Abstract
The number of peer-reviewed articles published during the 2017 solar year and retrieved using the “autoimmunity” key word increased significantly compared to 2016 while maintaining a stable share within the immunology field, following years with alternated fortunes. A detailed arbitrary analysis of the published articles in leading immunology and autoimmunity journals provides a privileged viewpoint on the current trends of research from both basic and clinical studies. Indeed, we are observing that major steps forward are found for rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis, among others. In particular, the new data on pregnancy success in lupus or biomarkers in systemic sclerosis are believed to change our management of these systemic conditions. In the case of rheumatoid arthritis, we have obtained data with significant implications in the understanding of the disease pathogenesis (as in the case of platelets), disease phenotyping, and new treatment options. Furthermore, the exponential growth of treatment options for cancer based on immune checkpoint modulation is paralleled by the need to address the potential autoimmunity side effects while taking advantage of new information obtained in paraneoplastic autoimmune conditions. Cumulatively, 2017 has been a very exciting year for autoimmune diseases, and we foresee that most of the data discussed herein will be followed by more extensive studies in the upcoming months.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
2017 and Autoimmunity
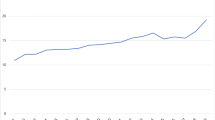
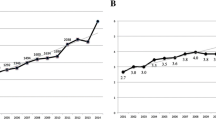
As in previous 10 years, an overview of the publications dedicated to autoimmunity over the past solar year is now provided in this article. Indeed, 2017 was a very productive year with a 21% increase in the absolute number of publications compared to 2016, with 2944 papers retrieved on PubMed using “autoimmunity” as the search word (Fig. 1). This enormous increase may be associated with a global rise in immunology as the ratio of “autoimmunity” over “immunology” hits remained stable in 2017, with a 5% prevalence (− 0.1%) (Fig. 2).
To retrieve the most important publications regarding autoimmunity in 2017, we performed a literature research on PubMed in June 2018 among the major journals in the areas of immunology (Nature Immunology, Journal of Immunology, Nature Medicine, Clinical Reviews in Allergy and Immunology) and autoimmunity (Autoimmunity Reviews, Journal of Autoimmunity) and divided the articles in the most important clinical topics: systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), systemic sclerosis (SSc), and cancer and autoimmune diseases. Indeed, this approach led to an underrepresentation of other important diseases, such as diabetes [1,2,3,4,5,6,7,8,9,10,11,12,13,14], inflammatory myopathies and anti-synthetase syndrome [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34], Sjogren’s syndrome [35,36,37,38,39,40,41,42], vasculitis [43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69], and seronegative spondyloarthritidies, psoriatic disease, and related conditions [70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90]. Similarly, we overlooked papers on immunodeficiency [91,92,93,94] and autoimmune liver diseases [95,96,97,98,99,100,101,102], gender medicine [103,104,105], as well as studies regarding autoantibodies [106,107,108,109,110,111,112,113,114,115,116,117], which were otherwise well represented in 2017. Taken altogether, we should be aware that the choice of the articles to be briefly discussed is arbitrary and will lead to some missing references. Nonetheless, we will discuss the most recent findings regarding the new insights on pathogenesis of autoimmunity and more specifically of SLE, RA, SSc, and cancer association with autoimmune diseases.
Getting Closer to the Mechanisms of Autoimmunity?
During the past year, several articles investigated novel mechanisms leading to autoimmunity [118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146], likely the most important knowledge gap and data have been provided on different cumulative hypotheses and specific cell types or genetic mechanisms.
The fascinating hypothesis based on the “nucleolus nexus” has been elegantly reviewed in 2017 and is based on chromatin disruption by nucleoli during cellular stress, when nucleoli can expand dramatically due to increased polyamine levels. Interestingly, an increase in polyamine levels has been reported in several autoimmune diseases. Moreover, the inactive X chromosome, a major epigenetic feature in female cells localized on the nuclear membrane, is in proximity with the nucleolus in up to 30% of cells throughout the cell cycle. In this view, the inactive X chromosome could abnormally interact with the nucleolus, which in turn will expand dramatically and engulf neighboring chromatin, leading to the disruption of the inactive X chromosome during cellular stress [147]. While the closeness with the inactive X chromosome is most suspect for autoimmune disease pathogenesis, other chromosomes could also be involved.
Other investigated mechanisms include small ubiquitin-like modifications (SUMOs) which are known to cause post-translational modifications that are crucial in activating protein functions. Through their interactions with innate immune pathways, SUMOs promote an efficient immune response to pathogenetic challenge avoiding an excess of immune response that could lead to the development of autoimmune diseases. Environmental factors, i.e., bacteria and viruses, also interfere with the sumoylation of their own proteins and by interfering with sumoylation of host proteins, both leading to a decreased immune defense and increased infective potential [148]. SUMOs interact with various signaling pathways involved in innate immune response, in particular NF-κB and interferon (IFN), while are important in avoiding an excessive immune response and subsequent autoimmunity, as shown by the regulation of T regulatory cells (Treg) [149]. SUMO has been shown to play a role in several autoimmune diseases, including RA, Bechet’s diseases, and type 1 diabetes (T1D) [148].
Third, long noncoding RNAs (lncRNAs), especially super-enhancer lncRNAs, are pervasive in autoimmune diseases, and their loci are in linkage with genome variants that confer a risk of autoimmunity, as it was reported by Aune et al. [150]. Interestingly, the transcriptional regulator Aire, which controls T cell tolerance by inducing the expression of a large repertoire of genes, has been shown to preferentially activate long chromatin stretchers, overloaded with transcriptional regulators, known as super-enhancers [151]. Additionally, topoisomerase 1 is a cardinal Aire partner, co-localizing on super-enhancers, and is required for the interaction of Aire with its associates [151].
Environmental factors are of great importance in the development of autoimmunity [152, 153], and the microbiome remains one of the most promising research areas, also because of the development of high-throughput sequencing, metagenome analysis, and other techniques. It is established that the microbiome influences the expression of Toll-like receptors (TLR) of antigen-presenting cells, and contributes to Th17/Treg imbalance. Moreover, it is well known that the gut microbiota and its metabolites can regulate immune cells and cytokines through epigenomic modifications, as short-chain fatty acids (SCFAs) promote the differentiation of naive T cells to Treg [154]. Recently, the salivary microbiota has been investigated in RA, and dysbiosis was found to be partially reversible after treatment [155]. The response to infections, tissue trauma, or inflammatory processes leads to the production of kinins, a group of potent autacoids that exert their action through two G-protein-coupled receptors, B1 and B2 receptors. Kinins have been involved in RA, multiple sclerosis (MS), T1D, and inflammatory bowel diseases (IBD) pathogenesis, and antagonists/agonists have emerged as new strategies for the treatment of autoimmunity with conflicting results [156].
Innate lymphoid cells (ILCs) have been recently defined as innate immune cells that function at mucosal tissue and organs, and secrete polarized cytokines and chemokines in rapid reaction to pathogens and infections, thereby contributing to the maintenance of homeostasis [157]. ILCs have been subdivided according to the type of cytokines produced: in group 1, similar to Th1 cells mainly producing IFN-gamma and TNF in response to IL-12 stimulation; group 2, similar to Th2 cells, producing IL-4, IL-5, IL-9, and IL-13; and group 3, similar to Th17 cells, producing IL-17 and IL-22. ILCs have also been found in association with several autoimmune diseases, in particular at early stages [157].
Endothelial cells, which are crucial mediators of SSc, show immunoregulatory properties and are involved in pathological angiogenesis, attraction of immune cells to sites of inflammation, and lymph node plasticity. The endothelium, in fact, actively participates to inflammation through antigen presentation, or through endothelial to mesenchymal transition [158]. All together, these findings suggest that targeting endothelial cells could contribute to control inflammation.
Besides, the endocrine system is in communication with the immune system and may influence the development of autoimmune diseases [159, 160]. Lines of evidence show that the development of autoimmune diseases is influenced by hormones, immunomodulators, and metabolic factors, such as vitamin D [161].
An important achievement of 2017 was the publication of a prospective study on the risk of developing autoimmune diseases after human papilloma virus (HPV) vaccination in France. The results of the present study show that there is no increased risk of developing any autoimmune condition after vaccination (adjusted odds ratio—OR 0.58, 95% confidence interval 0.41–0.83) in a 6-year follow-up [162].
Systemic Lupus Erythematosus
Articles on SLE were most relevant in the areas of disease pathogenesis, association with pregnancy, and medical treatment which will be reviewed separately.
Pathogenesis
While SLE is characterized mainly by B and T cell activation [163], also via IL-21 [164] and IL-23 [165], autoantibodies target multiple organ systems, resulting in chronic inflammation and organ damage, and the mechanisms underlying the dysregulation of the adaptive immune response remain enigmatic. Recent research on the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling pathway revealed an aberrant expression in SLE. STAT proteins are major components of the IFN-dependent gene expression profile, and the STAT4 gene has been associated to SLE development in genome-wide association studies (GWAS). Moreover, increased levels of STAT1 have been described in SLE T and B cells, while STAT3 has a crucial role in Th17 differentiation, T follicular helper, and B cells [166].
Neutrophils in SLE are known to mediate tissue damage and act as a source of self-nucleic acids driving plasmacytoid dendritic cell activation and IFN production. As many as 20% of patients with SLE express autoantibodies to neutrophil-specific components, likely due to the formation of neutrophil extracellular traps (NETosis). However, recent evidence shows that neutrophils may acquire an immunosuppressive phenotype and restrain the development of autoreactivity in the early phases of the disease. A recent work by Bird et al. showed that in an animal model of SLE, neutrophils accumulate in secondary lymphoid organs over the course of SLE progression, localizing preferentially near T cells in the early phases of the disease and subsequently near B cells, while changing their transcriptional program from anti-inflammatory in early stages to activation in the late stages. These findings were confirmed by the observation that neutrophil depletion in the early SLE phases leads to disease progression, while in more advanced disease, it does not affect progression [167].
Variable endothelial dysfunction is frequently found in SLE patients, that express higher cardiovascular risk [168,169,170], and recent findings suggest that IFN-alpha promotes endothelial dysfunction through the suppression of the transcription and mRNA instability of endothelial NO synthase (eNOS) and upregulation of MCP1 and VCAM1 [171].
Pregnancy
While in the past years pregnancy was invariably discouraged in women with SLE due to severe consequences for both the mother and the child, the current evidence supports the view that having a successful pregnancy is possible in SLE. However, SLE still has a high impact on maternal and fetal outcomes, as demonstrated by a recent systematic literature review and meta-analysis including 529,778 women [172], and this major issue should warrant special care in dedicated clinics. In patients with lupus nephritis, pregnancy should be carefully planned during inactive stages, when pregnancy does not seem to increase the rate of SLE flares, worsen renal prognosis, or increase organ damage [173]. With regard to fetal outcomes, a recent systematic literature review suggests that maternal SLE is associated with learning disorders, specifically dyslexia, autism spectrum disorders, and speech problems; however, the included studies were assigned a low or moderate evidence level; therefore, prospective studies are needed to address this finding [174].
Therapy
While the treatment of other autoimmune diseases has dramatically changed in the last years, corticosteroids remain the cornerstone for the therapy of lupus nephritis, and repeated methyl-prednisolone pulses help reduce the dose of oral glucocorticoids and enhance clinical response [175], while biologic treatments remain of limited efficacy, especially in case of pre-existing organ damage and smoking [176]. Recommendations for the use of biologics have been published in 2017 to help define patients who require a biologic treatment, the type of biologic and co-treatment to use, how to evaluate treatment efficacy, and when to consider discontinuation (Table 1) [177]. Importantly, rituximab should be considered in those patients with refractory and corticosteroid-dependent forms of kidney or central nervous system involvement or severe autoimmune thrombocytopenia associated with SLE [178, 179], while belimumab is effective mainly in articular and skin manifestations [180], but could also be useful in lupus nephritis [181]. In the future, new B-cell-depleting drugs are expected to become available (Table 2): obinutuzumab, a novel anti-CD20 biologic, elicits a broader action compared to rituximab, and a randomized clinical trial (RCT) is currently ongoing. Furthermore, a vaccination with CD20-mimotope peptides in animal models of SLE led to a reduction in B cells and prolonged the survival in the treated mice [193].
After numerous failures, targeting IFN has gained some new interest with anifrolumab, targeting type I IFN receptor (IFNARI), which achieved promising results in non-nephritic SLE, and phase II–III studies are ongoing. In addition, it has been shown that patients expressing a higher IFN signature were less likely to respond to placebo, while response to anifrolumab was comparable [189]. Other biomarkers of organ involvement have been identified, and in the future, we may foresee that these will serve as prognostic markers in therapeutic decisions [194].
Preclinical data suggest new therapeutic avenues in the near future. In animal models, the inhibition of serine/threonine kinase IL-1R-associated kinase (IRAK) 4, a critical regulator of innate immunity, through BMS-986126, repressed cytokine production and suppressed skin inflammation, also in combination with prednisolone, suggesting a potential steroid sparing effect [195]. Hematopoietic stem cell transplantation (HSCT) has been proposed as a therapeutic option for SLE patients refractory to standard therapy, and a recent systematic literature review including 279 patients, of which 54 have secondary anti-phospholipid syndrome, showed promising results with an improvement after HSCT in disease activity and overall survival, with conversely more than 30% of patients suffering from infections, of which one resulted in the death of the patient [196]. Comorbidity prevention is crucial in SLE, osteoporosis and bone fractures remain the major causes of injury, and vitamin D levels are often reduced and should be supplemented with bisphosphonates to prevent glucocorticoid-induced osteoporosis [197].
Rheumatoid Arthritis
Over the past solar year, research efforts on RA focused mostly on the fine mechanisms underlying disease development. From a genetic standpoint, single-nucleotide polymorphism (SNP) in the TRAF1 gene, a signaling adaptor known for TNF receptor-induced cell survival, is associated with RA, but also monocytes from non-RA subjects exhibiting this SNP show a reduction in TRAF1 protein and greater amounts of pro-inflammatory cytokine, suggesting a role in the increased inflammation [53]. Innate immunity plays a role in the early phases of RA, with TLR2 and TLR4 but also TLR5 and TLR7 capable to transform RA myeloid cells into M1 macrophages, which in collaboration with synovial fibroblasts secrete pro-inflammatory cytokines central to Th17 development [198]. Based on this growing amount of experimental evidence, novel approaches are being tested to target TLR function. Fibroblast-like synoviocytes from patients with RA express high levels of integrin alpha9 and its ligand, tenascin-C (Tn-C), and depletion of alpha9 suppresses the hyperplastic response of fibroblast-like synoviocytes and blunts the expression of MMP and IL-6 induced by TNF-alpha. B cells are crucial in RA pathogenesis, as shown also by the effectiveness of B-cell-depleting therapy and by numerous animal models. A new subset of memory B cell expressing Fc receptor like 4 (FcRL4) is enriched in RA joints and in the mucosa-associated lymphoid tissue. The FcRL4 + B cells, derived from the synovial fluid and tissue from patients with RA, manifest a larger proportion of recombinant antibodies and reactivity toward citrullinated autoantigens and an increased usage of the IgA isotype. These cells have been shown to produce high levels of RANKL, which could contribute in turn to the joint destruction and erosions observed in RA [199]. On the other hand, B regulatory cells (Breg) producing IL-10 play an important role in initiating and maintaining chronic inflammation in autoimmune diseases, such as RA. Breg are both numerically and functionally impaired in RA [200] and can convert into RANKL-producing cells, partially induced by TNF-alpha, thus impairing their immunosuppressive functions and exacerbating disease progression. These cells quantitatively correlate with RA disease activity and inversely with the number of Breg producing IL-10. Of note, RANKL-producing Bregs can promote osteoclast differentiation, which does not occur in healthy individuals [201].
Platelets are least known players that can modulate the immune response of leukocytes, particularly T cells, with a reduction of the proliferation and production of IFN-gamma and IL-17. This could have therapeutic potential, and Zamora et al. demonstrated that co-cultured platelets and lymphocytes lead to a reduction in the production of IFN-gamma and TNF-alpha, T cell proliferation, and the expression of CD25, PD-L1, and SLAM, while increasing CD39. The co-culture of platelets and RA synovial fluid cells reduces the inflammatory cytokines and increases IL-10 production [202].
Environmental factors are well known to contribute to RA pathogenesis, and smoking represents the major risk factor for the development of RA with serum anti-citrullinated peptide antibodies. A novel mechanism by which the tobacco habit may contribute to RA implies that smoking activates also CD8+ T cells, and causes the release of survivin [203]. Vitamin D, as for other autoimmune diseases [161], has a relationship with disease activity and patient-reported outcomes in RA [204] and could also play a role as a tolerogenic adjuvant in a model of RA [205].
Clinical research in RA is currently focused on biomarkers predicting the therapeutic response since up to 40% of patients with RA will discontinue drugs because of inefficacy or adverse events [206]; in this view, it has been reported that response to anti-TNF-alpha treatment is associated with high levels of GM-CSF and GM-CSF + T cells, while nonresponders are characterized by higher IL-17 levels, suggesting that RA not responding to anti-TNF is likely to be driven by different inflammatory pathways [207], and this is also suggested by the report that the response to abatacept, a fusion protein CTLA4-Ig, is associated with the levels of CD38 + CD27+ memory B cells [47, 208]. NETosis could also act as biomarker for therapeutic effectiveness in RA as it is increased in RA, and correlates with disease activity and autoantibodies positivity, while treatment with anti-TNF and IL-6 receptor inhibitor decreases the generation of NETs, in correlation with a reduction in disease activity and inflammatory markers [209].
Novel therapeutic targets in RA include, quite unexpectedly, risingly the programmed cell death-1 (PD-1) pathway which is currently central to cancer immunotherapy. In fact, PD-1 + T cells accumulate in RA synovial fluid, and PD-1 knockout animals develop inflammatory arthritis [210]. However, targeting PD-1 in cancer immunotherapy may induce autoimmune manifestations resembling RA, and further research is needed before a modulation of PD-1 in RA can be carried forward. Recent studies have demonstrated the anti-inflammatory properties of bee venom, a conglomeration of allergens, toxins, and other triggers of the immune response. In alternative medicine, bee venom has been used to retrieve pain with promising results, but more controlled trials are needed to determine the immune reaction with cells in the context of inflammation [211].
Systemic Sclerosis
SSc is a rare systemic disease characterized by the classical triad of immune system activation, vasculopathy, and altered collagen deposition resulting in widespread fibrosis [212,213,214] for which there is a large therapeutic unmet need. In the last year, an increasing body of literature is focusing on the identification of genetic and epigenetic markers of SSc, as well as the identification of biomarkers for the various disease manifestations and organ involvement [215].
Different from other autoimmune and chronic inflammatory diseases, various studies have supported the notion that genetic susceptibility is of modest importance in SSc, also supported by the low concordance in monozygotic twins. The strongest genetic association for SSc lies in the MHC region, with loci in the HLA-DRB1, HLA-DQB1, HLA-DPB1, and HLA-DOA1. Non-HLA genes associated with SSc include genes for B and T cell activation and innate immunity, while others are involved in extracellular matrix deposition, cytokines, and autophagy. Among these, STAT4 has the strongest association with SSc, but in addition to genetics, environmental factors, in particular exposure to heavy metals [216], have been shown to contribute to the development of SSc. Extensive epigenetic changes have been described in SSc, in particular regarding DNA methylation, histone modification, and dysregulated noncoding RNA levels, contributing to fibrosis, immune dysregulation, and impaired angiogenesis [217], while suggesting the possibility of therapeutic interventions to reprogram the epigenomic landscape.
The role of Tregs has also been investigated in SSc as it is known that Tregs have a decreased functional capacity, while they might be increased in number, especially in active SSc, possibly representing a failed regulatory attempt of the immune system. Conversely, in long-standing SSc, Treg frequency is reduced both in the peripheral blood and in the skin. Furthermore, it has been shown that Tregs may participate also in the inflammatory process as well as in transformation induced by the microenvironment in pathogenic T effector cells, with a Th2 or Th17 phenotype with pro-fibrotic and pro-inflammatory activity [218]. In this view, the evaluation of Tregs could represent possible future biomarkers for disease activity.
SSc is almost invariably associated with serum autoantibodies, especially anti-nuclear antibodies (ANA), but novel autoantibodies functionally active against cell-surface receptors have been identified and could have a possible pathogenetic link with the disease clinical manifestations. In particular, antibodies directed against the PDGF receptor have been shown to induce fibrosis in SSc animal models, fibrillin-1-directed antibodies have been found in SSc sera, while a number of antibodies directed toward the TGF-beta signaling pathway and to heterogeneous nuclear ribonucleoprotein A1 and superoxide dismutase 2 which protects against ROS have also been observed and could be linked all together to the fibrotic phenotype of SSc. In the search for clinical phenotype prediction, antibodies directed against the muscarinic acetylcholine (ACh) receptors have been linked to gastrointestinal dysfunction. Antibodies to endothelial cells (AECAs) have been identified in 86% of SSc sera and are associated with more severe disease, organ involvement, and pulmonary arterial hypertension, as well as with vascular and perivascular involvement and digital ulceration. AECAs are believed to have a role in the modulation of endothelial cell function and survival in SSc [212]. Finally, antibodies to angiotensin/endothelin receptors are present in 70% of SSc sera, with limited specificity for SSc, and are associated with a more severe disease and death. Cumulatively, these functional autoantibodies could act both as biomarkers and be responsible for specific disease manifestations and may further be investigated as therapeutic targets [219]. In this view, intravenous immunoglobulins (IVIGs) exhibit immunomodulatory and anti-fibrotic properties and are relevant in the treatment of musculoskeletal involvement, systemic inflammation, and digestive tract symptoms stemming from SSc [220, 221].
Cancer and Autoimmunity
The association between cancer and autoimmunity has been long suspected and then demonstrated in clinical and basic research, while recently published data from large registries and databases have changed our perspective on the association of most autoimmune diseases and cancer, as well as the presumed association with anti-TNF therapy. Meanwhile, the association between Sjögren syndrome and B cell lymphoma, especially MALT lymphoma, is well established and an early diagnosis is facilitated to identify patients at high risk by biomarkers, i.e., BAFF and beta2-microglobulin, and clinical manifestations such as parotid gland enlargement, purpura, lymphadenopathy, glomerulonephritis, peripheral neuropathy, or splenomegaly [222]. In the case of SSc, the positivity for anti-RNA polymerase III or Pm/Scl antibodies is associated with a paraneoplastic disease, particularly breast and lung cancer [223, 224]. The association of polymyositis/dermatomyositis with cancer is also well recognized, in particular for anti-TIF1-gamma antibody-positive subjects [222, 225].
Conversely, with the advent of effective immune checkpoint inhibitors for cancer, the resulting immunotoxicity and autoimmunity have been advocated as the Achilles heel of this revolutionary treatment [226]. These drugs have transformed the treatment for certain tumors, by blocking interactions that normally suppress T cell responses, i.e., CTLA4 and PD-1/PD-L1. However, these alterations in the adaptive immune response may lead to the development of forms of arthritis and other autoimmune manifestations, and the mechanisms have not been elucidated yet. In the future, we may speculate that checkpoint-based immunotherapy will be developed also for autoimmune diseases [227].
What to Expect in 2018
As in previous years, during 2017, common themes across autoimmune diseases significantly outnumbered differences and this supports the view that the several remaining unanswered questions in autoimmune diseases will be derived by research in unrelated areas. Indeed, common grounds include innate immunity, microbiota as a link to environmental factors, and shared treatment approaches. While we are excited by the significant increase in the number of publications that appeared in 2017 compared to the previous year, the quality of the research also continues to grow and more elegant solutions are expected to be reported in different areas.
References
Anquetil F, Sabouri S, Thivolet C, Rodriguez-Calvo T, Zapardiel-Gonzalo J, Amirian N, Schneider D, Castillo E, Lajevardi Y, von Herrath MG (2017) Alpha cells, the main source of IL-1beta in human pancreas. J Autoimmun 81:68–73
Hull CM, Nickolay LE, Estorninho M, Richardson MW, Riley JL, Peakman M, Maher J, Tree TIM (2017) Generation of human islet-specific regulatory T cells by TCR gene transfer. J Autoimmun 79:63–73
Kaminitz A, Ash S, Askenasy N (2017) Neutralization versus reinforcement of proinflammatory cytokines to arrest autoimmunity in type 1 diabetes. Clin Rev Allergy Immunol 52(3):460–472
Kracht MJ et al (2017) Autoimmunity against a defective ribosomal insulin gene product in type 1 diabetes. Nat Med 23(4):501–507
Kuhn C, Rezende RM, da Cunha AP, Valette F, Quintana FJ, Chatenoud L, Weiner HL (2017) Mucosal administration of CD3-specific monoclonal antibody inhibits diabetes in NOD mice and in a preclinical mouse model transgenic for the CD3 epsilon chain. J Autoimmun 76:115–122
Li CW, Osman R, Menconi F, Concepcion ES, Tomer Y (2017) Flexible peptide recognition by HLA-DR triggers specific autoimmune T-cell responses in autoimmune thyroiditis and diabetes. J Autoimmun 76:1–9
Li YY, Pearson JA, Chao C, Peng J, Zhang X, Zhou Z, Liu Y, Wong FS, Wen L (2017) Nucleotide-binding oligomerization domain-containing protein 2 (Nod2) modulates T1DM susceptibility by gut microbiota. J Autoimmun 82:85–95
Lombardi A, Tomer Y (2017) Interferon alpha impairs insulin production in human beta cells via endoplasmic reticulum stress. J Autoimmun 80:48–55
Marino E et al (2017) Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol 18(5):552–562
Ryden AK et al (2017) Anti-IL-21 monoclonal antibody combined with liraglutide effectively reverses established hyperglycemia in mouse models of type 1 diabetes. J Autoimmun 84:65–74
Wang Z, Xie Z, Lu Q, Chang C, Zhou Z (2017) Beyond genetics: what causes type 1 diabetes. Clin Rev Allergy Immunol 52(2):273–286
Wiles TA, Delong T, Baker RL, Bradley B, Barbour G, Powell RL, Reisdorph N, Haskins K (2017) An insulin-IAPP hybrid peptide is an endogenous antigen for CD4 T cells in the non-obese diabetic mouse. J Autoimmun 78:11–18
Zerif E, Maalem A, Gaudreau S, Guindi C, Ramzan M, Véroneau S, Gris D, Stankova J, Rola-Pleszczynski M, Mourad W, Dupuis G, Amrani A (2017) Constitutively active Stat5b signaling confers tolerogenic functions to dendritic cells of NOD mice and halts diabetes progression. J Autoimmun 76:63–74
Harbige J, Eichmann M, Peakman M (2017) New insights into non-conventional epitopes as T cell targets: the missing link for breaking immune tolerance in autoimmune disease? J Autoimmun 84:12–20
Afzali AM, Ruck T, Wiendl H, Meuth SG (2017) Animal models in idiopathic inflammatory myopathies: how to overcome a translational roadblock? Autoimmun Rev 16(5):478–494
Bartoloni E, Gonzalez-Gay MA, Scirè C, Castaneda S, Gerli R, Lopez-Longo FJ, Martinez-Barrio J, Govoni M, Furini F, Pina T, Iannone F, Giannini M, Nuño L, Quartuccio L, Ortego-Centeno N, Alunno A, Specker C, Montecucco C, Triantafyllias K, Balduzzi S, Sifuentes-Giraldo WA, Paolazzi G, Bravi E, Schwarting A, Pellerito R, Russo A, Selmi C, Saketkoo LA, Fusaro E, Parisi S, Pipitone N, Franceschini F, Cavazzana I, Neri R, Barsotti S, Codullo V, Cavagna L (2017) Clinical follow-up predictors of disease pattern change in anti-Jo1 positive anti-synthetase syndrome: results from a multicenter, international and retrospective study. Autoimmun Rev 16(3):253–257
Cavagna L, Monti S, Caporali R, Gatto M, Iaccarino L, Doria A (2017) How I treat idiopathic patients with inflammatory myopathies in the clinical practice. Autoimmun Rev 16(10):999–1007
Cavagna L et al (2017) Serum Jo-1 autoantibody and isolated arthritis in the antisynthetase syndrome: review of the literature and report of the experience of AENEAS Collaborative Group. Clin Rev Allergy Immunol 52(1):71–80
Cavazzana I, Fredi M, Selmi C, Tincani A, Franceschini F (2017) The clinical and histological spectrum of idiopathic inflammatory myopathies. Clin Rev Allergy Immunol 52(1):88–98
Ceribelli A, de Santis M, Isailovic N, Gershwin ME, Selmi C (2017) The immune response and the pathogenesis of idiopathic inflammatory myositis: a critical review. Clin Rev Allergy Immunol 52(1):58–70
Cucchiari D, Angelini C (2017) Renal involvement in idiopathic inflammatory myopathies. Clin Rev Allergy Immunol 52(1):99–107
Day J, Otto S, Proudman S, Hayball JD, Limaye V (2017) Dysregulated innate immune function in the aetiopathogenesis of idiopathic inflammatory myopathies. Autoimmun Rev 16(1):87–95
Gao S, Luo H, Zhang H, Zuo X, Wang L, Zhu H (2017) Using multi-omics methods to understand dermatomyositis/polymyositis. Autoimmun Rev 16(10):1044–1048
Gunawardena H (2017) The clinical features of myositis-associated autoantibodies: a review. Clin Rev Allergy Immunol 52(1):45–57
Hosono Y, Nakashima R, Serada S, Murakami K, Imura Y, Yoshifuji H, Ohmura K, Naka T, Mimori T (2017) Splicing factor proline/glutamine-rich is a novel autoantigen of dermatomyositis and associated with anti-melanoma differentiation-associated gene 5 antibody. J Autoimmun 77:116–122
Lu X, Tang Q, Lindh M, Dastmalchi M, Alexanderson H, Popovic Silwerfeldt K, Agerberth B, Lundberg IE, Wick C (2017) The host defense peptide LL-37 a possible inducer of the type I interferon system in patients with polymyositis and dermatomyositis. J Autoimmun 78:46–56
Mainetti C, Terziroli Beretta-Piccoli B, Selmi C (2017) Cutaneous manifestations of dermatomyositis: a comprehensive review. Clin Rev Allergy Immunol 53(3):337–356
Moghadam-Kia S, Oddis CV, Aggarwal R (2017) Modern therapies for idiopathic inflammatory myopathies (IIMs): role of biologics. Clin Rev Allergy Immunol 52(1):81–87
Pagnini I, Vitale A, Selmi C, Cimaz R, Cantarini L (2017) Idiopathic inflammatory myopathies: an update on classification and treatment with special focus on juvenile forms. Clin Rev Allergy Immunol 52(1):34–44
Santos E, Coutinho E, Martins da Silva A, Marinho A, Vasconcelos C, Taipa R, Pires MM, Gonçalves G, Lopes C, Leite MI (2017) Inflammatory myopathy associated with myasthenia gravis with and without thymic pathology: report of four cases and literature review. Autoimmun Rev 16(6):644–649
Satoh M, Tanaka S, Ceribelli A, Calise SJ, Chan EKL (2017) A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol 52(1):1–19
Suzuki S, Uruha A, Suzuki N, Nishino I (2017) Integrated diagnosis project for inflammatory myopathies: an association between autoantibodies and muscle pathology. Autoimmun Rev 16(7):693–700
Tansley SL, Simou S, Shaddick G, Betteridge ZE, Almeida B, Gunawardena H, Thomson W, Beresford MW, Midgley A, Muntoni F, Wedderburn LR, McHugh NJ (2017) Autoantibodies in juvenile-onset myositis: their diagnostic value and associated clinical phenotype in a large UK cohort. J Autoimmun 84:55–64
Tiniakou E, Mammen AL (2017) Idiopathic inflammatory myopathies and malignancy: a comprehensive review. Clin Rev Allergy Immunol 52(1):20–33
Espitia-Thibault A, Masseau A, Néel A, Espitia O, Toquet C, Mussini JM, Hamidou M (2017) Sjogren’s syndrome-associated myositis with germinal centre-like structures. Autoimmun Rev 16(2):154–158
Fujimura T, Fujimoto T, Itaya-Hironaka A, Miyaoka T, Yoshimoto K, Sakuramoto-Tsuchida S, Yamauchi A, Takeda M, Tsujinaka H, Tanaka Y, Takasawa S (2017) Significance of interleukin-6/STAT pathway for the gene expression of REG Ialpha, a new autoantigen in Sjogren’s syndrome patients, in salivary duct epithelial cells. Clin Rev Allergy Immunol 52(3):351–363
Garcia-Carrasco M, Jiménez-Herrera EA, Gálvez-Romero JL, de Lara LV, Mendoza-Pinto C, Etchegaray-Morales I, Munguía-Realpozo P, Ruíz-Argüelles A, Jose R, Vera-Recabarren M, Cervera R (2017) Vitamin D and Sjogren syndrome. Autoimmun Rev 16(6):587–593
Generali E, Costanzo A, Mainetti C, Selmi C (2017) Cutaneous and mucosal manifestations of Sjogren’s syndrome. Clin Rev Allergy Immunol 53(3):357–370
Haacke EA, Bootsma H, Spijkervet FKL, Visser A, Vissink A, Kluin PM, Kroese FGM (2017) FcRL4(+) B-cells in salivary glands of primary Sjogren’s syndrome patients. J Autoimmun 81:90–98
Roca F, Dominique S, Schmidt J, Smail A, Duhaut P, Lévesque H, Marie I (2017) Interstitial lung disease in primary Sjogren’s syndrome. Autoimmun Rev 16(1):48–54
Chen Y, Chauhan SK, Tan X, Dana R (2017) Interleukin-7 and -15 maintain pathogenic memory Th17 cells in autoimmunity. J Autoimmun 77:96–103
Vanoni F, Lava SAG, Fossali EF, Cavalli R, Simonetti GD, Bianchetti MG, Bozzini MA, Agostoni C, Milani GP (2017) Neonatal systemic lupus erythematosus syndrome: a comprehensive review. Clin Rev Allergy Immunol 53(3):469–476
Alba MA, Flores-Suárez LF, Henderson AG, Xiao H, Hu P, Nachman PH, Falk RJ, Charles Jennette J (2017) Interstital lung disease in ANCA vasculitis. Autoimmun Rev 16(7):722–729
Andre R et al (2017) Central nervous system involvement in eosinophilic granulomatosis with polyangiitis (Churg-Strauss): report of 26 patients and review of the literature. Autoimmun Rev 16(9):963–969
Chasset F, Frances C (2017) Cutaneous manifestations of medium- and large-vessel vasculitis. Clin Rev Allergy Immunol 53(3):452–468
Ciccia F, Rizzo A, Ferrante A, Guggino G, Croci S, Cavazza A, Salvarani C, Triolo G (2017) New insights into the pathogenesis of giant cell arteritis. Autoimmun Rev 16(7):675–683
Carvajal Alegria G, Gazeau P, Hillion S, Daïen CI, Cornec DYK (2017) Could lymphocyte profiling be useful to diagnose systemic autoimmune diseases? Clin Rev Allergy Immunol 53(2):219–236
Cornec D, Berti A, Hummel A, Peikert T, Pers JO, Specks U (2017) Identification and phenotyping of circulating autoreactive proteinase 3-specific B cells in patients with PR3-ANCA associated vasculitis and healthy controls. J Autoimmun 84:122–131
Danlos FX, Rossi GM, Blockmans D, Emmi G, Kronbichler A, Durupt S, Maynard C, Luca L, Garrouste C, Lioger B, Mourot-Cottet R, Dhote R, Arlet JB, Hanslik T, Rouvier P, Ebbo M, Puéchal X, Nochy D, Carlotti A, Mouthon L, Guillevin L, Vaglio A, Terrier B, French Vasculitis Study Group (2017) Antineutrophil cytoplasmic antibody-associated vasculitides and IgG4-related disease: a new overlap syndrome. Autoimmun Rev 16(10):1036–1043
Frumholtz L, Laurent-Roussel S, Aumaître O, Maurier F, le Guenno G, Carlotti A, Dallot A, Kemeny JL, Antunes L, Froment N, Fraitag S, London J, Berezne A, Terris B, le Jeunne C, Mouthon L, Aractingi S, Guillevin L, Dupin N, Terrier B, French Vasculitis Study Group (2017) Clinical and pathological significance of cutaneous manifestations in ANCA-associated vasculitides. Autoimmun Rev 16(11):1138–1146
Heineke MH, Ballering AV, Jamin A, Ben Mkaddem S, Monteiro RC, van Egmond M (2017) New insights in the pathogenesis of immunoglobulin A vasculitis (Henoch-Schonlein purpura). Autoimmun Rev 16(12):1246–1253
Hommada M, Mekinian A, Brillet PY, Abad S, Larroche C, Dhôte R, Fain O, Soussan M (2017) Aortitis in giant cell arteritis: diagnosis with FDG PET/CT and agreement with CT angiography. Autoimmun Rev 16(11):1131–1137
Abdul-Sater AA, Edilova MI, Clouthier DL, Mbanwi A, Kremmer E, Watts TH (2017) The signaling adaptor TRAF1 negatively regulates Toll-like receptor signaling and this underlies its role in rheumatic disease. Nat Immunol 18(1):26–35
Xue LJ, Wu R, du GL, Xu Y, Yuan KY, Feng ZC, Pan YL, Hu GY (2017) Effect and safety of TNF inhibitors in immunoglobulin-resistant Kawasaki disease: a meta-analysis. Clin Rev Allergy Immunol 52(3):389–400
Yilmaz I, Turk M, Bahcecioglu SN (2017) Classification of eosinophilic granulomatosis with polyangiitis phenotypes and treatment based on phenotypes. Autoimmun Rev 16(9):992–993
Kerstein A, Schüler S, Cabral-Marques O, Fazio J, Häsler R, Müller A, Pitann S, Moosig F, Klapa S, Haas C, Kabelitz D, Riemekasten G, Wolters S, Lamprecht P (2017) Environmental factor and inflammation-driven alteration of the total peripheral T-cell compartment in granulomatosis with polyangiitis. J Autoimmun 78:79–91
Lefevre G, Ackermann F, Kahn JE (2017) Hypereosinophilia with asthma and systemic (non-vasculitic) manifestations: eosinophilic granulomatosis with polyangiitis or hypereosinophilic syndrome? Autoimmun Rev 16(2):208–209
Legendre P, Régent A, Thiebault M, Mouthon L (2017) Anti-endothelial cell antibodies in vasculitis: a systematic review. Autoimmun Rev 16(2):146–153
Lule S, Colpak AI, Balci-Peynircioglu B, Gursoy-Ozdemir Y, Peker S, Kalyoncu U, Can A, Tekin N, Demiralp D, Dalkara T (2017) Behcet disease serum is immunoreactive to neurofilament medium which share common epitopes to bacterial HSP-65, a putative trigger. J Autoimmun 84:87–96
Martinez-Valle F et al (2017) IgG4-related disease: evidence from six recent cohorts. Autoimmun Rev 16(2):168–172
Marzano AV, Raimondo MG, Berti E, Meroni PL, Ingegnoli F (2017) Cutaneous manifestations of ANCA-associated small vessels vasculitis. Clin Rev Allergy Immunol 53(3):428–438
Mirouse A, Barete S, Monfort JB, Resche-Rigon M, Bouyer AS, Comarmond C, Sène D, Domont F, Ferfar Y, Cacoub P, Saadoun D (2017) Ustekinumab for Behcet’s disease. J Autoimmun 82:41–46
Misra DP, Sharma A, Kadhiravan T, Negi VS (2017) A scoping review of the use of non-biologic disease modifying anti-rheumatic drugs in the management of large vessel vasculitis. Autoimmun Rev 16(2):179–191
Regent A et al (2017) Molecular analysis of vascular smooth muscle cells from patients with giant cell arteritis: targeting endothelin-1 receptor to control proliferation. Autoimmun Rev 16(4):398–406
Restuccia G, Boiardi L, Cavazza A, Catanoso M, Macchioni P, Muratore F, Soriano A, Cimino L, Aldigeri R, Crescentini F, Pipitone N, Salvarani C (2017) Long-term remission in biopsy proven giant cell arteritis: a retrospective cohort study. J Autoimmun 77:39–44
Roccatello D (2017) “How I treat” autoimmune diseases: state of the art on the management of rare rheumatic diseases and ANCA-associated systemic idiopathic vasculitis. Autoimmun Rev 16(10):995–998
Salvarani C, Soriano A, Muratore F, Shoenfeld Y, Blockmans D (2017) Is PET/CT essential in the diagnosis and follow-up of temporal arteritis? Autoimmun Rev 16(11):1125–1130
Samson M, Corbera-Bellalta M, Audia S, Planas-Rigol E, Martin L, Cid MC, Bonnotte B (2017) Recent advances in our understanding of giant cell arteritis pathogenesis. Autoimmun Rev 16(8):833–844
Savioli B, Abdulahad WH, Brouwer E, Kallenberg CGM, de Souza AWS (2017) Are cytokines and chemokines suitable biomarkers for Takayasu arteritis? Autoimmun Rev 16(10):1071–1078
Argollo M, Fiorino G, Hindryckx P, Peyrin-Biroulet L, Danese S (2017) Novel therapeutic targets for inflammatory bowel disease. J Autoimmun 85:103–116
Chua-Aguilera CJ, Moller B, Yawalkar N (2017) Skin manifestations of rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritides. Clin Rev Allergy Immunol 53(3):371–393
Boehncke WH, Brembilla NC (2017) Unmet needs in the field of psoriasis: pathogenesis and treatment. Clin Rev Allergy Immunol
Greuter T, Navarini A, Vavricka SR (2017) Skin manifestations of inflammatory bowel disease. Clin Rev Allergy Immunol 53(3):413–427
Hindryckx P, Laukens D, D’Amico F, Danese S (2017) Unmet needs in IBD: the case of fatigue. Clin Rev Allergy Immunol
Lubrano E, De Socio A, and Perrotta FM (2017) Unmet needs in axial spondyloarthritis. Clin Rev Allergy Immunol
Martin JC, Wolk K, Bériou G, Abidi A, Witte-Händel E, Louvet C, Kokolakis G, Drujont L, Dumoutier L, Renauld JC, Sabat R, Josien R (2017) Limited presence of IL-22 binding protein, a natural IL-22 inhibitor, strengthens psoriatic skin inflammation. J Immunol 198(9):3671–3678
Martin-Esteban A et al (2017) Separate effects of the ankylosing spondylitis associated ERAP1 and ERAP2 aminopeptidases determine the influence of their combined phenotype on the HLA-B*27 peptidome. J Autoimmun 79:28–38
McArdle A, Pennington S, and FitzGerald O (2017) Clinical features of psoriatic arthritis: a comprehensive review of unmet clinical needs. Clin Rev Allergy Immunol
Oka T, Sugaya M, Takahashi N, Takahashi T, Shibata S, Miyagaki T, Asano Y, Sato S (2017) CXCL17 attenuates Imiquimod-induced psoriasis-like skin inflammation by recruiting myeloid-derived suppressor cells and regulatory T cells. J Immunol 198(10):3897–3908
Park JH, Jeong DY, Peyrin-Biroulet L, Eisenhut M, Shin JI (2017) Insight into the role of TSLP in inflammatory bowel diseases. Autoimmun Rev 16(1):55–63
Park JH, Peyrin-Biroulet L, Eisenhut M, Shin JI (2017) IBD immunopathogenesis: a comprehensive review of inflammatory molecules. Autoimmun Rev 16(4):416–426
Peyrin-Biroulet L, Christopher R, Behan D, Lassen C (2017) Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun Rev 16(5):495–503
Pollock RA, Abji F, Gladman DD (2017) Epigenetics of psoriatic disease: a systematic review and critical appraisal. J Autoimmun 78:29–38
Prinz JC (2017) Autoimmune aspects of psoriasis: heritability and autoantigens. Autoimmun Rev 16(9):970–979
Raychaudhuri SP, Wilken R, Sukhov AC, Raychaudhuri SK, Maverakis E (2017) Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun 76:21–37
Robert M, Miossec P (2017) Effects of interleukin 17 on the cardiovascular system. Autoimmun Rev 16(9):984–991
Sakkas LI, Bogdanos DP (2017) Are psoriasis and psoriatic arthritis the same disease? The IL-23/IL-17 axis data. Autoimmun Rev 16(1):10–15
Shabgah AG, Navashenaq JG, Shabgah OG, Mohammadi H, Sahebkar A (2017) Interleukin-22 in human inflammatory diseases and viral infections. Autoimmun Rev 16(12):1209–1218
Szentpetery A et al (2017) Effects of targeted therapies on the bone in arthritides. Autoimmun Rev 16(3):313–320
Zanin-Zhorov A, Weiss JM, Trzeciak A, Chen W, Zhang J, Nyuydzefe MS, Arencibia C, Polimera S, Schueller O, Fuentes-Duculan J, Bonifacio KM, Kunjravia N, Cueto I, Soung J, Fleischmann RM, Kivitz A, Lebwohl M, Nunez M, Woodson J, Smith SL, West RF, Berger M, Krueger JG, Ryan JL, Waksal SD (2017) Cutting edge: selective oral ROCK2 inhibitor reduces clinical scores in patients with psoriasis vulgaris and normalizes skin pathology via concurrent regulation of IL-17 and IL-10. J Immunol 198(10):3809–3814
Song J, Lleo A, Yang GX, Zhang W, Bowlus CL, Gershwin ME, Leung PSC (2017) Common variable immunodeficiency and liver involvement. Clin Rev Allergy Immunol
Taraldsrud E, Fevang B, Jørgensen SF, Moltu K, Hilden V, Taskén K, Aukrust P, Myklebust JH, Olweus J (2017) Defective IL-4 signaling in T cells defines severe common variable immunodeficiency. J Autoimmun 81:110–119
Vignesh P, Rawat A, Singh S (2017) An update on the use of Immunomodulators in primary immunodeficiencies. Clin Rev Allergy Immunol 52(2):287–303
Wong GK, Heather JM, Barmettler S, Cobbold M (2017) Immune dysregulation in immunodeficiency disorders: the role of T-cell receptor sequencing. J Autoimmun 80:1–9
Dvir R, Sautto GA, Mancini N, Racca S, Diotti RA, Clementi M, Memoli M (2017) Autoimmune hepatitis and occult HCV infection: a prospective single-centre clinical study. Autoimmun Rev 16(3):323–325
Hardtke-Wolenski M, Dywicki J, Fischer K, Hapke M, Sievers M, Schlue J, Anderson MS, Taubert R, Noyan F, Manns MP, Jaeckel E (2017) The influence of genetic predisposition and autoimmune hepatitis inducing antigens in disease development. J Autoimmun 78:39–45
Lian M, Li B, Xiao X, Yang Y, Jiang P, Yan L, Sun C, Zhang J, Wei Y, Li Y, Chen W, Jiang X, Miao Q, Chen X, Qiu D, Sheng L, Hua J, Tang R, Wang Q, Eric Gershwin M, Ma X (2017) Comparative clinical characteristics and natural history of three variants of sclerosing cholangitis: IgG4-related SC, PSC/AIH and PSC alone. Autoimmun Rev 16(8):875–882
Terziroli Beretta-Piccoli B, Invernizzi P, Gershwin ME, Mainetti C (2017) Skin manifestations associated with autoimmune liver diseases: a systematic review. Clin Rev Allergy Immunol 53(3):394–412
Wang Z, Sheng L, Yang Y, Yang F, Xiao X, Hua J, Guo C, Wei Y, Tang R, Miao Q, Zhang J, Li Y, Fang J, Qiu D, Krawitt EL, Bowlus CL, Gershwin ME, Wang Q, Ma X (2017) The management of autoimmune hepatitis patients with decompensated cirrhosis: real-world experience and a comprehensive review. Clin Rev Allergy Immunol 52(3):424–435
Chung BK, Guevel BT, Reynolds GM, Gupta Udatha DBRK, Henriksen EKK, Stamataki Z, Hirschfield GM, Karlsen TH, Liaskou E (2017) Phenotyping and auto-antibody production by liver-infiltrating B cells in primary sclerosing cholangitis and primary biliary cholangitis. J Autoimmun 77:45–54
Li Y, Tang R, Leung PSC, Gershwin ME, Ma X (2017) Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmun Rev 16(9):885–896
Ma HD, Ma WT, Liu QZ, Zhao ZB, Liu MZY, Tsuneyama K, Gao JM, Ridgway WM, Ansari AA, Gershwin ME, Fei YY, Lian ZX (2017) Chemokine receptor CXCR3 deficiency exacerbates murine autoimmune cholangitis by promoting pathogenic CD8(+) T cell activation. J Autoimmun 78:19–28
Jaillon S, Berthenet K, and Garlanda C (2017) Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol
Margery-Muir AA, Bundell C, Nelson D, Groth DM, Wetherall JD (2017) Gender balance in patients with systemic lupus erythematosus. Autoimmun Rev 16(3):258–268
Castaneda S, Gonzalez-Juanatey C, Gonzalez-Gay MA (2017) Sex and cardiovascular involvement in inflammatory joint diseases. Clin Rev Allergy Immunol
Ma WT, Chang C, Gershwin ME, Lian ZX (2017) Development of autoantibodies precedes clinical manifestations of autoimmune diseases: a comprehensive review. J Autoimmun 83:95–112
Conrad K, Röber N, Andrade LEC, Mahler M (2017) The clinical relevance of anti-DFS70 autoantibodies. Clin Rev Allergy Immunol 52(2):202–216
Tozzoli R, Villalta D, Bizzaro N (2017) Challenges in the standardization of autoantibody testing: a comprehensive review. Clin Rev Allergy Immunol 53(1):68–77
Takeuchi A, Matsushita T, Kaji K, Okamoto Y, Yasui M, Hirata M, Oishi N, Higashi A, Seishima M, Asano T, Fujimoto M, Kuwana M, Takehara K, Hamaguchi Y (2017) Autoantibody to scaffold attachment factor B (SAFB): a novel connective tissue disease-related autoantibody associated with interstitial lung disease. J Autoimmun 76:101–107
Sperotto F, Seguso M, Gallo N, Plebani M, Zulian F (2017) Anti-DFS70 antibodies in healthy schoolchildren: a follow-up analysis. Autoimmun Rev 16(2):210–211
Sowa M, Hiemann R, Schierack P, Reinhold D, Conrad K, Roggenbuck D (2017) Next-generation autoantibody testing by combination of screening and confirmation-the CytoBead(R) technology. Clin Rev Allergy Immunol 53(1):87–104
Hoxha A, Banzato A, Ruffatti A, Pengo V (2017) Detection of lupus anticoagulant in the era of direct oral anticoagulants. Autoimmun Rev 16(2):173–178
Novak GV, Marques M, Balbi V, Gormezano NWS, Kozu K, Sakamoto AP, Pereira RMR, Terreri MT, Magalhães CS, Guariento A, Sallum AME, Marini R, Ferriani VPL, Barbosa CM, de Castro TCM, Ramos VC, Bonfá E, Silva CA (2017) Anti-RO/SSA and anti-La/SSB antibodies: association with mild lupus manifestations in 645 childhood-onset systemic lupus erythematosus. Autoimmun Rev 16(2):132–135
Reed JH, Gorny MK, Li L, Cardozo T, Buyon JP, Clancy RM (2017) Ro52 autoantibodies arise from self-reactive progenitors in a mother of a child with neonatal lupus. J Autoimmun 79:99–104
Alivernini S, Galeazzi M, Peleg H, Tolusso B, Gremese E, Ferraccioli G, Naparstek Y (2017) Is ACPA positivity the main driver for rheumatoid arthritis treatment? Pros and cons. Autoimmun Rev 16(11):1096–1102
Verheul MK, Yee A, Seaman A, Janssen GM, van Veelen PA, Drijfhout JW, Toes REM, Mahler M, Trouw LA (2017) Identification of carbamylated alpha 1 anti-trypsin (A1AT) as an antigenic target of anti-CarP antibodies in patients with rheumatoid arthritis. J Autoimmun 80:77–84
Pfeifle R, Rothe T, Ipseiz N, Scherer HU, Culemann S, Harre U, Ackermann JA, Seefried M, Kleyer A, Uderhardt S, Haugg B, Hueber AJ, Daum P, Heidkamp GF, Ge C, Böhm S, Lux A, Schuh W, Magorivska I, Nandakumar KS, Lönnblom E, Becker C, Dudziak D, Wuhrer M, Rombouts Y, Koeleman CA, Toes R, Winkler TH, Holmdahl R, Herrmann M, Blüml S, Nimmerjahn F, Schett G, Krönke G (2017) Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat Immunol 18(1):104–113
Dahan S, Segal Y, Watad A, Azrielant S, Shemer A, Maymon D, Stroev YI, Sobolevskaya PA, Korneva EA, Blank M, Gilburd B, Shovman O, Amital H, Ehrenfeld M, Tanay A, Kivity S, Pras E, Chapman J, Damoiseaux J, Cervera R, Putterman C, Shapiro I, Mouthon L, Perricone R, Bizzaro N, Koren O, Riemekasten G, Chereshnev VA, Mazurov VI, Goloviznin M, Gurevich V, Churilov LP, Shoenfeld Y (2017) Novelties in the field of autoimmunity—1st Saint Petersburg congress of autoimmunity, the bridge between east and west. Autoimmun Rev 16(12):1175–1184
Doria A, Gatto M, Iaccarino L, Sarzi-Puttini P (2017) Unresolved and critical issues in autoimmune rheumatic diseases. Autoimmun Rev 16(11):1093–1095
Generali E, Ceribelli A, Stazi MA, Selmi C (2017) Lessons learned from twins in autoimmune and chronic inflammatory diseases. J Autoimmun 83:51–61
Elieh Ali Komi D and Grauwet K (2017) Role of mast cells in regulation of T cell responses in experimental and clinical settings. Clin Rev Allergy Immunol
Ferreira RC, Simons HZ, Thompson WS, Rainbow DB, Yang X, Cutler AJ, Oliveira J, Castro Dopico X, Smyth DJ, Savinykh N, Mashar M, Vyse TJ, Dunger DB, Baxendale H, Chandra A, Wallace C, Todd JA, Wicker LS, Pekalski ML (2017) Cells with Treg-specific FOXP3 demethylation but low CD25 are prevalent in autoimmunity. J Autoimmun 84:75–86
Fortner KA, Bond JP, Austin JW, Boss JM, Budd RC (2017) The molecular signature of murine T cell homeostatic proliferation reveals both inflammatory and immune inhibition patterns. J Autoimmun 82:47–61
Gattinoni L, Speiser DE, Lichterfeld M, Bonini C (2017) T memory stem cells in health and disease. Nat Med 23(1):18–27
Geng J, Yu S, Zhao H, Sun X, Li X, Wang P, Xiong X, Hong L, Xie C, Gao J, Shi Y, Peng J, Johnson RL, Xiao N, Lu L, Han J, Zhou D, Chen L (2017) The transcriptional coactivator TAZ regulates reciprocal differentiation of TH17 cells and Treg cells. Nat Immunol 18(7):800–812
Gravina G, Wasén C, Garcia-Bonete MJ, Turkkila M, Erlandsson MC, Töyrä Silfverswärd S, Brisslert M, Pullerits R, Andersson KM, Katona G, Bokarewa MI (2017) Survivin in autoimmune diseases. Autoimmun Rev 16(8):845–855
Heink S, Yogev N, Garbers C, Herwerth M, Aly L, Gasperi C, Husterer V, Croxford AL, Möller-Hackbarth K, Bartsch HS, Sotlar K, Krebs S, Regen T, Blum H, Hemmer B, Misgeld T, Wunderlich TF, Hidalgo J, Oukka M, Rose-John S, Schmidt-Supprian M, Waisman A, Korn T (2017) Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic TH17 cells. Nat Immunol 18(1):74–85
Hemon P, Renaudineau Y, Debant M, le Goux N, Mukherjee S, Brooks W, Mignen O (2017) Calcium signaling: from normal B cell development to tolerance breakdown and autoimmunity. Clin Rev Allergy Immunol 53(2):141–165
Iizuka-Koga M, Nakatsukasa H, Ito M, Akanuma T, Lu Q, Yoshimura A (2017) Induction and maintenance of regulatory T cells by transcription factors and epigenetic modifications. J Autoimmun 83:113–121
Jamilloux Y, et al (2017) Geoepidemiology and immunologic features of autoinflammatory diseases: a comprehensive review. Clin Rev Allergy Immunol
Jorch SK, Kubes P (2017) An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med 23(3):279–287
Kaur G, Mohindra K, Singla S (2017) Autoimmunity—basics and link with periodontal disease. Autoimmun Rev 16(1):64–71
Lee KH, Kronbichler A, Park DDY, Park YM, Moon H, Kim H, Choi JH, Choi YS, Shim S, Lyu IS, Yun BH, Han Y, Lee D, Lee SY, Yoo BH, Lee KH, Kim TL, Kim H, Shim JS, Nam W, So H, Choi SY, Lee S, Shin JI (2017) Neutrophil extracellular traps (NETs) in autoimmune diseases: a comprehensive review. Autoimmun Rev 16(11):1160–1173
Manthiram K, Zhou Q, Aksentijevich I, Kastner DL (2017) The monogenic autoinflammatory diseases define new pathways in human innate immunity and inflammation. Nat Immunol 18(8):832–842
Mantovani A (2017) Wandering pathways in the regulation of innate immunity and inflammation. J Autoimmun 85:1–5
Morell M, Varela N, Maranon C (2017) Myeloid populations in systemic autoimmune diseases. Clin Rev Allergy Immunol 53(2):198–218
Oftedal BE, Ardesjö Lundgren B, Hamm D, Gan PY, Holdsworth SR, Hahn CN, Schreiber AW, Scott HS (2017) T cell receptor assessment in autoimmune disease requires access to the most adjacent immunologically active organ. J Autoimmun 81:24–33
Papp G, Boros P, Nakken B, Szodoray P, Zeher M (2017) Regulatory immune cells and functions in autoimmunity and transplantation immunology. Autoimmun Rev 16(5):435–444
Park SH, Kang K, Giannopoulou E, Qiao Y, Kang K, Kim G, Park-Min KH, Ivashkiv LB (2017) Type I interferons and the cytokine TNF cooperatively reprogram the macrophage epigenome to promote inflammatory activation. Nat Immunol 18(10):1104–1116
Petersen F, Yue X, Riemekasten G, Yu X (2017) Dysregulated homeostasis of target tissues or autoantigens—a novel principle in autoimmunity. Autoimmun Rev 16(6):602–611
Picard C, Belot A (2017) Does type-I interferon drive systemic autoimmunity? Autoimmun Rev 16(9):897–902
Renaudineau Y (2017) Immunophenotyping as a new tool for classification and monitoring of systemic autoimmune diseases. Clin Rev Allergy Immunol 53(2):177–180
Zikherman J, Lowell CA (2017) B cell autoimmunity at the extremes. Nat Immunol 18(10):1065–1066
Xiang Z, Yang Y, Chang C, Lu Q (2017) The epigenetic mechanism for discordance of autoimmunity in monozygotic twins. J Autoimmun 83:43–50
Veldhoen M (2017) Interleukin 17 is a chief orchestrator of immunity. Nat Immunol 18(6):612–621
Theofilopoulos AN, Kono DH, Baccala R (2017) The multiple pathways to autoimmunity. Nat Immunol 18(7):716–724
Brooks WH (2017) A review of autoimmune disease hypotheses with introduction of the “nucleolus” hypothesis. Clin Rev Allergy Immunol 52(3):333–350
Adorisio S, Fierabracci A, Muscari I, Liberati AM, Ayroldi E, Migliorati G, Thuy TT, Riccardi C, Delfino DV (2017) SUMO proteins: guardians of immune system. J Autoimmun 84:21–28
Chen K, Liu J, Cao X (2017) Regulation of type I interferon signaling in immunity and inflammation: a comprehensive review. J Autoimmun 83:1–11
Aune TM, Crooke PS III, Patrick AE, Tossberg JT, Olsen NJ, Spurlock CF III (2017) Expression of long non-coding RNAs in autoimmunity and linkage to enhancer function and autoimmune disease risk genetic variants. J Autoimmun 81:99–109
Bansal K, Yoshida H, Benoist C, Mathis D (2017) The transcriptional regulator Aire binds to and activates super-enhancers. Nat Immunol 18(3):263–273
Crowe W, Allsopp PJ, Watson GE, Magee PJ, Strain JJ, Armstrong DJ, Ball E, McSorley EM (2017) Mercury as an environmental stimulus in the development of autoimmunity—a systematic review. Autoimmun Rev 16(1):72–80
Watad A, Azrielant S, Bragazzi NL, Sharif K, David P, Katz I, Aljadeff G, Quaresma M, Tanay G, Adawi M, Amital H, Shoenfeld Y (2017) Seasonality and autoimmune diseases: the contribution of the four seasons to the mosaic of autoimmunity. J Autoimmun 82:13–30
Qiao YC, Pan YH, Ling W, Tian F, Chen YL, Zhang XX, Zhao HL (2017) The yin and Yang of regulatory T cell and therapy progress in autoimmune disease. Autoimmun Rev 16(10):1058–1070
Chen B, Sun L, Zhang X (2017) Integration of microbiome and epigenome to decipher the pathogenesis of autoimmune diseases. J Autoimmun 83:31–42
Dutra RC (2017) Kinin receptors: key regulators of autoimmunity. Autoimmun Rev 16(2):192–207
Li S, Yang D, Peng T, Wu Y, Tian Z, Ni B (2017) Innate lymphoid cell-derived cytokines in autoimmune diseases. J Autoimmun 83:62–72
Al-Soudi A, Kaaij MH, Tas SW (2017) Endothelial cells: from innocent bystanders to active participants in immune responses. Autoimmun Rev 16(9):951–962
Coronel-Restrepo N, Posso-Osorio I, Naranjo-Escobar J, Tobón GJ (2017) Autoimmune diseases and their relation with immunological, neurological and endocrinological axes. Autoimmun Rev 16(7):684–692
Torres-Ruiz J, Sulli A, Cutolo M, Shoenfeld Y (2017) Air travel, circadian rhythms/hormones, and autoimmunity. Clin Rev Allergy Immunol 53(1):117–125
Colotta F, Jansson B, Bonelli F (2017) Modulation of inflammatory and immune responses by vitamin D. J Autoimmun 85:78–97
Grimaldi-Bensouda L, Rossignol M, Koné-Paut I, Krivitzky A, Lebrun-Frenay C, Clet J, Brassat D, Papeix C, Nicolino M, Benhamou PY, Fain O, Costedoat-Chalumeau N, Courcoux MF, Viallard JF, Godeau B, Papo T, Vermersch P, Bourgault-Villada I, Breart G, Abenhaim L, Abbas F, Abdelmoumni A, Hilliquin P, Requeda E, Adoue D, Brassat D, Agard C, Masseau A, Aladjidi N, Clet J, Fernandes H, Lemasson G, Perel Y, Raymond I, Richer O, Vital A, Allain-Launay E, Bru M, Nicolino M, Thomas C, Altman JJ, Amsallem D, Aras N, Boukari L, Dubrel M, Fain O, Letellier E, Lucidarme N, Mekinian A, Morin AS, Stirnemann J, Atlan C, Audry D, Augustin J, Bakir R, Bartolucci P, Chevalier X, Godeau B, Guillaud C, Khellaf M, Limal N, Lousteau V, Mahevas M, Méliksetyan G, Michel M, Roumier M, Bayart S, Bonnet F, Decaux O, Bekherraz A, Brihaye B, Dachez R, Daugas E, Hayem G, Meyer O, Papo T, Pasqualoni E, Sacre K, Travert F, Bellon H, Beltrand J, Lefrere F, Simon A, Benhamou PY, Benveniste O, Bolgert F, Costedoat-Chalumeau N, de Paz R, Demeret S, Fautrel B, Jacqueminet S, Louapre C, Maillart E, Morel N, Papeix C, Rigabert J, Bensaid P, Berger C, Berquin P, le Moing AG, Berroir S, Besson G, Boutte C, Casez O, Bonnotte B, Audia S, Bossu-Estour C, Bourgarit A, Dupuy A, Keshmandt H, Bourre B, Brac A, Perrin A, Pondarré C, Villar-Fimbel S, Bruckert I, Cosson A, Magy-Bertrand N, Tisserand G, Camu W, Carlander B, Morales RJ, Cances C, Pasquet M, Castilla Lievre MA, Chabroux S, Charif M, Chatelus E, Sibilia J, Chevrant-Breton J, Clavel S, Bille-Turc F, Cohen J, Courcoux MF, Leverger G, Machet L, Cuisset JM, Cony-Makhoul P, Darsy P, Favre S, Giraud P, Leitenschenck L, Monteiro I, Morati C, DeSeze J, Dinulescu M, Dhaoui T, Dommange-Romero F, Drevard E, Dupuis C, Dumuis ML, Durand JM, Farad S, Lecomte P, Pierre P, Fouyssac F, Gaudin P, Gautier A, Gellen-Dautremer J, Jarrin I, Richette P, Georget E, Gras P, Moreau T, Giraud E, Hacini M, Mayer A, Guillaumat C, Guillaume S, Guitton C, Kone-Paut I, Marsaud C, Rossi L, Guyot MH, Hassler P, Heimfert C, Heinzlef O, Hillion B, Hocquelet C, Husson H, Ichai P, Jeziorski E, Deslandre CJ, le Guern V, Kamenov K, Kerlan V, Lemoine P, Misery L, Pan-Petesch B, Krivitzky A, Labauge P, Rodier M, Lacade C, Razafimahefa B, Lachgar K, Larmarau MP, Leblanc T, Lebrun-Frenay C, Lefèbvre P, Lejoyeux P, Leske C, Ly K, Magy L, Mansuy S, Marechaud R, Martin Negrier ML, Sole G, Maupetit J, Mazingue F, Mochon S, Moktar B, Morcamp D, Morlet-Barla N, Nicolas G, Pautot V, Pellier I, Verret JL, Outteryck O, Vermersch P, Pallot-Prades B, Paquet JM, Puechal X, Sortais A, Pelletier J, Rico A, Pez D, Stankoff B, Quittet P, Rémy C, Roba E, Rosario H, Roudaut N, Sonnet E, Ruel M, Sebban S, Schaepelynck P, Simonin MJ, Vial C, Viallard JF, Ladedan I, Zenone T (2017) Risk of autoimmune diseases and human papilloma virus (HPV) vaccines: six years of case-referent surveillance. J Autoimmun 79:84–90
De Groof A et al (2017) Dysregulated lymphoid cell populations in mouse models of systemic lupus erythematosus. Clin Rev Allergy Immunol 53(2):181–197
Choi JY, Seth A, Kashgarian M, Terrillon S, Fung E, Huang L, Wang LC, Craft J (2017) Disruption of pathogenic cellular networks by IL-21 blockade leads to disease amelioration in murine lupus. J Immunol 198(7):2578–2588
Dai H, He F, Tsokos GC, Kyttaris VC (2017) IL-23 limits the production of IL-2 and promotes autoimmunity in lupus. J Immunol 199(3):903–910
Goropevsek A, Holcar M, Avcin T (2017) The role of STAT signaling pathways in the pathogenesis of systemic lupus erythematosus. Clin Rev Allergy Immunol 52(2):164–181
Bird AK, Chang M, Barnard J, Goldman BI, Meednu N, Rangel-Moreno J, Anolik JH (2017) Neutrophils slow disease progression in murine lupus via modulation of autoreactive germinal centers. J Immunol 199(2):458–466
Bakshi J, Segura BT, Wincup C, Rahman A (2017) Unmet needs in the pathogenesis and treatment of systemic lupus erythematosus. Clin Rev Allergy Immunol
Giannelou M, Mavragani CP (2017) Cardiovascular disease in systemic lupus erythematosus: a comprehensive update. J Autoimmun 82:1–12
Tektonidou MG, Kravvariti E, Konstantonis G, Tentolouris N, Sfikakis PP, Protogerou A (2017) Subclinical atherosclerosis in systemic lupus erythematosus: comparable risk with diabetes mellitus and rheumatoid arthritis. Autoimmun Rev 16(3):308–312
Buie JJ, Renaud LL, Muise-Helmericks R, Oates JC (2017) IFN-alpha negatively regulates the expression of endothelial nitric oxide synthase and nitric oxide production: implications for systemic lupus erythematosus. J Immunol 199(6):1979–1988
Bundhun PK, Soogund MZ, Huang F (2017) Impact of systemic lupus erythematosus on maternal and fetal outcomes following pregnancy: a meta-analysis of studies published between years 2001-2016. J Autoimmun 79:17–27
Gianfreda D, Quaglini S, Frontini G, Raffiotta F, Messa P, Moroni G (2017) Does pregnancy have any impact on long term damage accrual and on the outcome of lupus nephritis? J Autoimmun 84:46–54
Yousef Yengej FA, van Royen-Kerkhof A, Derksen RHWM, Fritsch-Stork RDE (2017) The development of offspring from mothers with systemic lupus erythematosus. A systematic review. Autoimmun Rev 16(7):701–711
Ruiz-Irastorza G, Ugarte A, Saint-Pastou Terrier C, Lazaro E, Iza A, Couzi L, Saenz R, Richez C, Porta S, Blanco P (2017) Repeated pulses of methyl-prednisolone with reduced doses of prednisone improve the outcome of class III, IV and V lupus nephritis: an observational comparative study of the lupus-Cruces and lupus-Bordeaux cohorts. Autoimmun Rev 16(8):826–832
Parodis I, Sjöwall C, Jönsen A, Ramsköld D, Zickert A, Frodlund M, Sohrabian A, Arnaud L, Rönnelid J, Malmström V, Bengtsson AA, Gunnarsson I (2017) Smoking and pre-existing organ damage reduce the efficacy of belimumab in systemic lupus erythematosus. Autoimmun Rev 16(4):343–351
Kleinmann JF, Tubach F, le Guern V, Mathian A, Richez C, Saadoun D, Sacre K, Sellam J, Seror R, Amoura Z, Andres E, Audia S, Bader-Meunier B, Blaison G, Bonnotte B, Cacoub P, Caillard S, Chiche L, Chosidow O, Costedoat-Chalumeau N, Daien C, Daugas E, Derdèche N, Doria A, Fain O, Fakhouri F, Farge D, Gabay C, Guillo S, Hachulla E, Hajjaj-Hassouni N, Hamidou M, Houssiau FA, Jourde-Chiche N, Koné-Paut I, Ladjouz-Rezig A, Lambotte O, Lipsker D, Mariette X, Martin-Silva N, Martin T, Maurier F, Meckenstock R, Mékinian A, Meyer O, Mohamed S, Morel J, Moulin B, Mulleman D, Papo T, Poindron V, Puéchal X, Punzi L, Quartier P, Sailler L, Smail A, Soubrier M, Sparsa A, Tazi-Mezalek Z, Zakraoui L, Zuily S, Sibilia J, Gottenberg JE (2017) International and multidisciplinary expert recommendations for the use of biologics in systemic lupus erythematosus. Autoimmun Rev 16(6):650–657
Kronbichler A, Windpessl M, Pieringer H, Jayne DRW (2017) Rituximab for immunologic renal disease: what the nephrologist needs to know. Autoimmun Rev 16(6):633–643
Zampeli E, Klinman DM, Gershwin ME, Moutsopoulos HM (2017) A comprehensive evaluation for the treatment of lupus nephritis. J Autoimmun 78:1–10
Ribero S, Sciascia S, Borradori L, Lipsker D (2017) The cutaneous spectrum of lupus erythematosus. Clin Rev Allergy Immunol 53(3):291–305
Sciascia S, Radin M, Yazdany J, Levy RA, Roccatello D, Dall'Era M, Cuadrado MJ (2017) Efficacy of belimumab on renal outcomes in patients with systemic lupus erythematosus: a systematic review. Autoimmun Rev 16(3):287–293
Narain S, Furie R (2016) Update on clinical trials in systemic lupus erythematosus. Curr Opin Rheumatol 28(5):477–487
Isenberg D, Gordon C, Licu D, Copt S, Rossi CP, Wofsy D (2015) Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann Rheum Dis 74(11):2006–2015
Merrill JT, Wallace DJ, Wax S, Kao A, Fraser PA, Chang P, Isenberg D, on behalf of the ADDRESS II Investigators (2018) Efficacy and safety of atacicept in patients with systemic lupus erythematosus: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled, parallel-arm, phase IIb study. Arthritis Rheumatol 70(2):266–276
Merrill JT, Shanahan WR, Scheinberg M, Kalunian KC, Wofsy D, Martin RS (2018) Phase III trial results with blisibimod, a selective inhibitor of B-cell activating factor, in subjects with systemic lupus erythematosus (SLE): results from a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis 77(6):883–889
Isenberg D (2016) Further thoughts about the ILLUMINATE studies of tabalumab in SLE. Ann Rheum Dis 75(2):e11
Isenberg DA, Petri M, Kalunian K, Tanaka Y, Urowitz MB, Hoffman RW, Morgan-Cox M, Iikuni N, Silk M, Wallace DJ (2016) Efficacy and safety of subcutaneous tabalumab in patients with systemic lupus erythematosus: results from ILLUMINATE-1, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis 75(2):323–331
Mysler EF, Spindler AJ, Guzman R, Bijl M, Jayne D, Furie RA, Houssiau FA, Drappa J, Close D, Maciuca R, Rao K, Shahdad S, Brunetta P (2013) Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study. Arthritis Rheum 65(9):2368–2379
Doria A, Cervera R, Gatto M, Chehab G, Schneider M (2017) The new targeted therapy in systemic lupus erythematosus: is the glass half-full or half-empty? Autoimmun Rev 16(11):1119–1124
Du FH, Mills EA, Mao-Draayer Y (2017) Next-generation anti-CD20 monoclonal antibodies in autoimmune disease treatment. Auto Immun Highlights 8(1):12
Reddy V, Klein C, Isenberg DA, Glennie MJ, Cambridge G, Cragg MS, Leandro MJ (2017) Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology (Oxford) 56(7):1227–1237
Baker KF, Isaacs JD (2018) Novel therapies for immune-mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann Rheum Dis 77(2):175–187
Favoino E, Prete M, Marzullo A, Millo E, Shoenfeld Y, Perosa F (2017) CD20-mimotope peptide active immunotherapy in systemic lupus erythematosus and a reappraisal of vaccination strategies in rheumatic diseases. Clin Rev Allergy Immunol 52(2):217–233
Wu Q, Wang Q, Mao G, Dowling CA, Lundy SK, Mao-Draayer Y (2017) Dimethyl fumarate selectively reduces memory T cells and shifts the balance between Th1/Th17 and Th2 in multiple sclerosis patients. J Immunol 198(8):3069–3080
Dudhgaonkar S, Ranade S, Nagar J, Subramani S, Prasad DS, Karunanithi P, Srivastava R, Venkatesh K, Selvam S, Krishnamurthy P, Mariappan TT, Saxena A, Fan L, Stetsko DK, Holloway DA, Li X, Zhu J, Yang WP, Ruepp S, Nair S, Santella J, Duncia J, Hynes J, McIntyre KW, Carman JA (2017) Selective IRAK4 inhibition attenuates disease in murine lupus models and demonstrates steroid sparing activity. J Immunol 198(3):1308–1319
Leone A, Radin M, Almarzooqi AM, al-Saleh J, Roccatello D, Sciascia S, Khamashta M (2017) Autologous hematopoietic stem cell transplantation in systemic lupus erythematosus and antiphospholipid syndrome: a systematic review. Autoimmun Rev 16(5):469–477
Salman-Monte TC, Torrente-Segarra V, Vega-Vidal AL, Corzo P, Castro-Dominguez F, Ojeda F, Carbonell-Abelló J (2017) Bone mineral density and vitamin D status in systemic lupus erythematosus (SLE): a systematic review. Autoimmun Rev 16(11):1155–1159
Elshabrawy HA, Essani AE, Szekanecz Z, Fox DA, Shahrara S (2017) TLRs, future potential therapeutic targets for RA. Autoimmun Rev 16(2):103–113
Amara K, Clay E, Yeo L, Ramsköld D, Spengler J, Sippl N, Cameron JA, Israelsson L, Titcombe PJ, Grönwall C, Sahbudin I, Filer A, Raza K, Malmström V, Scheel-Toellner D (2017) B cells expressing the IgA receptor FcRL4 participate in the autoimmune response in patients with rheumatoid arthritis. J Autoimmun 81:34–43
Banko Z et al (2017) Induction and differentiation of IL-10-producing regulatory B cells from healthy blood donors and rheumatoid arthritis patients. J Immunol 198(4):1512–1520
Hu F, Liu H, Liu X, Zhang X, Xu L, Zhu H, Li Y, Shi L, Ren L, Zhang J, Li Z, Jia Y (2017) Pathogenic conversion of regulatory B10 cells into osteoclast-priming cells in rheumatoid arthritis. J Autoimmun 76:53–62
Zamora C, Cantó E, Nieto JC, Bardina J, Diaz-Torné C, Moya P, Magallares B, Ortiz MA, Julià G, Juarez C, Llobet JM, Vidal S (2017) Binding of platelets to lymphocytes: a potential anti-inflammatory therapy in rheumatoid arthritis. J Immunol 198(8):3099–3108
Wasen C et al (2017) Smoking activates cytotoxic CD8(+) T cells and causes survivin release in rheumatoid arthritis. J Autoimmun 78:101–110
Vojinovic J, Tincani A, Sulli A, Soldano S, Andreoli L, Dall'Ara F, Ionescu R, Pasalic KS, Balcune I, Ferraz-Amaro I, Tlustochowicz M, Butrimiene I, Punceviciene E, Toroptsova N, Grazio S, Morovic-Vergles J, Masaryk P, Otsa K, Bernardes M, Boyadzhieva V, Salaffi F, Cutolo M (2017) European multicentre pilot survey to assess vitamin D status in rheumatoid arthritis patients and early development of a new Patient Reported Outcome questionnaire (D-PRO). Autoimmun Rev 16(5):548–554
Ishikawa LLW, Colavite PM, Fraga-Silva TFC, Mimura LAN, França TGD, Zorzella-Pezavento SFG, Chiuso-Minicucci F, Marcolino LD, Penitenti M, Ikoma MRV, Sartori A (2017) Vitamin D deficiency and rheumatoid arthritis. Clin Rev Allergy Immunol 52(3):373–388
Favalli EG, Raimondo MG, Becciolini A, Crotti C, Biggioggero M, Caporali R (2017) The management of first-line biologic therapy failures in rheumatoid arthritis: current practice and future perspectives. Autoimmun Rev 16(12):1185–1195
Bystrom J, Clanchy FI, Taher TE, al-Bogami MM, Muhammad HA, Alzabin S, Mangat P, Jawad AS, Williams RO, Mageed RA (2017) Response to treatment with TNFalpha inhibitors in rheumatoid arthritis is associated with high levels of GM-CSF and GM-CSF(+) T lymphocytes. Clin Rev Allergy Immunol 53(2):265–276
Gazeau P, Alegria GC, Devauchelle-Pensec V, Jamin C, Lemerle J, Bendaoud B, Brooks WH, Saraux A, Cornec D, Renaudineau Y (2017) Memory B cells and response to abatacept in rheumatoid arthritis. Clin Rev Allergy Immunol 53(2):166–176
Perez-Sanchez C et al (2017) Diagnostic potential of NETosis-derived products for disease activity, atherosclerosis and therapeutic effectiveness in rheumatoid arthritis patients. J Autoimmun 82:31–40
Sandigursky S, Silverman GJ, Mor A (2017) Targeting the programmed cell death-1 pathway in rheumatoid arthritis. Autoimmun Rev 16(8):767–773
Elieh Ali Komi D, Shafaghat F, Zwiener RD (2017) Immunology of bee venom. Clin Rev Allergy Immunol
Mostmans Y, Cutolo M, Giddelo C, Decuman S, Melsens K, Declercq H, Vandecasteele E, de Keyser F, Distler O, Gutermuth J, Smith V (2017) The role of endothelial cells in the vasculopathy of systemic sclerosis: a systematic review. Autoimmun Rev 16(8):774–786
Cossu M, Beretta L, Mosterman P, de Hair MJH, Radstake TRDJ (2017) Unmet needs in systemic sclerosis understanding and treatment: the knowledge gaps from a scientist’s, clinician’s, and patient’s perspective. Clin Rev Allergy Immunol
Ferreli C, Gasparini G, Parodi A, Cozzani E, Rongioletti F, Atzori L (2017) Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol 53(3):306–336
Soulaidopoulos S, Triantafyllidou E, Garyfallos A, Kitas GD, Dimitroulas T (2017) The role of nailfold capillaroscopy in the assessment of internal organ involvement in systemic sclerosis: a critical review. Autoimmun Rev 16(8):787–795
Marie I, Gehanno JF, Bubenheim M, Duval-Modeste AB, Joly P, Dominique S, Bravard P, Noël D, Cailleux AF, Benichou J, Levesque H, Goullé JP (2017) Systemic sclerosis and exposure to heavy metals: a case control study of 100 patients and 300 controls. Autoimmun Rev 16(3):223–230
Tsou PS, Sawalha AH (2017) Unfolding the pathogenesis of scleroderma through genomics and epigenomics. J Autoimmun 83:73–94
Slobodin G, Rimar D (2017) Regulatory T cells in systemic sclerosis: a comprehensive review. Clin Rev Allergy Immunol 52(2):194–201
Berger M, Steen VD (2017) Role of anti-receptor autoantibodies in pathophysiology of scleroderma. Autoimmun Rev 16(10):1029–1035
Sanges S, Rivière S, Mekinian A, Martin T, le Quellec A, Chatelus E, Lescoat A, Jego P, Cazalets C, Quéméneur T, le Gouellec N, Senet P, Francès C, Deroux A, Imbert B, Fain O, Boukari L, Sené T, Deligny C, Mathian A, Agard C, Pugnet G, Speca S, Dubucquoi S, Hatron PY, Hachulla É, Launay D (2017) Intravenous immunoglobulins in systemic sclerosis: data from a French nationwide cohort of 46 patients and review of the literature. Autoimmun Rev 16(4):377–384
Rossi D, Zanatta E, Marson P, Sciascia S, Polito P, Roccatello D, Cozzi F (2017) How I treat patients with systemic sclerosis in clinical practice. Autoimmun Rev 16(10):1024–1028
Giat E, Ehrenfeld M, Shoenfeld Y (2017) Cancer and autoimmune diseases. Autoimmun Rev 16(10):1049–1057
Boonstra M, Huizinga TWJ, de Vries-Bouwstra JK (2017) Auto-antibodies and cancer in systemic sclerosis. Autoimmun Rev 16(8):883–884
Bernal-Bello D, de Tena JG, Guillén-del Castillo A, Selva-O’Callaghan A, Callejas-Moraga EL, Marín-Sánchez AM, Fonollosa-Pla V, Simeón-Aznar CP (2017) Novel risk factors related to cancer in scleroderma. Autoimmun Rev 16(5):461–468
Wu J, Li X, Song W, Fang Y, Yu L, Liu S, Churilov LP, Zhang F (2017) The roles and applications of autoantibodies in progression, diagnosis, treatment and prognosis of human malignant tumours. Autoimmun Rev 16(12):1270–1281
June CH, Warshauer JT, Bluestone JA (2017) Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med 23(5):540–547
He XS, Gershwin ME, Ansari AA (2017) Checkpoint-based immunotherapy for autoimmune diseases—opportunities and challenges. J Autoimmun 79:1–3
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that he has no conflict of interest.
Ethical Approval and Informed Consent
The present article is a review; thus, no informed consent was obtained.
Rights and permissions
About this article
Cite this article
Selmi, C. Autoimmunity in 2017. Clinic Rev Allerg Immunol 55, 239–253 (2018). https://doi.org/10.1007/s12016-018-8699-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-018-8699-7