNs are pretreated with 10 μM TSA, 10 μM TSA + 1 mM ITSA-1, 1 μM MS275, 20 μM MC1568, 1.25 μM NU9056, 5 μg/ml CN, CN + TSA or CN + ITSA-1 for 1 h and then subjected to H0.5R24 for cell viability analysis. H0 refers to no OGD normal control. Data are mean ± SD of at least 3 independent experiments performed in triplicate. **P < 0.01 versus H0.5R24 vehicle control (Con)
Histone deacetylases (HDACs) are divided into 4 groups: class I isomers (HDAC1, 2, 3 and 8) are localized to the nucleus; class IIa isomers (HDAC4, 5, 7 and 9) are found in both nucleus and cytoplasm; class IIb isoforms (HDAC6 and 10) are confined to the cytoplasm; class IV isoform (HDAC11) has properties of both class I and class II; and class III isoforms (or sirtuins) form a structurally distinct class of NAD-dependent and TSA-insensitive enzymes, which are different from the Zn-dependent and TSA-sensitive HDACs (Thiagalingam et al. 2003).
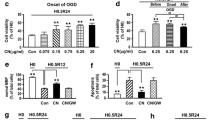
To ascertain whether the beneficial effect of CN is HDAC dependent, we examine the mRNA levels of the TDA-sensitive HDAC1 to 11, after hypoxic insult (Fig. 3). A significant increase in the level of HDAC1, 2, 3, 6 and 8 was noted in PNs after H0.5R4 challenge. Among them, only HDAC1 and HDAC6 mRNA levels were repressed by CN (Fig. 3).
C. nutans (CN) selectively inhibited OGD-induced HDAC1 and HDAC6 mRNAs upregulation in primary cortical neurons. PNs are pretreated with 5 μg/ml CN for 1 h and then subjected to H0.5R4 for isoforms of HDAC mRNA analysis. Representative RT-PCR mRNA bands are semi-quantified by densitometry and normalized to that of β-actin. H0 refers to no OGD normal control. Data are mean ± SD of at least 3 independent experiments performed in triplicate. *P < 0.05, **P < 0.01 versus vehicle control (Con)
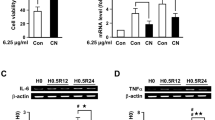
We then examined the temporal profile for CN on HDAC1 and HDAC6 expression following transient hypoxia insult. When PNs were subjected to 30-min OGD followed by different reoxygenation times, there was a transient induction of both HDAC1 and HDAC6 mRNAs, which was already maximal at 30 min after OGD (R0), and then gradually declined (Fig. 4a, b). CN pretreatment protected PNs from the transient increase in HDAC1 and HDAC6. In addition, OGD not only changed mRNA, HDAC1 and HDAC6 protein levels were also marked increased 4 h after H0.5R4 insult, and pretreatment of CN nearly completely abrogated the increase due to OGD (Fig. 4c, d).
C. nutans (CN) time-dependently suppressed OGD-induced HDAC1 and HDAC6 mRNAs upregulation in primary cortical neurons. PNs are pretreated with 5 μg/ml CN for 1 h and then subjected to 0.5-h OGD and various reoxygenation periods for HDAC1 and HDAC6 mRNAs (a, b) and proteins (c, d) analysis. Representative mRNA or protein bands are semi-quantified by densitometry and normalized to that of β-actin or GAPDH, respectively. H0 refers to no OGD normal control. H0 refers to no OGD normal control. Data are mean ± SD of at least three independent experiments performed in triplicate. *P < 0.05, **P < 0.01 versus vehicle control (Con)
The aforementioned data suggested CN might regulate HDAC1 and HDAC6 at the transcription level. To test this hypothesis, we sub-cloned 5′-flanking regions of mouse HDAC1 genome into pGL4 luciferase reporter vector: (1) p1000-Luc, a 1000-bp (−1000 to 1); (2) p520-Luc, a 520-bp (−520 to 1); and (3) p240-Luc, a 240-bp (−240 to 1) 5′ region of HDAC1 genome, as well as mouse HDAC6 gene: (4) p1000-Luc, a 1000-bp (−1000 to 1) 5′ region of HDAC6 genome (Fig. 5). To determine the role of CN on HDAC1 and HDAC6 transcription, the reporter constructs were transfected to PNs 24 h before subjected to H0.5R24 (Fig. 5). HDAC1 p1000-Luc reporter was turn on after H0.5R24 challenge, while CN attenuated hypoxia-induced reporter activation. CN’s reporter inhibitory effect was not observed when PNs were transfected with truncated p520-Luc and p240-Luc reporter, suggesting −1000 to −520 region harbored critical site(s) for HDAC1 transcription. Similarly, HDAC6 p1000-Luc reporter was also turned on after H0.5R24 challenge, and reporter activation was inhibited by CN (Fig. 5).
C. nutans (CN) silences OGD-activated HDAC1 and HDAC6 transcription. PNs are transfected with 0.5 μg of serial deleted HDAC1 or HDAC6 reporter plasmids for 24 h, incubated with 5 μg/ml CN for another h and then subjected to H0.5R24 for reporter analysis. Reporter activity is expressed as relative light unit (RLU) with β-gal (b-Gal) as normalization control. H0 refers to no OGD normal control. Data are mean ± SD of at least three independent experiments performed in triplicate. **P < 0.01 versus vehicle control (Con)
-
C.
HDAC1 siRNA knockdown ameliorates, while HDAC1 activation or overexpression exaggerates hypoxia-induced neuron death after H0.5R24 insult
In order to study the impact of HDAC1 on cell survival, PNs were transfected with HDAC1 siRNA for 24 h and then subjected to OGD–reoxygenation. Pretreatment of HDAC1 siRNA decreased HDAC1 protein level (Fig. 6a), and this was marked by a reciprocal increase in the viability of PNs after H0.5R24 (Fig. 6b). On the other hand, when PNs were pretreated with 10 μM theophylline, a known HDAC1 activator, expression of HDAC1 was enhanced (Fig. 6c), but this condition led to a decrease in cell viability as compared with control group (Fig. 6d). Similarly, increased HDAC1 mRNA was observed when theophylline was added to cells pretreated with CN, and the presence of theophylline abrogated the effects of CN (Fig. 6c, d). Furthermore, the beneficial effect of CN was abrogated in PNs transfected with wild-type HDAC1, but not with mut-HDAC1 plasmid after H0.5R24 insult (Fig. 6e). In addition, PNs transfected with wild-type HDAC 1 plasmid showed less viability than transfected with mut-HDAC1 plasmid at H0.5R24 (Fig. 6e).
HDAC1 knockdown increases, while over-expression decreases neuronal survival after OGD insult. The efficacy of HDAC1 siRNA is evaluated by western blot (a). PNs are transfected with 40 nM HDAC1 scRNA or siRNA for 24 h and subjected to H0.5R24 for cell viability analyses (b). PNs are incubated with 5 μg/ml CN or/and 10 μM theophylline (TH) for another h and then subjected to H0.5R24 for HDAC1 mRNA (c) and cell viability analysis (d). PNs are transfected with 0.5 μg HDAC1 or mut-HDAC1 plasmid for 24 h and then incubated with CN for 1 h before subjected to H0.5R24 for cell viability analyses (e). H0 refers to no OGD normal control. Data are mean ± SD of at least 3 independent experiments performed in triplicate. **P < 0.01 versus vehicle control (Con)
Discussion
There is evidence that cerebral ischemia is marked by a general decrease in histone acetylation suggesting possible activation of histone deacetylases (HDACs) or subsequent transcription repression (Fessler et al. 2013; Schweizer et al. 2013; Ziemka-Nalecz and Zalewska 2014; Aune et al. 2015). These findings led to use of pan-HDAC inhibitors (HDACi), such as trichostatin/TSA and valproic acid/VPA, to reduce ischemic damage (Fessler et al. 2013; Schweizer et al. 2013; Aune et al. 2015). Others reported that knocking-in HDAC4 and HDAC5 can protect neurons from OGD injury and HDAC9 SNP is associated with large vessel ischemic stroke (Bellenguez et al. 2012; He et al. 2013; Aune et al. 2015). Thus, despite implication of HDACs in ischemic stroke, the exact mechanism remains elusive.
In the present study, we showed that primary cortical neurons subjected to OGD–reoxygenation led to a transient induction of HDAC1/2/3/8 (class I) and HDAC6 (class IIb) and demonstrated for the first time that knockdown of HDAC1 increased cell viability, while HDAC1 overexpression decreased cell survival. Our findings are in line with previous report that HDAC3 and HDAC6 were increased during the early phase of experimental stroke, while HDAC3/6 knockdown increased neuronal survival (Chen et al. 2012); in addition, HDAC6 inhibition alleviated stroke-induced brain infarction and functional deficits (Wang et al. 2016). Whether induction of HDAC2/8 also contributes to neuronal death deserves further investigation.
Recent epidemiological studies showed that some natural herbs can confer prophylactic effects against stroke incidence (Chen et al. 2012). Despite the use of C. nutans in treating snake bite and viral infection, and recently for cancer therapy, whether this herbal plant may exert protective effects on injury of the central nervous system has not been explored in detail. In the present study, we demonstrated for the first time that ethanol extract of leaves from C. nutans protected not only neurons but also astrocyte and endothelial cells from hypoxia-induced cell death.
Results further show that C. nutans attenuated neuronal death via inhibiting hypoxia-induced early HDAC1 and HDAC6 transcription. C. nutans extract is known to contain many components including lupeol, vitexin, orientin and cerebrosides and has been reported to have anti-inflammatory effects (Saleem 2009; Loizou et al. 2010; Borghi et al. 2013; Mandal et al. 2014; Zhou et al. 2014). Further studies are needed to identify the active ingredient(s) responsible for the neuroprotective effects. Once the key components are identified, it will be important to further investigate the transcription factor(s) responsible for inhibiting HDAC gene expression.
In summary, the present study provided evidence that inhibitors of epigenetic regulator HDACs can confer multifaceted neuroprotective actions. In this study, we further provide initial findings that C. nutans, a medicinal herb, can act as a HDACs inhibitor to attenuate the transcriptional activity of HDAC1 and HDAC6 and subsequent ameliorate hypoxic neuronal death. This neuroprotective signaling cascade brings this herb to a new horizon and opens a new avenue for possibility to use this product to treat ischemic brain injury.
References
Aune, S. E., Herr, D. J., Kutz, C. J., & Menick, D. R. (2015). Histone deacetylases exert class-specific roles in conditioning the brain and heart against acute ischemic injury. Frontiers Neurology, 6, 145.
Bellenguez, C., Bevan, S., Gschwendtner, A., & ISGC, WTCCC2. (2012). Genome-wide association study identifies a variant in HDAC9 associated with large vessel ischemic stroke. Nature Genetics, 44(3), 328–333.
Borghi, S. M., Carvalho, T. T., Staurengo-Ferrari, L., Hohmann, M. S., Pinge-Filho, P., Casagrande, R., et al. (2013). Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. Journal of Natural Products, 76(6), 1141–1149.
Chen, Y. C., Wu, J. S., Yang, S. T., Huang, C. Y., Chang, C., Sun, G. Y., & Lin, T. N. (2012a). Stroke, angiogenesis and phytochemicals. Frontiers in Bioscience (Schol Ed)., 4, 599–610.
Chen, Y. T., Zang, X. F., Pan, J., Zhu, X. L., Chen, F., Chen, Z. B., & Xu, Y. (2012b). Expression patterns of histone deacetylases in experimental stroke and potential targets for neuroprotection. Clinical and Experimental Pharmacology and Physiology, 39(9), 751–758.
Daduang, S., Sattayasai, N., Sattayasai, J., Tophrom, P., Thammathaworn, A., Chaveerach, A., et al. (2005). Screening of plants containing Naja Naja siamensis cobra venom inhibitory activity using modified ELISA technique. Analytical Biochemistry, 341(2), 316–325.
Fessler, E. B., Chibane, F. L., Wang, Z., & Chuang, D. M. (2013). Potential roles of HDAC inhibitors in mitigating ischemia-induced brain damage and facilitating endogenous regeneration and recovery. Current Pharmaceutical Design, 19(28), 5105–5120.
Goldberg, M. P., & Choi, D. W. (1993). Combined oxygen and glucose deprivation in cortical cell culture: Calcium-dependent and calcium-independent mechanisms of neuronal injury. The Journal of neuroscience: The official journal of the Society for Neuroscience, 13(8), 3510–3524.
He, M., Zhang, B., Wei, X., Wang, Z., Fan, B., et al. (2013). HDAC4/5-HMGB1 signaling mediated by NADPH oxidase activity contributes to cerebral ischemia/reperfusion injury. Journal of Cellular and Molecular Medicine, 17(4), 531–542.
Hu, C. J., Chen, S. D., Yang, D. I., Lin, T. N., Chen, C. M., Huang, T. H., & Hsu, C. Y. (2006). Promoter region methylation and reduced expression of thrombospondin-1 after oxygen-glucose deprivation in murine cerebral endothelial cells. Journal of Cerebral Blood Flow and Metabolism, 26(12), 1519–1526.
Huang, C. Y., Chen, J. J., Wu, J. S., Tsai, H. D., Lin, H., Yan, Y. T., et al. (2015). Novel Link of Anti-apoptotic ATF3 with Pro-apoptotic CTMP in the Ischemic Brain. Molecular Neurobiology, 51(2), 543–557.
Ito, K., Lim, S., Caramori, G., Cosio, B., Chung, K. F., Adcock, I. M., & Barnes, P. J. (2002). A molecular mechanism of action of theophylline: induction of histone deacetylase activity to decrease inflammatory gene expression. Proceedings of the National Academy of Sciences, 99(13), 8921–8926.
Loizou, S., Lekakis, I., Chrousos, G. P., & Moutsatsou, P. (2010). Beta-sitosterol exhibits anti-inflammatory activity in human aortic endothelial cells. Molecular Nutrition and Food Research, 54(4), 551–558.
Mandal, A., Ojha, D., Lalee, A., Kaity, S., Das, M., Chattopadhyay, D., et al. (2015). Bioassay directed isolation of a novel anti-inflammatory cerebroside from the leaves of Aerva sanguinolenta. Medicinal Chemistry Research, 24, 1952–1963.
Mehta, S. L., Manhas, N., & Raghubir, R. (2007). Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Research Reviews, 54(1), 34–66.
Pongphasuk, N., & Khunkitti, W. (1996). Anti-inflammatory and analgesic activities of the extract from Garcinia mangostana Linn. Traditional Medicine and Nutraceuticals, 6, 125–130.
Sakdarat, S., Shuyprom, A., Ayudhya, T., Waterman, P. G., & Karagianis, G. (2006). Chemical composition investigation of the Clinacanthus nutans Lindau leaves. Thai Journal of Phytopharmacy, 13(2), 14–24.
Saleem, M. (2009). Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Letters, 285(2), 109–115.
Schweizer, S., Meisel, A., & Märschenz, S. (2013). Epigenetic mechanisms in cerebral ischemia. Journal of Cerebral Blood Flow and Metabolism, 33(9), 1335–1346.
Tan, C. S., Ng, Y. K., & Ong, W. Y. (2015). Epigenetic regulation of cytosolic phospholipase A in SH-SY5Y human neuroblastoma cells. Molecular Neurobiology. doi:10.1007/s12035-015-9314-z.
Tan, C. S, Ho, C. F, Heng, S. S, Wu, J. S, Tan, B. K, Ng, Y. K, Sun, G. Y, Lin, T. N, Ong, W. Y. Clinacanthus nutans extracts modulate epigenetic link to cytosolic phospholipase A2 expression in SH-SY5Y cells and primary neurons (submitted).
Thiagalingam, S., Cheng, K. H., Lee, H. J., Mineva, N., Thiagalingam, A., & Ponte, J. F. (2003). Histone deacetylases: Unique players in shaping the epigenetic histone code. Annals of the New York Academy of Sciences, 983, 84–100.
Tou, L., Liu, Q., & Shivdasani, R. A. (2004). Regulation of mammalian epithelial differentiation and intestine development by class I histone deacetylases. Molecular and Cellular Biology, 24(8), 3132–3139.
Uawonggul, N., Chaveerach, A., Thammasirirak, S., Arkaravichien, T., Chuachan, C., & Daduang, S. (2006). Screening of plants acting against Heterometrus laoticus scorpion venom activity on fibroblast cell lysis. Journal of Ethnopharmacology, 103(2), 201–207.
Wang, Z., Leng, Y., Wang, J., Liao, H. M., Bergman, J., Leeds, P., et al. (2016). Tubastatin A, an HDAC6 inhibitor, alleviates stroke-induced brain infarction and functional deficits: Potential roles of α-tubulin acetylation and FGF-21 up-regulation. Sci Rep., 6, 19626.
Wu, J. S., Cheung, W. M., Tsai, Y. S., Chen, Y. T., Fong, W. H., Tsai, H. D., et al. (2009). Ligand-activated PPAR-γ protects against ischemic cerebral infarction and neuronal apoptosis by 14-3-3ε upregulation. Circulation, 119(8), 1124–1134.
Wu, J. S, Tsai, H. D, Cheung, W. M, Hsu, C. Y, Lin, T. N. (2015). PPAR-γ ameliorates neuronal apoptosis and ischemic brain injury via suppressing NF-κB-driven p22phox transcription. Molecular Neurobiology (Epub) PMID: 26108185.
Wu, J. S., Tsai, H. D., Huang, C. Y., Chen, J. J., & Lin, T. N. (2014). 15-deoxy-∆12,14-PGJ2 via activating peroxisome proliferator-activated receptor-gamma suppresses p22phox transcription to protect brain endothelial cells against hypoxia-induced apoptosis. Molecular Neurobiology, 50(1), 221–238.
Yin, K. J., Cirrito, J. R., Yan, P., Hu, X., Xiao, Q., Pan, X., et al. (2006). Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. Journal of Neuroscience, 26(43), 10939–10948.
Yong, Y. K., Tan, J. J., Teh, S. S., Mah, S. H., Ee, G. C., Chiong, H. S., et al. (2013). Clinacanthus nutans extracts are antioxidant with antiproliferative effect on cultured human cancer cell lines. Evidence Based Complementary Alternative Med, 2013, 462751. doi:10.1155/2013/462751.
Zhou, X., Gan, P., Hao, L., Tao, L., Jia, J., Gao, B., et al. (2014). Antiinflammatory effects of orientin-2″-O-galactopyranoside on lipopolysaccharide-stimulated microglia. Biological and Pharmaceutical Bulletin, 37(8), 1282–1294.
Ziemka-Nalecz, M., & Zalewska, T. (2014). Neuroprotective effects of histone deacetylase inhibitors in brain ischemia. Acta Neurobiology Express (Wars)., 74(4), 383–395.
Acknowledgments
This study was supported by grants from Academia Sinica, and Minister of Science and Technology ROC (MOST 104-2320-B-001-006-MY3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Wei-Yi Ong and Teng-Nan Lin have equally contributed to this work.
Rights and permissions
About this article
Cite this article
Tsai, HD., Wu, JS., Kao, MH. et al. Clinacanthus nutans Protects Cortical Neurons Against Hypoxia-Induced Toxicity by Downregulating HDAC1/6. Neuromol Med 18, 274–282 (2016). https://doi.org/10.1007/s12017-016-8401-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-016-8401-2