Abstract
House dust mites are an unsurpassed cause of atopic sensitization and allergic illness throughout the world. The major allergenic dust mites Dermatophagoides pteronyssinus, Dermatophagoides farinae, Euroglyphus maynei, and Blomia tropicalis are eight-legged members of the Arachnid class. Their approximately 3-month lifespan comprises egg, larval, protonymph, tritonymph, and adult stages, with adults, about one fourth to one third of a millimeter in size, being at the threshold of visibility. The geographic and seasonal distributions of dust mites are determined by their need for adequate humidity, while their distribution within substrates is further determined by their avoidance of light. By contacting the epithelium of the eyes, nose, lower airways, skin, and gut, the allergen-containing particles of dust mites can induce sensitization and atopic symptoms in those organs. Various mite allergens, contained primarily in mite fecal particles but also in shed mite exoskeletons and decaying mite body fragments, have properties that include proteolytic activity, homology with the lipopolysaccharide-binding component of Toll-like receptor 4, homology with other invertebrate tropomyosins, and chitin-cleaving and chitin-binding activity. Mite proteases have direct epithelial effects including the breaching of tight junctions and the stimulation of protease-activated receptors, the latter inducing pruritus, epithelial dysfunction, and cytokine release. Other components, including chitin, unmethylated mite and bacterial DNA, and endotoxin, activate pattern recognition receptors of the innate immune system and act as adjuvants promoting sensitization to mite and other allergens. Clinical conditions resulting from mite sensitization and exposure include rhinitis, sinusitis, conjunctivitis, asthma, and atopic dermatitis. Systemic allergy symptoms can also occur from the ingestion of cross-reacting invertebrates, such as shrimp or snail, or from the accidental ingestion of mite-contaminated foods. Beyond their direct importance as a major allergen source, an understanding of dust mites leads to insights into the nature of atopy and of allergic sensitization in general.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
House dust mites are arguably the masters of allergenicity. Atopic reactivity to their products is one of the most common causes of allergy in the world [1, 2], affecting the eyes, upper and lower airways, skin, and on occasion the systemic circulation. Unique attributes of dust mites have allowed them to colonize the indoor environment in most homes in the temperate regions of the world, while other attributes bring their allergens into close contact with the epithelium of humans. They produce an unmatched assortment of allergens and adjuvants that seems perfectly suited to inducing both innate and adaptive immune reactions. This review will discuss the classification, biology, and distribution of house dust mites and will focus on those features of dust mites and their products that are relevant to atopic sensitization. Dust mites produce diverse allergens that are not only immunogenic but often have proteolytic activity and are packaged with bacterial DNA, endotoxin, chitin, and other materials that induce immune responses.
Classification of House Dust Mites
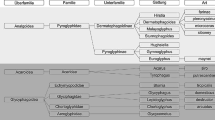
Although house dust mites share the Arthropod phylum with insects and crustaceans, they are in the Arachnid class, the eight-legged members of which are no more closely related to the six-legged members of the insect class than humans are related to sea squirts. Arachnids comprise the spiders, scorpions, and acarii, the latter order containing several suborders including parasitic mites, ticks, chiggers, soil mites and the “astigmata” [3]. (Fig. 1). Although ticks have recently been shown to be capable of provoking IgE-mediated reactions to alpha gal [4], it is the members of the Astigmata suborder—house dust mites, storage mites, and scabies mites—that are responsible for inducing classical atopic reactions. Storage mites and house dust had both been recognized since the 1920s as being capable of causing allergic reactions [5], but it was not until the 1960s that Voorhorst et al. [6] in the Netherlands and Miyamoto et al. [7] in Japan identified specific house dust mites as the major source of house dust allergen, and not until 1981 that Tovey, Chapman, and Platts-Mills identified the mite fecal particle as a major source of house dust mite allergens [8].
The term “domestic mites” refers to both house dust mites and storage mites. The major allergenic house dust mites include Dermatophagoides pteronyssinus, Dermatophagoides farinae, and Euroglyphus maynei; although technically categorized as a storage mite, Blomia tropicalis is a source of mite allergen in houses in tropical and semitropical locations.
Structure and Biology of House Dust Mites
General Anatomy
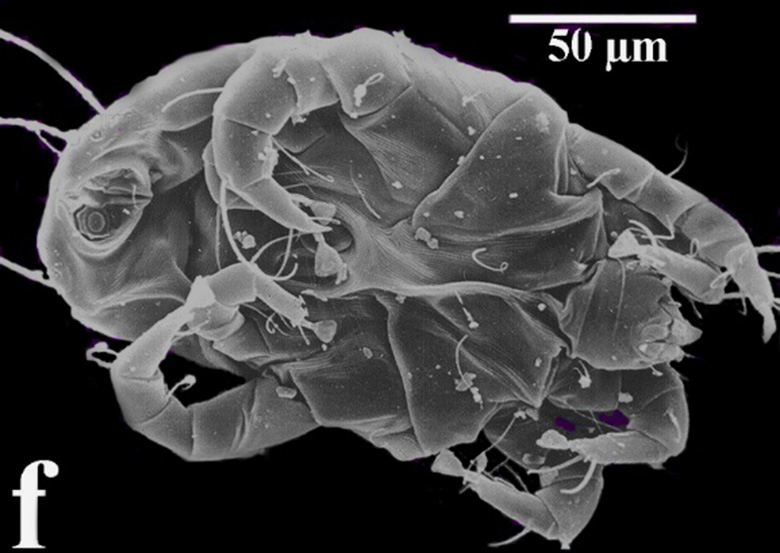
There are two common misconceptions about dust mites, both resulting from their being usually seen in electron microscope images: they are neither as small, nor as opaque, as commonly believed. The unsegmented bodies of adult mites measure 250–350 μm in length, i.e., approximately one fourth to one third of a millimeter. Although very small indeed for a multicellular organism, they are actually fairly large for a microorganism, on the threshold of visibility. They are easily seen under low power (10–60×) light microscopy, the electron microscope being required only to discern fine details such as the ridges on their outer cuticle. About the size of a small point made with a very sharp pencil, they would be visible with the naked eye were it not for their translucency, and the fact that their photophobic response to light prevents them from being on exposed surfaces during daylight hours.
In contrast to their solid gray appearance in electron micrographs (Fig. 2a), dust mites under light microscopy are pale amber in color and are translucent, reminiscent of tiny nasal polyps (Fig. 2b). Their high water content and watery appearance makes understandable their humidity dependency, to be discussed below. Mites do not have eyes, and although their light receptor organ has not been defined they are photophobic, moving until they are in a dark area. It is this aversion to light that determines their distribution within the indoor environment. Dust mites ambulate on four pairs of legs, with hair-like “setae” on their body, legs and feet functioning as feelers (Fig. 3), and with suction cup-like feet (Fig. 4a), allowing them to adhere to surfaces and fibers (Fig. 4b).
Reproduction and Life Cycle
Dust mites reproduce sexually, with copulation lasting up to 48 h (!), the consequence of the mites’ tiny size necessitating single-file exit of the sperm from the male [9]. The normal life span of a house dust mite is approximately 65–100 days, with each life stage progressing more rapidly at higher temperature and more constant humidity. At room temperature, females lay approximately 50–80 eggs (Fig. 5a, b) during their lifetime [10], with a six-legged larval stage emerging from the egg after about 8 days. Larvae develop into first stage nymphs (“protonymphs”), which in turn develop into the second stage nymph (“tritonymph”), before finally reaching adulthood (Fig. 6a, b). The total lifespan is approximately equally divided between development (new egg to adulthood) and adulthood, with each of the developmental stages (egg, larva, protonymph, and tritonymph) again being roughly equal in duration. Just as a lobster must mold its hard external skeleton in order to grow, so does each immature mite stage need to shed its exoskeleton before passing onto the next stage (Fig. 7). As will be discussed, these shed exoskeletons are sources of chitin and additional mite allergen.
Respiration, Water Balance, and Temperature
As members of the suborder Astigmata (“lacking stigma”), dust mites lack respiratory passages connecting their inner tissues to openings on the mite’s surface. The small size of dust mites results in minimal oxygen requirements and allows gas exchange to occur directly through their outer cuticle.
Mites are critically dependent on ambient water vapor to meet their water needs. A specialized gland, the supracoxal gland, is present bilaterally between the side of the mouth and the base of the first leg (Fig. 8). These glands concentrate sodium and potassium chloride, which in turn osmotically absorb water vapor from the ambient air. The humidity requirements of dust mites have been extensively studied by Arlian [11] and account for their global and local distribution as well as their seasonal fluctuations. Dust mites proliferate maximally at a relative humidity of 75%. Humidity levels above 75% allow mites to maintain water equilibrium, but favor competing mold growth. Humidity levels below the 75% optimum (but above the critical level of 50%) result in a progressive decrease in feeding, reproduction, and allergen production by dust mites [11]. At constant relative humidity levels below 50%, the supracoxal glands are unable to maintain positive water balance, and adult dust mites will dehydrate and eventually die if the humidity remains below that level. However, dust mites do survive and complete their life cycle, albeit on a slower time scale, if humidity is adequate for only part of the day [12], for example 20 h per day at 35% relative humidity but 4 h per day at 75% relative humidity [13]. Mites survive the dry winter months in temperate climates through the survival of a quiescent, immotile, and desiccation-resistant nymph stage [14].
Metabolism, reproductive rate, and allergen production are similarly affected by temperature, with higher temperatures within the optimal range leading to greater proliferation, as long as the relative humidity remains adequate. Dermatophagoides farinae completes its egg-to-adult cycle in 35 days at 23 °C (74 °F), but in only half that time at 30 °C (95 °F) [15]. Outside of that optimal range, temperature extremes are lethal to dust mite eggs [16] and to mites [17]. Eighty percent of D. pteronyssinus eggs will hatch at 40 °C (104 °F), whereas dry heat at 50 °C (122 °F) kills all eggs within a few hours and dust mites within 20 min; exposure to wet heat at 60 °C (140 °F) instantly kills eggs and rapidly kills mites [18]. At the cold end of the temperature spectrum, typical refrigerator temperatures of 3 °C (37 °F) make dust mites immotile but do not kill them. Dust mites die at home-freezer temperatures of − 17 °C (0 °F) [19], but deep-freezer temperatures (− 70 °C) are required to kill their eggs [16].
Food, Digestion, and Excretion
The dust mite GI tract comprises a mouth with movable chelicerae that allow the grasping and moving of food (Fig. 9a), salivary glands, an esophagus, small and large intestines, and an anal opening (Fig. 9b) [9]. The food of dust mites includes the skin scales shed by warm-blooded animals, along with fungi, bacteria, and yeast. Dust mites live in the wild in dog dens and bird nests and in modern homes near humans and pets, with increased numbers of dust mites found near humans suffering from skin diseases that increase skin shedding [20, 21]. Dust mites can also share the dietary habits of their storage mite relatives, and live on grain. Clues to these presumed dietary preferences are present in their genus and species names: Dermatophagoides = “skin-eating”; pteronyssinus = “feather-loving” (or more precisely “wing loving,” as in Pterodactyl); farinae = “wheat.”
Importantly from the viewpoint of the atopic person, during their lifetime mites produce approximately 1000 solid fecal waste particles (Fig. 10a, b), each about 25 μ in diameter and each surrounded by a peritrophic membrane [22] containing proteolytic digestive enzymes. From the dust mite’s viewpoint, these digestive enzymes allow the dust mite, at least under certain conditions of food availability, to benefit from coprophagia, the ingestion of its own fecal particles. The enzymes in the peritrophic membrane allow additional digestion of nutrients to occur extra-corporeally, with those nutrients then becoming available for absorption by the mite when the fecal particle is ingested several days after deposition. From the atopic individual’s viewpoint, however, the peritrophic membrane presents significant problems, to be discussed.
Distribution of Dust Mites and Mite Allergens
Geographic Distribution
Mites are ancient organisms that have evolved diverse lifestyles and diets and have colonized diverse environments. There is evidence that the house dust mite suborder Astigmata evolved from mites that had been parasites of warm-blooded vertebrates, but regained the capacity to be free living [23]. The major determinants of dust mite location in the world are humidity [24] and temperature. Dust mites are present in both hemispheres, excluding the Arctic and Antarctic. They have been found in the homes of indigenous people of Papua New Guinea, living in blankets brought in from the modern world [25].
High altitude is generally not hospitable to dust mites, and it is interesting to note that even before the discovery of dust mites, sanitaria for asthma and other respiratory diseases were often built at high attitudes, e.g., the Alps in Europe and Denver, CO, in the USA. Dust mite growth [26, 27] and sensitization [28] are much lower in the Alps than at sea level, the apparent result of the lower indoor humidity at high altitude. However, altitude per se is not crucial, the “exception that proves the rule” being the occurrence of mites at high altitude in the tropics, where humidity is high despite the altitude [29, 30]. A similar situation exists in the USA, where a study of homes in the Rocky Mountains showed few or no dust mites, the only exceptions being homes with unusually high indoor humidity [31]. D. pteronyssinus and D. farinae are present throughout most of the USA, with B. tropicalis and E. maynei limited to the southern states [32].
Local and Seasonal Distribution
Within a given geographical region, dust mites vary in their distribution among specific locations and in their seasonal distribution. The highest mite numbers are found in private homes, and there is an association between higher socioeconomic levels and dust mite exposure, with higher mite allergen exposure correlated with higher education, higher household income, and lower population density in a private home with fewer people sharing a room [33]. Mite allergen levels are higher in homes without air-conditioning than in homes with it [34] and are higher in older homes [35]. Apartments in the inner city are low in dust mites, with mouse and cockroach allergens predominating [36].
Variable dust mite numbers are found in areas outside of the home. US hospitals have no mites in the winter and very few mites in the summer [37], presumably the result of air-conditioning, uncarpeted floors, plastic-covered mattresses, lack of fabric-upholstered furniture, and sanitary laundering. Compared to homes, less mite allergen is found in public places such as schools, trains, buses and pubs, exceptions being day care centers [38] and the upholstered seats in movie theaters [39]. University dormitory rooms have fewer mites than homes, but still have significant numbers, particularly if carpeted [40]. Most work environments have much less dust mite allergen than do homes, with understandable exceptions being workplaces performing textile recycling, feather-bed filling, and carpet and upholstery cleaning [41]. In keeping with their cosmopolitan nature, dust mites or mite allergens have been found in submarines [42] and even in the space station [43].
Varying with seasonal humidity, the highest dust mite numbers in the northern USA occur during the humid summer months and the lowest numbers during the dry winter months [44]. There is a corresponding seasonal variation in dust mite allergen [45], with the seasonal drop in mite numbers preceding the drop in allergen levels [46]. This seasonal variation can be blunted by using air-conditioners and dehumidifiers to keep relative humidity below 50% for all [47] or part of [48] the day. Homes in arid desert environments have low levels of dust mites and mite sensitization, except in homes using evaporative (swamp) coolers, which increase indoor humidity [49, 50].
Location in Substrates
On the level of particular substrates, dust mites and mite allergen are found in objects that meet the mites’ requirement for high humidity and low light. Within homes, the highest mite numbers are found in the most heavily used upholstered and carpeted areas of the living room and bedroom [44], with high dust mite numbers and allergen levels in couches and beds [51] being mirrored by high mite numbers in the floor beneath them [52, 53]. Dust mites numbers [54] and allergen levels [55] are high in mattresses, especially innerspring and wool mattresses, with new mattresses accumulating clinically significant levels of mite allergen with 4 months. Old mattresses have the highest mite allergen levels [56], presumably the result of many generations of dust mites having provided accumulated allergenic waste. Pillows have long been considered an important site of dust mite growth and allergen exposure, especially considering their proximity to the sleeper’s airway, but the traditional advice to avoid feather pillows as a source of feather-loving dust mites was upended when it was found that much higher levels of dust mite allergen are recoverable from synthetic pillows than from feather pillows [57, 58]. The apparent paradox was resolved by the discovery that the outer ticking on feather pillows, a tightly woven fabric used to prevent feathers from poking through, was acting as a barrier to dust mite entry and allergen escape [59]. Dust mites inhabit blankets and comforters [60], but shun the drying heat of electric blankets [61]. Dust mites and their allergens are extremely high in the sheepskin bedding used for infants in Australia and New Zealand [62, 63]. Also particularly problematic is the bottom level of a bunk bed, where the occupant is exposed to allergen from both the top and bottom mattresses [64]. Mattress bases (boxsprings) beneath the mattress generally have more mites [65] and mite allergen [66] than the mattress itself.
Dust mites and mite allergens are much greater on carpeted than on smooth floors, with the height and type of the carpet pile being of little importance [67]. Although some dust mites move temporarily to the surface of the carpet during the dark of night, most dust mites reside deep within the carpet, and the highest mite allergen levels occur closest to the carpet’s base [67, 68].
Clothing is a major and underappreciated locus of dust mite growth and mite allergen accumulation, with mite allergen being highest in wool garments that have not been recently cleaned [69]. Clothing also acts as a vector that disperses live mites through the home [70]. Children’s stuffed toys harbor dust mites, with one study finding mite allergen levels three times as high as those in mattresses [71]. Just as dust mites eat human dander and colonize human beds, so do they eat animal dander [72] and colonize pet beds [73]. Similarly, dust mites can colonize wheat products in the home for human consumption [74] and also colonize stored pet food [75]. Mites have been found in the upholstered seats of cars [76] and taxis [77]. As might be expected from the physiology of dust mites, there is little mite allergen found on hard, wipeable surfaces such as home walls [78, 79] or school desktops [80]. Where present, mite allergen is long lasting, persisting for years unless steps have been taken to remove it [81].
Personal Exposure to Mite Allergens
It is crucial to remember that from the viewpoint of the atopic individual, what matters is not the mite allergen within a particular substrate, but rather the mite allergen that directly contacts the epithelium of that person’s eyes, upper and lower airways, skin, and gut. More than 80% of mite allergen is on particles > 10 μ, and is undetectable in the air of undisturbed rooms [82], becoming airborne only after the disturbance of the soft substrates in which the allergen was produced. Although the high dust mite allergen concentrations in beds have led to the assumption that most exposure occurs while asleep, recent data using personal allergen samplers attached to a shoulder strap during the day and taped to the head of the bed at night suggest that only 10% of airborne exposure occurs in bed, with most occurring during daytime activities [83]. Although sampling by a device attached to a headboard may not replicate the inhalation of allergen through a nose in direct contact with a pillow, such studies do serve to remind us of the critical distinction between measured mite allergen concentrations in reservoirs and the individual’s actual end-organ mite allergen exposure.
Atopic Sensitization to Dust Mites
Dust Mite Particles as Allergen Delivery Systems
Atopic sensitization, and the subsequent elicitation of symptoms, can occur by allergen contact with the conjunctivae, skin, upper airway, lower airway, or gut. Regarding inhalational exposure, Platts-Mills has made the important point that under natural circumstances, people do not inhale pure allergens; they inhale particles containing allergens [84]. In the case of house dust mites, the primary allergen-containing particles are the fecal waste pellets. These 20–25 μ particles are approximately the same size as pollen grains, although as with pollen it is at times fragments rather than the intact particles that are inhaled. The weight and size of mite fecal particles are such that they become airborne when the soft substrate in which they are present is disturbed—for example by making a bed, moving one’s face on a pillow, walking on or vacuuming a carpet, hugging a stuffed toy, or putting on a sweater—and then settle to the ground within 20–30 min [85]. Although particles containing mite allergen are generally much larger, and therefore quicker to settle, than particles carrying cat dander allergen, some dust mite allergen is present on smaller particles [86], possibly representing fragments of shed exoskeletons or of dead dust mite bodies. It is also important to remember that it is not only small particles that are respirable; large particles can also be inhaled, although in smaller numbers. The relationship between mite allergen exposure and the development of sensitization is not linear, but rather is bell shaped, with less sensitization occurring at both very low and very high mite allergen concentrations [87].
Routes of sensitization and symptom provocation need not be the same, as demonstrated by allergy to peanuts [88] and to mammalian meat [89], where sensitization occurs via the skin but symptoms occur after oral exposure. Skin sensitization can also lead to respiratory reactivity; epicutaneous sensitization to ovalbumin in mice induces not only an allergic dermatitis but also airway eosinophilia and airway hyper-reactivity on subsequent inhalation of that allergen [90]. Considering that many airborne particles that settle in the nose and throat are eventually swallowed, it is perhaps not surprising that dust mite allergen is frequently detectable in the adult human gut, where it potentially affects the intestinal epithelium [91]. Although GI exposure is usually considered to induce tolerance, it is not inconceivable that sensitivity to “respiratory” allergens could be elicited in the gut. House dust mite allergens are present in breast milk [92], and children breast fed by a mother who has a history of allergy or asthma, and who also has higher than average levels of house dust mite allergen in her breast milk, are more likely to become allergically sensitized and to develop asthma or allergic rhinitis themselves [93]. In mice, GI sensitivity to ovalbumin increases airway reactivity not only to ovalbumin but also to house dust mite extract [94]. The situation may be even more complicated, as illustrated by the finding of an association between frequent vacuum cleaning and changes in the gut microbiome [95].
Dust Mite Allergens
Allergens from any organism are given standardized names by a WHO/International Union of Immunological Societies committee based on the order of their discovery and their sequence homology. That list, available at www.allergen.org, currently shows 31 allergens for D. farinae, 20 allergens for D. pteronyssinus, 14 allergens for B. tropicalis, and 5 allergens for E. maynei, with recent additions having resulted from new techniques of transcriptome and proteome analysis [96]. The major allergens of D. pteronyssinus are listed in Table 1, along with their physiologic function and specific characteristics. Der p1 and Der p2 have the highest rates of sensitization. Dust mite allergens with proteolytic activity include Der p1, a cysteine protease, and Der p3, p6 and p9, which are serine proteases [97]. This proteolytic property has significant consequences for allergic sensitization, as will be discussed below. Der p2 is unusual in that it has sequence homology with MD-2, which, when bound to lipopolysaccharide (LPS), acts as the ligand binding to TLR-4, the pattern recognition receptor for endotoxin [98]. Der p2 may therefore be acting as an “auto-adjuvant.” Der p10 is tropomyosin, a muscle protein present in other invertebrates [99] and responsible for the cross-reactivity between dust mites and shrimp [100]. Der p11, another muscle allergen, has the unusually high molecular weight of 100 kDa, has homology with the paramyosin proteins found in invertebrates, is present in dust mite bodies rather than feces, and is the significant dust mite allergen in atopic dermatitis [101]. In contrast to humans, dogs with atopic dermatitis are reactive primarily to Der p15, a chitinase, and Der p18, a chitin-binding protein [102]. Der p23, a fairly recently identified dust mite allergen, is a peritrophin from the mite gut and is present in the outer membrane of mite feces. Although IgE directed against it is common [103], it represents only a relatively small percentage of the total anti-dust mite IgE [104]. The most recently identified dust mite allergen is Der f 24, discovered in 2015 after the sequencing the D. farinae genome [105].
It is not simply the structure of an individual dust mite protein that makes it allergenic, but rather the total effects of all of the components of the allergenic particle acting on the contacted epithelium. Dust mite particles contain two such categories of active components, proteolytic enzymes and pathogen-associated molecular patterns (PAMPS), which act on protease-activated receptors (PARs) and pattern recognition receptors (PRRs), respectively [106]. As will be discussed, these PRRs include TLR-2. TLR-4, TLR-9, C-type lectin, and formyl peptide receptor.
Dust Mite Proteolytic Enzymes and Their Effects on the Epithelium
Many allergens are, or occur in association with, enzymes. Pollen grains contain proteolytic enzymes needed for the pollen tube of the germinating pollen to move through the stigma and style of the female plant, allowing the gamete within the pollen to reach the female plant’s ovary [107]. Molds digest extracellularly by secreting enzymes that break down the substrate on which they are growing [108]. And dust mites have proteolytic digestive enzymes in the peritrophic membrane surrounding their fecal particles, which allow extra-corporeal digestion prior to coprophagy and which may also play a protective role within the mite GI tract.
In addition to being allergens, the mite cysteine protease Der p1 and serine proteases Der p3, p6, and p9 have direct epithelial effects that are important in atopy. It was formerly thought that the effect of proteases on epithelium was limited to breaching the “tight junction” between epithelial cells, thereby allowing the allergens to reach allergen-presenting dendritic cells [109]. However, it is now recognized that these proteases have many additional consequences [110]. In atopic dermatitis these effects include disrupting the skin barrier [111]; activating PAR-2 (protease-activated-receptor-2) in epidermal keratinocytes and dermal nerves, leading to a non-histamine-mediated itch [112] (and thus explaining the poor response of the pruritus of atopic dermatitis to antihistamines); promoting inflammation by directly inducing cytokine release [113]; and, again via PAR-2 activation, delaying healing and recovery of epidermal permeability barrier function in barrier-disrupted skin [114]. In atopic asthma, dust mite proteases directly stimulate protease-activated receptors in the bronchial epithelium of asthmatics [115], leading to cysteinyl leukotriene mediated hypertrophy of bronchial smooth muscle [116]. There is a correlation between asthma severity, airway smooth muscle enlargement, and the expression of PAR-2 and its ligands [117].
Mite proteases also play a role in eosinophilic chronic rhinosinusitis (ECRS), where they overwhelm endogenous protease inhibitors and drive Th-2 type inflammation even in the absence IgE [118]. Dust mite proteolytic enzymes also cleave receptors on B-cells, including the IL-2 receptor CD25, thereby moving the balance away from Th1 and towards Th2 [119], and the low affinity IgE receptor CD23, thereby inhibiting a feedback loop that would otherwise limit IgE synthesis [120]. Other effects of proteases include causing the release of the proinflammatory cytokines IL-8, IL-6, MCP-1, and GM-CSF from bronchial epithelial cells and degrading lung surfactant proteins that would otherwise bind inhaled allergens and prevent them from reaching IgE bound to mast cells [121].
Dust Mite Activators of Innate Immune Response Pattern Recognition Receptors
Multiple components in dust mite particles act as pathogen-associated molecular patterns (PAMPS) that bind to pattern recognition receptors (PRRs) on the epithelium and on antigen presenting cells including dendritic cells. Even on initial exposure, these PRRs recognize PAMPS as belonging to non-self primitive organisms and can lead to a Th2 directed, IgE producing immune response [122]. Known PAMPS in house dust mite feces and bodies include chitin, mite DNA, bacterial DNA, and endotoxin.
Chitin, present in insects, shellfish, fungi, and parasitic intestinal worms, is also present in dust mite exoskeletons and activates the innate immune system via PRRs including TLR-2 and C-type lectin [123], thereby inducing Th2 responses [124]. Chitin, being absent in mammals, stimulates the production of both the enzyme acidic mammalian chitinase (AMC), which cleaves chitin, and of the chitinase-related protein YKL-40, which binds to but does not cleave chitin. AMC and YKL-40 are high in asthmatics and correlate with its severity [125]. By inactivating chitin, AMC minimizes the pulmonary eosinophilia [126] and fibrosis [127] that would otherwise be induced by chitin. In contrast, YLK-40 stimulates bronchial smooth muscle proliferation and is involved in airway remodeling [128].
Mite and bacterial DNA are both unmethylated and thus act as PAMPS activating TLR-9 [129]. Endotoxin (bacterial lipopolysaccharide, LPS) in mite feces, aided by the homology of Der p2 to the LPS-binding portion of TLR-4, acts as a ligand to TLR-4 receptors on pulmonary epithelial cells, resulting in the release of epithelial-derived Th-2 promoting cytokines including thymic stromal lymphopoietin (TSLP), IL-25 and IL-33, and leading to airway inflammation and bronchial hyper-reactivity [130]. Bacterial signal peptides [131] present in dust mite extract act as PAMPS to activate the formyl peptide receptor (FPR) on the surface of human eosinophils [132]. In addition, dust mites carry Gram-positive and Gram-negative bacteria in their own microbiome that induce IgE antibodies to those bacteria, with such sensitization to bacterial antigens being more frequent in dust mite sensitive than in dust mite negative allergic patients [133].
Dust Mites as Facilitators of Polysensitization
The adjuvants in dust mites promote sensitization not only to the allergens of the mites themselves but also to other potential allergens. The ability of dust mite products to induce inflammation and to promote Th2 polarization can lead to a cascade of spreading sensitization to other allergens, thereby magnifying the atopic state.
In mice, the proteolytic activity of Der p1 increases the IgE response to injected ovalbumin (OVA) [134]. Intranasal exposure to dust mite extract changes the usual tolerance to inhaled OVA into a strong inflammatory response with eosinophilia, bronchial hyper-reactivity, and Th2 cytokine production [135]. House dust mite chitin also produces an increase in OVA-induced airway inflammation [136].
Atopy is both an epithelial barrier disease and an immunologic disease [137]. Dust mites can thus promote sensitization to other allergens both by virtue of their proteases disrupting epithelial barriers, whether in the skin, the airway or the gut, and by virtue of their adjuvants polarizing dendritic cells towards Th2 responses [138, 139]. IgE antibodies against a single antigen can potentiate the formation of IgE to other antigens via the effect of CD23 on epithelium and B cells [140] and can augment IgE responses in general via upregulation of the high-affinity receptor for IgE, FcεRI, on basophils and mast cells [141]. With their multiple allergens, proteases, PAMPs, and bacteria, house dust mites are likely playing a significant role in the progression of the “atopic march.”
Dust Mites and Atopic Diseases
In a sensitized person, mite allergens can provoke symptoms by direct external contact (conjunctivitis, eczema), inhalation (rhinitis, asthma, eczema), and ingestion (urticaria, anaphylaxis).
Asthma
In patients with existing asthma and dust mite sensitivity, bronchospasm and bronchial hyper-reactivity worsen on exposure to mite allergen and lessen in a mite-allergen-free environment [142]. Symptoms in dust mite sensitive asthmatic children [143], and abnormal pulmonary function and bronchial reactivity in mite-sensitive adult asthmatics [144], correlate with the mite allergen level in their home. Seasonal increases in dust mite allergen exposure also lead to seasonal increases in bronchial hyper-reactivity [145]. The combined presence of mite sensitivity and mite exposure correlates with severity of asthma symptoms [146], with increased exhaled nitric oxide and bronchial hyper-reactivity [147], and with acute exacerbations requiring hospital admission [148]. There is an additional synergism between sensitization, exposure, and viral infection leading to acute wheezing [149] or hospitalization [150, 151]. Beyond its bronchospastic effects, the inhalation of dust mite allergen increases the deposition of other inhaled particles while decreasing mucociliary clearance [152], inhibits the bronchodilating effect of deep inhalation [153], and induces the proliferation of asthmatic bronchial smooth muscle [116]. Interestingly, some effects of mite exposure are present in patients without skin test reactivity to dust mite allergen. Increased bronchial hyper-reactivity correlates with high mite allergen exposure even in non-mite-sensitized asthmatics [154], and functional IgE against mite allergens is present in the sputum of “intrinsic” asthmatics (albeit with a lack of bronchoconstriction on inhalational challenge) [155].
In contrast to the clear ability of dust mite exposure to exacerbate existing asthma, the evidence for the role of mite exposure in the development of that multifactorial disease is somewhat less clear [156]. A prospective UK study in 1990 showed that there was a relationship between high dust mite allergen exposure in infancy and the likelihood of wheezing 11 years later [157]. A subsequent study in Australia demonstrated a doubling of the risk of asthma in mite-sensitized children with each doubling of mite allergen in their bed [158], and a prospective US study of children with parental atopy showed an association between exposure to high levels of mite allergen in infancy and the development of asthma at age 7 [159]. However, a different US study found no relationship between mite allergen levels measured repeatedly during the first 2 years of life and the presence of asthma or bronchial hyper-reactivity at 6 or 7 years of age [160], and other studies have also failed to find a relationship between early allergen exposure and subsequent development of asthma, at least in children without parental atopy [161,162,163,164]. Post-natal exposure may be only part of the story, as suggested by a murine model indicating increased airway reactivity to mite allergen in offspring whose mothers were exposed to that allergen during pregnancy [165].
Apart from the role of dust mite exposure, dust mite sensitization is a risk factor for asthma. A study in Virginia, where 80% of homes had elevated dust mite allergen levels, showed that sensitization to dust mites was the primary risk factor for asthma in adolescents [166]; another study showed sensitization to dust mites (and other allergens) to be a risk factor for asthma in adults [167]. The degree of sensitization, as determined by allergen-specific IgE levels to mite and dander allergens at 3 years of age, is a risk factor for wheezing, decreased pulmonary function, and persistent wheezing at age 5 years [168], and for bronchial hyper-reactivity and decreased pulmonary function at 7 and at 13 years of age, respectively [169]. Two more-recent studies have shown that the presence of skin test reactivity to dust mites in wheezy toddlers increases the risk of persistent wheezing in later childhood [170, 171].
Rhinitis and Conjunctivitis
Nasal challenge with mite allergen produces obstruction and rhinorrhea that correlate with mite skin test reactivity [172]. A group of 108 patients evaluated for chronic rhinitis or chronic rhinosinusitis comprised 39% with allergic rhinitis, 21% with non-allergic rhinitis, and 40% with chronic rhinosinusitis (of whom 40% were also allergic); 52% of the allergic rhinitis patients and 65% of the chronic rhinosinusitis patients with allergy were sensitized to house dust mites [173]. In keeping with the unified airway concept, 92% of patients with mite-sensitive asthma recruited for an allergen avoidance study also had symptoms of allergic rhinitis [174]. Conversely, in two studies of the effectiveness of sublingual immunotherapy, approximately 30 and 12% of dust mite sensitive allergic rhinitics also had asthma [175, 176]. However, a murine study suggests that the airway may not be totally “unified,” in that the PAMPs triggering mite-induced allergic rhinitis and mite-induced allergic asthma differ, the former being beta-glucans acting via TLR2 and the latter lipopolysaccharides acting via TLR4 [177].
There is little data on the occurrence of ocular symptoms in association with allergic rhinitis, but one study of patients with allergic rhinitis from various sources, including dust mites, indicated that the majority also had ocular involvement including pruritus, tearing, conjunctival injection, and eyelid edema [178]. Even during asymptomatic periods, patients with dust mite reactivity and a history of allergic rhinitis show persistent inflammatory cell infiltrates of the nose and eyes [179]. Dust mite allergy can also cause vernal conjunctivitis, with symptoms peaking in concert with elevated dust mite levels during the humid months [180].
Atopic Dermatitis
The relationship between dust mites and atopic dermatitis was first observed almost 30 years ago, when a correlation was observed between the number of dust mites in the home and the presence and severity of atopic dermatitis in patients [20, 181]. Such observations do not indicate the direction of causality, as the increase in mites could simply be the result of increased patient desquamation providing additional food for dust mites. However, the relationship is apparently bidirectional, as patch testing with mite extract elicits eczematous skin lesions in a majority of patients with atopic dermatitis, particularly if their eczema is in an air-exposed distribution [182], suggesting that aeroallergens can cause pathology by direct skin contact. Sensitization to mites is frequent in atopic dermatitis, with IgE to mite allergen found in 95% of patients with atopic dermatitis, compared to 42% of asthmatics and 17% of controls [183]. Although dust mites are generally considered to live near people rather than on them, one study using transparent adhesive tape sampling found at least one house dust mite on the skin of 84% of pediatric atopic dermatitis patients vs. 14% of controls [184].
The ability of mite proteases to decrease skin barrier function has already been discussed [111, 113, 114]. In addition, in infants 3–12 months old with atopic dermatitis, transepidermal water loss through uninvolved skin (a measure of skin permeability) and sensitization to mites and other aeroallergens correlate, suggesting that the increase in skin permeability may increase the risk of sensitization to aeroallergens, initiating a vicious cycle [185]. Even without sensitization, mite extracts affect human keratinocytes, releasing pro-inflammatory [186] and pro-Th2 [187] cytokines and activating the innate immune system’s NLRP3 inflammasome [188]. Chitin also initiates innate immune responses in keratinocytes, being sensed by TLR2 and inducing chemokine release and TLR4 expression [189]. Epicutaneous application of house dust mite allergens induces expression of thymic stromal lymphopoietin in nonlesional skin of atopic dermatitis patients [190].
Systemic Allergy from Oral Ingestion
Atopic sensitivity to house dust mite allergens can lead to symptoms following oral ingestion in two situations: the ingestion of invertebrates exhibiting cross-reactivity with mite allergens, and the ingestion of foods contaminated with dust mites.
Cross-reactivity has been reported among dust mites and their fellow arthropods crustaceans (shrimp, crab, and lobster) and insects (cockroach, grasshopper), as well as members of the mollusk phylum (snails, clams, oysters, and squid), with clinical symptoms ranging from oral allergy syndrome to severe asthma and anaphylaxis [191]. The muscle protein tropomyosin, represented by Der p10 in dust mites, has been considered to be the “pan-allergen” responsible for these reactions to invertebrates [99, 192], sharing sequence homology with shrimp tropomyosin Pen a1, American cockroach tropomyosin Per a7, and lobster tropomyosin Hom a1 [193] (but with vertebrate tropomyosin being non-allergenic). However, there are reasons to believe that tropomyosin is not the only relevant allergen, there being dust mite sensitive patients with shrimp allergy but not snail allergy and with snail allergy but not shrimp allergy [194]. Furthermore, a 20-kDa allergen [195], and other allergens [196], have been identified in shrimp- and house dust mite sensitive patients without IgE to tropomyosin. There is also variability in the primary sensitizer; symptomatic dust mite sensitive individuals who had never eaten shellfish for religious reasons had IgE to shrimp tropomyosin [197], while residents of Iceland with IgE to shrimp who had never been exposed to dust mites had IgE to mites [198]. In inner city populations, IgE to shrimp has a greater correlation with exposure to cockroach than with exposure to dust mites [199]. Of potential practical importance are reports of reactivity to snails developing [200, 201], or increasing from mild to life-threatening [202], following subcutaneous dust mite immunotherapy. In contrast is a report suggesting a decrease in shrimp allergy following high dose sublingual mite immunotherapy [203]. Also noteworthy, particularly in view of the trend towards ethnic foods and new dietary protein sources, is the report of severe anaphylaxis in two mite-sensitive patients with rhinitis, asthma, and crustacean allergy following their first ingestion of “chapulines,” a Mexican dish of roasted grasshopper [204].
Systemic allergic symptoms can follow the inadvertent ingestion of dust mites themselves in the form of a food that has been colonized with mites, the so-called oral mite anaphylaxis. This was first reported in 1993, following the ingestion of a beignet (a fried pastry) made from flour that had been contaminated with D. farinae [205]. Since then, there have been well over 100 reports of systemic allergy from the ingestion of mite-contaminated foods including pancakes [206], wheat and corn flour [207, 208], and grits [209]. Cases are more common in tropical or semitropical areas where the high humidity facilitates dust mite growth in foodstuffs [210], but have also been reported in the northeastern USA [211]. Cooking the food does not eliminate the problem, as group 2 mite allergens are relatively heat stabile [212]. Episodes have occurred in children as well as adults [213], with symptoms including urticaria, wheezing, anaphylaxis, and exercise-induced anaphylaxis [214, 215]. For reasons that are unclear, a large percentage of these patients also have sensitivity to NSAIDs [210]. They generally do not have concomitant wheat allergy, and current guidelines suggest that patients presenting with symptoms suggestive of IgE-mediated disease following the ingestion grain flour products be tested for dust mite reactivity, regardless of whether or not they have IgE to wheat [216]. If the offending food substance is available, it can be examined microscopically for the presence of live mites, or sent for an assay of mite allergens. (A microscope video-recording of contaminated pancake mix from a published case is available online [217].) Mite colonization of the offending foods appears to have occurred in the home, in packages that had been left open at room temperature for prolonged periods [74, 211], rather than during manufacturing [205, 208]. It is therefore prudent for mite-sensitive patients to store any opened packages of pancake mix, grains, or flour in a refrigerator, where mites are unable to reproduce, rather than in a cupboard.
The role, if any, played by the ingestion of dust mite allergen in eosinophilic esophagitis [218, 219] or inflammatory bowel disease [91, 220] is currently unclear.
Specific Treatments for Mite-Related Diseases
Although a detailed discussion of the treatment of allergic diseases caused by exposure to dust mites is beyond the scope of this review, it can be briefly mentioned that in addition to the non-specific pharmacologic treatments available for all atopic diseases, those cases in which dust mite allergy is playing a major role may be specifically treated with mite allergen avoidance measures and/or with subcutaneous or sublingual immunotherapy. The steps involved in the preparation of mite allergen extracts for immunotherapy include culturing, harvesting, inactivation, drying, purification, fractionation, characterization, and standardization, all of which have recently been reviewed by experts involved in these commercial processes [221]. Appropriate home environmental interventions for house dust mites have also recently been reviewed [216, 222] and include encasing mattresses and pillows; frequent washing of bedding and clothing; removing carpeting, upholstered furniture, drapes, and other habitats for dust mites; and dehumidification.
Conclusion
Apart from their interest as biological organisms, house dust mites open a window into atopy in general. As an allergen of unsurpassed importance, they have major lessons to teach us about allergenicity, proteases, adjuvants, PAMPs, chitin, and the immunologic role of the epithelium. An understanding of their biology and physiology is necessary to make sense of allergen avoidance studies, and they are a portal to an understanding not only of allergic rhinoconjuctivitis, allergic asthma, atopic dermatitis, and food allergy but also of the atopic march and the nature of atopy itself.
References
Calderon MA, Linneberg A, Kleine-Tebbe J et al (2015) Respiratory allergy caused by house dust mites: what do we really know? J Allergy Clin Immunol 136:38–48
Sanchez-Borges M, Fernandez-Caldas E, Thomas WR et al (2017) International consensus (ICON) on: clinical consequences of mite hypersensitivity, a global problem. World Allergy Organ J 10:14
Colloff MJ (1998) Taxonomy and identification of dust mites. Allergy 53:7–12
Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, Woodfolk JA, Platts-Mills TAE (2009) Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol 123:426–433
Spieksma FT, Dieges PH (2004) The history of the finding of the house dust mite. J Allergy Clin Immunol 113:573–576
Voorhorst R, Spieksma FT, Varekamp H, Leupen M, Lyklema A (1967) The house-dust mite (Dermatophagoides pteronyssinus) and the allergens it produces. Identity with the house-dust allergen. J Allergy (Cairo) 39:325–339
Miyamoto T, Oshima S, Ishizaki T, Sato SH (1968) Allergenic identity between the common floor mite (Dermatophagoides farinae Hughes, 1961) and house dust as a causative antigen in bronchial asthma. J Allergy 42:14–28
Tovey ER, Chapman MD, Platts-Mills TA (1981) Mite faeces are a major source of house dust allergens. Nature 289:592–593
Colloff M (2009) Dust mites. CSIRO Pub, Collingwood
Arlian L, Rapp C, Ahmed S (1990) Development of Dermatophagoides pteronyssinus (Acari: Pyroglyphidae). J Med Entomol 27:1035–1040
Arlian LG (1992) Water balance and humidity requirements of house dust mites. Exp Appl Acarol 16:15–35
De Boer R, Kuller K, Kahl O (1998) Water balance of Dermatophagoides pteronyssinus (Acari: Pyroglyphidae) maintained by brief daily spells of elevated air humidity. J Med Entomol 35:905–910
Arlian LG, Neal JS, Vyszenski-Moher DL (1999) Fluctuating hydrating and dehydrating relative humidities effects on the life cycle of Dermatophagoides farinae (Acari: Pyroglyphidae). J Med Entomol 36:457–461
Ellingsen IJ (1976) Permeability to water in different adaptive phases of the same instar in the American house-dust mite. Acarologia 17:734–744
Arlian LG, Dippold JS (1996) Development and fecundity of Dermatophagoides farinae (Acari: Pyroglyphidae). J Med Entomol 33:257–260
Mahakittikun V, Boitano JJ, Ninsanit P, Wangapai T, Ralukruedej K (2011) Effects of high and low temperatures on development time and mortality of house dust mite eggs. Exp Appl Acarol 55:339–347
Mahakittikun V, Wongkamchai S, Ahamad MH, Vichyanond P (2001) Killing mites with heat. Allergy 56:262
McDonald LG, Tovey E (1992) The role of water temperature and laundry procedures in reducing house dust mite populations and allergen content of bedding. J Allergy Clin Immunol 90:599–608
Feichtner CR, Arlian LG, Morgan MS, Vyszenski-Moher DL (2018) Home freezers kill house dust mites. J Allergy Clin Immunol 141:451–454
Colloff MJ (1992) Exposure to house dust mites in homes of people with atopic dermatitis. Br J Dermatol 127:322–327
Beck HI, Bjerring P (1987) House dust mites and human dander. Allergy 42:471–472
Wharton GW, Brody AR (1972) The peritrophic membrane of the mite, Dermatophagoides farinae: Acariformes. J Parasitol 58:801–804
Klimov PB, B OC (2013) Is permanent parasitism reversible?—critical evidence from early evolution of house dust mites. Syst Biol 62:411–423
Murray AB, Ferguson AC, Morrison BJ (1985) Sensitization to house dust mites in different climatic areas. J Allergy Clin Immunol 76:108–112
Green W, Woolcock AJ, Dowse G (1982) House dust mites in blankets and houses in the highlands of Papua New Guinea. P N G Med J 25:219–222
Spieksma FT, Zuidema P, Leupen MJ (1971) High altitude and house-dust mites. Br Med J 1:82–84
Vervloet D, Penaud A, Razzouk H et al (1982) Altitude and house dust mites. J Allergy Clin Immunol 69:290–296
Charpin D, Birnbaum J, Haddi E, Genard G, Lanteaume A, Toumi M, Faraj F, van der Brempt X, Vervloet D (1991) Altitude and allergy to house-dust mites. A paradigm of the influence of environmental exposure on allergic sensitization. Am Rev Respir Dis 143:983–986
Gitoho F, Rees P (1971) High altitude and house-dust mites. Br Med J 3:475
Sanchez-Medina M, Zarante I (1996) Dust mites at high altitude in a tropical climate. J Allergy Clin Immunol 97:1167–1168
Nelson HS, Fernandez-Caldas E (1995) Prevalence of house dust mites in the Rocky Mountain states. Ann Allergy Asthma Immunol 75:337–339
Arlian LG, Bernstein D, Bernstein IL, Friedman S, Grant A, Lieberman P, Lopez M, Metzger J, Platts-Mills T, Schatz M, Spector S, Wasserman SI, Zeiger RS (1992) Prevalence of dust mites in the homes of people with asthma living in eight different geographic areas of the United States. J Allergy Clin Immunol 90:292–300
Leaderer BP, Belanger K, Triche E, Holford T, Gold DR, Kim Y, Jankun T, Ren P, McSharry JE, Platts-Mills TAE, Chapman MD, Bracken MB (2002) Dust mite, cockroach, cat, and dog allergen concentrations in homes of asthmatic children in the northeastern United States: impact of socioeconomic factors and population density. Environ Health Perspect 110:419–425
van Strien RT, Gehring U, Belanger K, Triche E, Gent J, Bracken MB, Leaderer BP (2004) The influence of air conditioning, humidity, temperature and other household characteristics on mite allergen concentrations in the northeastern United States. Allergy 59:645–652
Wilson J, Dixon SL, Breysse P, Jacobs D, Adamkiewicz G, Chew GL, Dearborn D, Krieger J, Sandel M, Spanier A (2010) Housing and allergens: a pooled analysis of nine US studies. Environ Res 110:189–198
Chew GL, Reardon AM, Correa JC, Young M, Acosta L, Mellins R, Chew FT, Perzanowski MS (2009) Mite sensitization among Latina women in New York, where dust-mite allergen levels are typically low. Indoor Air 19:193–197
Babe KS Jr, Arlian LG, Confer PD, Kim R (1995) House dust mite (Dermatophagoides farinae and Dermatophagoides pteronyssinus) prevalence in the rooms and hallways of a tertiary care hospital. J Allergy Clin Immunol 95:801–805
Sander I, Lotz A, Neumann HD, Czibor C, Flagge A, Zahradnik E, Raulf M (2018) Indoor allergen levels in settled airborne dust are higher in day-care centers than at home. Allergy 73:1263–1275
Custovic A, Taggart SC, Woodcock A (1994) House dust mite and cat allergen in different indoor environments. Clin Exp Allergy 24:1164–1168
Massey DG, Furumizo RT, Fournier-Massey G, Kwock D, Harris ST (1988) House dust mites in university dormitories. Ann Allergy 61:229–232
Sander I, Zahradnik E, Kraus G, Mayer S, Neumann HD, Fleischer C, Brüning T, Raulf-Heimsoth M (2012) Domestic mite antigens in floor and airborne dust at workplaces in comparison to living areas: a new immunoassay to assess personal airborne allergen exposure. PLoS One 7:e52981
Engelhart ST, Wilmes-Link M, Gilges S, Exner M, Kramer MH (1999) Exposure of submarine personnel to house dust mite allergens. J Allergy Clin Immunol 104:242–243
Ott CM, Bruce RJ, Pierson DL (2004) Microbial characterization of free floating condensate aboard the Mir space station. Microb Ecol 47:133–136
Arlian LG, Bernstein IL, Gallagher JS (1982) The prevalence of house dust mites, Dermatophagoides spp, and associated environmental conditions in homes in Ohio. J Allergy Clin Immunol 69:527–532
Lintner TJ, Brame KA (1993) The effects of season, climate, and air-conditioning on the prevalence of Dermatophagoides mite allergens in household dust. J Allergy Clin Immunol 91:862–867
Platts-Mills TA, Hayden ML, Chapman MD, Wilkins SR (1987) Seasonal variation in dust mite and grass-pollen allergens in dust from the houses of patients with asthma. J Allergy Clin Immunol 79:781–791
Arlian LG, Neal JS, Morgan MS, Vyszenski-Moher DL, Rapp CM, Alexander AK (2001) Reducing relative humidity is a practical way to control dust mites and their allergens in homes in temperate climates. J Allergy Clin Immunol 107:99–104
Arlian LG, Neal JS, Vyszenski-Moher DL (1999) Reducing relative humidity to control the house dust mite Dermatophagoides farinae. J Allergy Clin Immunol 104:852–856
Johnston JD, Barney TP, Crandall JH, Brown MA, Westover TR, Paulson SM, Smith MS, Weber KS (2018) Prevalence of house dust mite allergens in low-income homes with evaporative coolers in a semiarid climate. Arch Environ Occup Health 73:38–41
Prasad C, Hogan MB, Peele K, Wilson NW (2009) Effect of evaporative coolers on skin test reactivity to dust mites and molds in a desert environment. Allergy Asthma Proc 30:624–627
Arbes SJ Jr, Cohn RD, Yin M, Muilenberg ML, Burge HA, Friedman W, Zeldin DC (2003) House dust mite allergen in US beds: results from the First National Survey of Lead and Allergens in Housing. J Allergy Clin Immunol 111:408–414
Arlian LG, Brandt RL, Bernstein R (1978) Occurrence of house dust mites, Dermatophagoides spp. (Acari: Pyroglyphidae), during the heating season. J Med Entomol 15:35–42
Wickens K, Siebers R, Ellis I et al (1997) Determinants of house dust mite allergen in homes in Wellington, New Zealand. Clin Exp Allergy 27:1077–1085
Abbott J, Cameron J, Taylor B (1981) House dust mite counts in different types of mattresses, sheepskins and carpets, and a comparison of brushing and vacuuming collection methods. Clin Allergy 11:589–595
Garrett MH, Hooper BM, Hooper MA (1998) Indoor environmental factors associated with house-dust-mite allergen (Der p 1) levels in south-eastern Australian houses. Allergy 53:1060–1065
Mihrshahi S, Marks G, Vanlaar C, Tovey E, Peat J (2002) Predictors of high house dust mite allergen concentrations in residential homes in Sydney. Allergy 57:137–142
Kemp TJ, Siebers RW, Fishwick D, O'Grady GB, Fitzharris P, Crane J (1996) House dust mite allergen in pillows. BMJ 313:916
Hallam C, Custovic A, Simpson B, Houghton N, Simpson A, Woodcock A (1999) Mite allergens in feather and synthetic pillows. Allergy 54:407–408
Siebers R, Nam HS, Crane J (2004) Permeability of synthetic and feather pillows to live house dust mites and house dust. Clin Exp Allergy 34:888–890
Mills S, Siebers R, Wickens K, Crane J, Purdie G, Fitzharris P (2002) House dust mite allergen levels in individual bedding components in New Zealand. N Z Med J 115:151–153
Mosbech H, Korsgaard J, Lind P (1988) Control of house dust mites by electrical heating blankets. J Allergy Clin Immunol 81:706–710
Ingham PE, Ingham DM (1976) House dust mites and infant-use sheepskins. Med J Aust 1:302–304
Sawyer G, Kemp T, Shaw R, Patchett K, Siebers R, Lewis S, Beasley R, Crane J, Fitzharris P (1998) Biologic pollution in infant bedding in New Zealand: high allergen exposure during a vulnerable period. J Allergy Clin Immunol 102:765–770
Gaig P, Enrique E, Garcia-Ortega P, Olona M, del Mar San Miguel M, Richart C (1999) Asthma, mite sensitization, and sleeping in bunks. Ann Allergy Asthma Immunol 82:531–533
de Oliveira CH, Binotti RS, Muniz JR, dos Santos JC, do Prado AP, de Pinho AJ Jr (2003) Comparison of house dust mites found on different mattress surfaces. Ann Allergy Asthma Immunol 91:559–562
Pauli G, de Blay F, Bessot JC, Ott M, Gries P (1997) The role of mattress bases in the mite infestation of dwellings. J Allergy Clin Immunol 99:261–263
Causer S, Shorter C, Sercombe J (2006) Effect of floorcovering construction on content and vertical distribution of house dust mite allergen, Der p I. J Occup Environ Hyg 3:161–168 quiz D45
Sercombe JK, Liu-Brennan D, Causer SM, Tovey ER (2007) The vertical distribution of house dust mite allergen in carpet and the effect of dry vacuum cleaning. Int J Hyg Environ Health 210:43–50
Tovey ER, Mahmic A, McDonald LG (1995) Clothing—an important source of mite allergen exposure. J Allergy Clin Immunol 96:999–1001
Mollet JA, Robinson WH (1996) Dispersal of American house dust mites (Acari:Pyroglyphidae) in a residence. J Med Entomol 33:844–847
Wu FF, Wu MW, Ting MH, Crane J, Siebers R (2014) Cat, dog and house dust mite allergen levels on children’s soft toys. J Asthma 51:75–78
Spieksma FT (1976) Cultures of house-dust mites on animal skin scales. Allergol Immunopathol 4:419–428
Eaton KK, Downing FS, Griffiths DA, Hockland S, Lynch S (1985) Housedust mites (D. pteronyssinus) in pets’ beds and their relation to dust allergy. Clin Allergy 15:151–154
Matsumoto T, Satoh A (2004) The occurrence of mite-containing wheat flour. Pediatr Allergy Immunol 15:469–471
Gill C, McEwan N, McGarry J, Nuttall T (2011) House dust and storage mite contamination of dry dog food stored in open bags and sealed boxes in 10 domestic households. Vet Dermatol 22:162–172
Neal JS, Arlian LG, Morgan MS (2002) Relationship among house-dust mites, Der 1, Fel d 1, and Can f 1 on clothing and automobile seats with respect to densities in houses. Ann Allergy Asthma Immunol 88:410–415
Taketomi EA, Justino CM, Pereira FL, Segundo GR, Sopelete MC, Sung SJ, Silva DA (2006) Taxis but not private cars are mite allergen reservoirs in Brazil. J Investig Allergol Clin Immunol 16:34–36
Arlian LG, Neal JS, Morgan MS, Rapp CM, Clobes AL (2001) Distribution and removal of cat, dog and mite allergens on smooth surfaces in homes with and without pets. Ann Allergy Asthma Immunol 87:296–302
Wood RA, Mudd KE, Eggleston PA (1992) The distribution of cat and dust mite allergens on wall surfaces. J Allergy Clin Immunol 89:126–130
Kanchongkittiphon W, Sheehan WJ, Friedlander J, Chapman MD, King EM, Martirosyan K, Baxi SN, Permaul P, Gaffin JM, Kopel L, Bailey A, Fu C, Petty CR, Gold DR, Phipatanakul W (2014) Allergens on desktop surfaces in preschools and elementary schools of urban children with asthma. Allergy 69:960–963
de Boer R, van der Hoeven WA, Stapel SO (1995) The decay of house dust mite allergens, Der p I and Der p II, under natural conditions. Clin Exp Allergy 25:765–770
Tovey ER, Chapman MD, Wells CW, Platts-Mills TA (1981) The distribution of dust mite allergen in the houses of patients with asthma. Am Rev Respir Dis 124:630–635
Tovey ER, Willenborg CM, Crisafulli DA, Rimmer J, Marks GB (2013) Most personal exposure to house dust mite aeroallergen occurs during the day. PLoS One 8:e69900
Woodfolk JA, Commins SP, Schuyler AJ, Erwin EA, Platts-Mills TA (2015) Allergens, sources, particles, and molecules: why do we make IgE responses? Allergol Int 64:295–303
de Blay F, Heymann PW, Chapman MD, Platts-Mills TA (1991) Airborne dust mite allergens: comparison of group II allergens with group I mite allergen and cat-allergen Fel d I. J Allergy Clin Immunol 88:919–926
Custovic A, Woodcock H, Craven M, Hassall R, Hadley E, Simpson A, Woodcock A (1999) Dust mite allergens are carried on not only large particles. Pediatr Allergy Immunol 10:258–260
Tovey ER, Almqvist C, Li Q, Crisafulli D, Marks GB (2008) Nonlinear relationship of mite allergen exposure to mite sensitization and asthma in a birth cohort. J Allergy Clin Immunol 122:114–118 8 e1–5
Lack G, Fox D, Northstone K, Golding J, Avon Longitudinal Study of P, Children Study T (2003) Factors associated with the development of peanut allergy in childhood. N Engl J Med 348:977–985
Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, Kocan KM, Fahy JV, Nganga LW, Ronmark E, Cooper PJ, Platts-Mills TAE (2011) The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol 127:1286–93 e6
Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS (1998) Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest 101:1614–1622
Tulic MK, Vivinus-Nebot M, Rekima A et al (2016) Presence of commensal house dust mite allergen in human gastrointestinal tract: a potential contributor to intestinal barrier dysfunction. Gut 65:757–766
Macchiaverni P, Rekima A, Turfkruyer M, Mascarell L, Airouche S, Moingeon P, Adel-Patient K, Condino-Neto A, Annesi-Maesano I, Prescott SL, Tulic MK, Verhasselt V (2014) Respiratory allergen from house dust mite is present in human milk and primes for allergic sensitization in a mouse model of asthma. Allergy 69:395–398
Baiz N, Macchiaverni P, Tulic MK et al (2017) Early oral exposure to house dust mite allergen through breast milk: a potential risk factor for allergic sensitization and respiratory allergies in children. J Allergy Clin Immunol 139:369–72 e10
Brandt EB, Scribner TA, Akei HS, Rothenberg ME (2006) Experimental gastrointestinal allergy enhances pulmonary responses to specific and unrelated allergens. J Allergy Clin Immunol 118:420–427
Avershina E, Ravi A, Storro O, Oien T, Johnsen R, Rudi K (2015) Potential association of vacuum cleaning frequency with an altered gut microbiota in pregnant women and their 2-year-old children. Microbiome 3:65
Bordas-Le Floch V, Le Mignon M, Bussieres L et al (2017) A combined transcriptome and proteome analysis extends the allergome of house dust mite Dermatophagoides species. PLoS One 12:e0185830
Reithofer M, Jahn-Schmid B (2017) Allergens with protease activity from house dust mites. Int J Mol Sci 18:E1368
Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, Karp CL (2009) Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 457:585–588
Reese G, Ayuso R, Lehrer SB (1999) Tropomyosin: an invertebrate pan-allergen. Int Arch Allergy Immunol 119:247–258
Wong L, Huang CH, Lee BW (2016) Shellfish and house dust mite allergies: is the link tropomyosin? Allergy, Asthma Immunol Res 8:101–106
Banerjee S, Resch Y, Chen KW, Swoboda I, Focke-Tejkl M, Blatt K, Novak N, Wickman M, van Hage M, Ferrara R, Mari A, Purohit A, Pauli G, Sibanda EN, Ndlovu P, Thomas WR, Krzyzanek V, Tacke S, Malkus U, Valent P, Valenta R, Vrtala S (2015) Der p 11 is a major allergen for house dust mite-allergic patients suffering from atopic dermatitis. J Investig Dermatol 135:102–109
Fernandez-Caldas E (2013) On mite allergy in dogs and humans. Int Arch Allergy Immunol 160:329–330
Weghofer M, Grote M, Resch Y, Casset A, Kneidinger M, Kopec J, Thomas WR, Fernandez-Caldas E, Kabesch M, Ferrara R, Mari A, Purohit A, Pauli G, Horak F, Keller W, Valent P, Valenta R, Vrtala S (2013) Identification of Der p 23, a peritrophin-like protein, as a new major Dermatophagoides pteronyssinus allergen associated with the peritrophic matrix of mite fecal pellets. J Immunol 190:3059–3067
Mueller GA, Randall TA, Glesner J, Pedersen LC, Perera L, Edwards LL, DeRose EF, Chapman MD, London RE, Pomés A (2016) Serological, genomic and structural analyses of the major mite allergen Der p 23. Clin Exp Allergy 46:365–376
Chan TF, Ji KM, Yim AK et al (2015) The draft genome, transcriptome, and microbiome of Dermatophagoides farinae reveal a broad spectrum of dust mite allergens. J Allergy Clin Immunol 135:539–548
Papazian D, Hansen S, Wurtzen PA (2015) Airway responses towards allergens—from the airway epithelium to T cells. Clin Exp Allergy 45:1268–1287
Gunawan H, Takai T, Ikeda S, Okumura K, Ogawa H (2008) Protease activity of allergenic pollen of cedar, cypress, juniper, birch and ragweed. Allergol Int 57:83–91
Yike I (2011) Fungal proteases and their pathophysiological effects. Mycopathologia 171:299–323
Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, Stewart GA, Taylor GW, Garrod DR, Cannell MB, Robinson C (1999) Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest 104:123–133
Chapman MD, Wunschmann S, Pomes A (2007) Proteases as Th2 adjuvants. Curr Allergy Asthma Rep 7:363–367
Nakamura T, Hirasawa Y, Takai T, Mitsuishi K, Okuda M, Kato T, Okumura K, Ikeda S, Ogawa H (2006) Reduction of skin barrier function by proteolytic activity of a recombinant house dust mite major allergen Der f 1. J Investig Dermatol 126:2719–2723
Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, Luger TA, Schmelz M (2003) Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci 23:6176–6180
Kato T, Takai T, Fujimura T, Matsuoka H, Ogawa T, Murayama K, Ishii A, Ikeda S, Okumura K, Ogawa H (2009) Mite serine protease activates protease-activated receptor-2 and induces cytokine release in human keratinocytes. Allergy 64:1366–1374
Jeong SK, Kim HJ, Youm JK, Ahn SK, Choi EH, Sohn MH, Kim KE, Hong JH, Shin DM, Lee SH (2008) Mite and cockroach allergens activate protease-activated receptor 2 and delay epidermal permeability barrier recovery. J Investig Dermatol 128:1930–1939
Grunstein MM, Veler H, Shan X, Larson J, Grunstein JS, Chuang S (2005) Proasthmatic effects and mechanisms of action of the dust mite allergen, Der p 1, in airway smooth muscle. J Allergy Clin Immunol 116:94–101
Trian T, Allard B, Dupin I, Carvalho G, Ousova O, Maurat E, Bataille J, Thumerel M, Begueret H, Girodet PO, Marthan R, Berger P (2015) House dust mites induce proliferation of severe asthmatic smooth muscle cells via an epithelium-dependent pathway. Am J Respir Crit Care Med 191:538–546
Aubier M, Thabut G, Hamidi F, Guillou N, Brard J, Dombret MC, Borensztajn K, Aitilalne B, Poirier I, Roland-Nicaise P, Taillé C, Pretolani M (2016) Airway smooth muscle enlargement is associated with protease-activated receptor 2/ligand overexpression in patients with difficult-to-control severe asthma. J Allergy Clin Immunol 138:729–39 e11
Pfeffer PE, Corrigan CJ (2017) An imbalance between proteases and endogenous protease inhibitors in eosinophilic airway disease. Am J Respir Crit Care Med 195:707–708
Schulz O, Sewell HF, Shakib F (1998) Proteolytic cleavage of CD25, the alpha subunit of the human T cell interleukin 2 receptor, by Der p 1, a major mite allergen with cysteine protease activity. J Exp Med 187:271–275
Hewitt CR, Brown AP, Hart BJ, Pritchard DI (1995) A major house dust mite allergen disrupts the immunoglobulin E network by selectively cleaving CD23: innate protection by antiproteases. J Exp Med 182:1537–1544
Shakib F, Ghaemmaghami AM, Sewell HF (2008) The molecular basis of allergenicity. Trends Immunol 29:633–642
Wills-Karp M, Nathan A, Page K, Karp CL (2010) New insights into innate immune mechanisms underlying allergenicity. Mucosal Immunol 3:104–110
Lee CG (2009) Chitin, chitinases and chitinase-like proteins in allergic inflammation and tissue remodeling. Yonsei Med J 50:22–30
Kim LK, Morita R, Kobayashi Y, Eisenbarth SC, Lee CG, Elias J, Eynon EE, Flavell RA (2015) AMCase is a crucial regulator of type 2 immune responses to inhaled house dust mites. Proc Natl Acad Sci U S A 112:E2891–E2899
Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, Cullen M, Grandsaigne M, Dombret MC, Aubier M, Pretolani M, Elias JA (2007) A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med 357:2016–2027
Van Dyken SJ, Garcia D, Porter P et al (2011) Fungal chitin from asthma-associated home environments induces eosinophilic lung infiltration. J Immunol 187:2261–2267
Van Dyken SJ, Liang HE, Naikawadi RP et al (2017) Spontaneous chitin accumulation in airways and age-related fibrotic lung disease. Cell 169:497–509 e13
Lee CG, Dela Cruz CS, Herzog E, Rosenberg SM, Ahangari F, Elias JA (2012) YKL-40, a chitinase-like protein at the intersection of inflammation and remodeling. Am J Respir Crit Care Med 185:692–694
El Kebir D, Jozsef L, Pan W, Wang L, Filep JG (2009) Bacterial DNA activates endothelial cells and promotes neutrophil adherence through TLR9 signaling. J Immunol 182:4386–4394
Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN (2009) House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med 15:410–416
Bufe B, Schumann T, Kappl R, Bogeski I, Kummerow C, Podgórska M, Smola S, Hoth M, Zufall F (2015) Recognition of bacterial signal peptides by mammalian formyl peptide receptors: a new mechanism for sensing pathogens. J Biol Chem 290:7369–7387
Svensson L, Redvall E, Bjorn C et al (2007) House dust mite allergen activates human eosinophils via formyl peptide receptor and formyl peptide receptor-like 1. Eur J Immunol 37:1966–1977
Dzoro S, Mittermann I, Resch-Marat Y, Vrtala S, Nehr M, Hirschl AM, Wikberg G, Lundeberg L, Johansson C, Scheynius A, Valenta R (2018) House dust mites as potential carriers for IgE sensitization to bacterial antigens. Allergy 73:115–124
Gough L, Sewell HF, Shakib F (2001) The proteolytic activity of the major dust mite allergen Der p 1 enhances the IgE antibody response to a bystander antigen. Clin Exp Allergy 31:1594–1598
Fattouh R, Pouladi MA, Alvarez D, Johnson JR, Walker TD, Goncharova S, Inman MD, Jordana M (2005) House dust mite facilitates ovalbumin-specific allergic sensitization and airway inflammation. Am J Respir Crit Care Med 172:314–321
Choi JP, Lee SM, Choi HI, Kim MH, Jeon SG, Jang MH, Jee YK, Yang S, Cho YJ, Kim YK (2016) House dust mite-derived chitin enhances Th2 cell response to inhaled allergens, mainly via a TNF-alpha-dependent pathway. Allergy, Asthma Immunol Res 8:362–374
Mattila P, Joenvaara S, Renkonen J, Toppila-Salmi S, Renkonen R (2011) Allergy as an epithelial barrier disease. Clin Transl Allergy 1:5
Hammad H, Smits HH, Ratajczak C, Nithiananthan A, Wierenga EA, Stewart GA, Jacquet A, Tonnel AB, Pestel J (2003) Monocyte-derived dendritic cells exposed to Der p 1 allergen enhance the recruitment of Th2 cells: major involvement of the chemokines TARC/CCL17 and MDC/CCL22. Eur Cytokine Netw 14:219–228
Ghaemmaghami AM, Gough L, Sewell HF, Shakib F (2002) The proteolytic activity of the major dust mite allergen Der p 1 conditions dendritic cells to produce less interleukin-12: allergen-induced Th2 bias determined at the dendritic cell level. Clin Exp Allergy 32:1468–1475
Willumsen N, Holm J, Christensen LH, Wurtzen PA, Lund K (2012) The complexity of allergic patients’ IgE repertoire correlates with serum concentration of allergen-specific IgE. Clin Exp Allergy 42:1227–1236
Galli SJ, Tsai M (2012) IgE and mast cells in allergic disease. Nat Med 18:693–704
Platts-Mills TA, Tovey ER, Mitchell EB, Moszoro H, Nock P, Wilkins SR (1982) Reduction of bronchial hyperreactivity during prolonged allergen avoidance. Lancet 2:675–678
Chan-Yeung M, Manfreda J, Dimich-Ward H, Lam J, Ferguson A, Warren P, Simons E, Broder I, Chapman M, Platts-Mills T (1995) Mite and cat allergen levels in homes and severity of asthma. Am J Respir Crit Care Med 152:1805–1811
Custovic A, Taggart SC, Francis HC, Chapman MD, Woodcock A (1996) Exposure to house dust mite allergens and the clinical activity of asthma. J Allergy Clin Immunol 98:64–72
van der Heide S, De Monchy JG, De Vries K, Dubois AE, Kauffman HF (1997) Seasonal differences in airway hyperresponsiveness in asthmatic patients: relationship with allergen exposure and sensitization to house dust mites. Clin Exp Allergy 27:627–633
Tunnicliffe WS, Fletcher TJ, Hammond K, Roberts K, Custovic A, Simpson A, Woodcock A, Ayres JG (1999) Sensitivity and exposure to indoor allergens in adults with differing asthma severity. Eur Respir J 13:654–659
Langley SJ, Goldthorpe S, Craven M, Morris J, Woodcock A, Custovic A (2003) Exposure and sensitization to indoor allergens: association with lung function, bronchial reactivity, and exhaled nitric oxide measures in asthma. J Allergy Clin Immunol 112:362–368
Sporik R, Platts-Mills TA, Cogswell JJ (1993) Exposure to house dust mite allergen of children admitted to hospital with asthma. Clin Exp Allergy 23:740–746
Soto-Quiros M, Avila L, Platts-Mills TA et al (2012) High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J Allergy Clin Immunol 129:1499–1505 e5
Green RM, Custovic A, Sanderson G, Hunter J, Johnston SL, Woodcock A (2002) Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ 324:763
Murray CS, Poletti G, Kebadze T, Morris J, Woodcock A, Johnston SL, Custovic A (2006) Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in children. Thorax 61:376–382
Bennett WD, Herbst M, Alexis NE, Zeman KL, Wu J, Hernandez ML, Peden DB (2011) Effect of inhaled dust mite allergen on regional particle deposition and mucociliary clearance in allergic asthmatics. Clin Exp Allergy 41:1719–1728
Milanese M, Peroni D, Costella S, Aralla R, Loiacono A, Barp C, Boner A, Brusasco V (2004) Improved bronchodilator effect of deep inhalation after allergen avoidance in asthmatic children. J Allergy Clin Immunol 114:505–511
Langley SJ, Goldthorpe S, Craven M, Woodcock A, Custovic A (2005) Relationship between exposure to domestic allergens and bronchial hyperresponsiveness in non-sensitised, atopic asthmatic subjects. Thorax 60:17–21
Mouthuy J, Detry B, Sohy C, Pirson F, Pilette C (2011) Presence in sputum of functional dust mite-specific IgE antibodies in intrinsic asthma. Am J Respir Crit Care Med 184:206–214
Dick S, Friend A, Dynes K, AlKandari F, Doust E, Cowie H, Ayres JG, Turner SW (2014) A systematic review of associations between environmental exposures and development of asthma in children aged up to 9 years. BMJ Open 4:e006554
Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ (1990) Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med 323:502–507
Peat JK, Tovey E, Toelle BG, Haby MM, Gray EJ, Mahmic A, Woolcock AJ (1996) House dust mite allergens. A major risk factor for childhood asthma in Australia. Am J Respir Crit Care Med 153:141–146
Celedon JC, Milton DK, Ramsey CD et al (2007) Exposure to dust mite allergen and endotoxin in early life and asthma and atopy in childhood. J Allergy Clin Immunol 120:144–149
Carter PM, Peterson EL, Ownby DR, Zoratti EM, Johnson CC (2003) Relationship of house-dust mite allergen exposure in children's bedrooms in infancy to bronchial hyperresponsiveness and asthma diagnosis by age 6 to 7. Ann Allergy Asthma Immunol 90:41–44
Cole Johnson C, Ownby DR, Havstad SL, Peterson EL (2004) Family history, dust mite exposure in early childhood, and risk for pediatric atopy and asthma. J Allergy Clin Immunol 114:105–110
Brussee JE, Smit HA, van Strien RT, Corver K, Kerkhof M, Wijga AH, Aalberse RC, Postma D, Gerritsen J, Grobbee DE, de Jongste JC, Brunekreef B (2005) Allergen exposure in infancy and the development of sensitization, wheeze, and asthma at 4 years. J Allergy Clin Immunol 115:946–952
Polk S, Sunyer J, Munoz-Ortiz L et al (2004) A prospective study of Fel d1 and Der p1 exposure in infancy and childhood wheezing. Am J Respir Crit Care Med 170:273–278
Cullinan P, MacNeill SJ, Harris JM, Moffat S, White C, Mills P, Newman Taylor AJ (2004) Early allergen exposure, skin prick responses, and atopic wheeze at age 5 in English children: a cohort study. Thorax 59:855–861
Richgels PK, Yamani A, Chougnet CA, Lewkowich IP (2017) Maternal house dust mite exposure during pregnancy enhances severity of house dust mite-induced asthma in murine offspring. J Allergy Clin Immunol 140:1404–1415.e9
Squillace SP, Sporik RB, Rakes G et al (1997) Sensitization to dust mites as a dominant risk factor for asthma among adolescents living in Central Virginia. Multiple regression analysis of a population-based study. Am J Respir Crit Care Med 156:1760–1764
Simpson BM, Custovic A, Simpson A, Hallam CL, Walsh D, Marolia H, Campbell J, Woodcock A (2001) NAC Manchester Asthma and Allergy Study (NACMAAS): risk factors for asthma and allergic disorders in adults. Clin Exp Allergy 31:391–399
Simpson A, Soderstrom L, Ahlstedt S, Murray CS, Woodcock A, Custovic A (2005) IgE antibody quantification and the probability of wheeze in preschool children. J Allergy Clin Immunol 116:744–749
Illi S, von Mutius E, Lau S, Niggemann B, Grüber C, Wahn U (2006) Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet 368:763–770
Lodge CJ, Lowe AJ, Gurrin LC, Hill DJ, Hosking CS, Khalafzai RU, Hopper JL, Matheson MC, Abramson MJ, Allen KJ, Dharmage SC (2011) House dust mite sensitization in toddlers predicts current wheeze at age 12 years. J Allergy Clin Immunol 128:782–8 e9
Llanora GV, Ming LJ, Wei LM, van Bever HP (2012) House dust mite sensitization in toddlers predict persistent wheeze in children between eight to fourteen years old. Asia Pac Allergy 2:181–186
Chusakul S, Phannaso C, Sangsarsri S, Aeumjaturapat S, Snidvongs K (2010) House-dust mite nasal provocation: a diagnostic tool in perennial rhinitis. Am J Rhinol Allergy 24:133–136
Rolla G, Guida G, Heffler E, Badiu I, Bommarito L, de Stefani A, Usai A, Cosseddu D, Nebiolo F, Bucca C (2007) Diagnostic classification of persistent rhinitis and its relationship to exhaled nitric oxide and asthma: a clinical study of a consecutive series of patients. Chest 131:1345–1352
Terreehorst I, Oosting AJ, Tempels-Pavlica Z, de Monchy JGR, Bruijnzeel-Koomen CAFM, Hak E, van Wijk RG (2002) Prevalence and severity of allergic rhinitis in house dust mite-allergic patients with bronchial asthma or atopic dermatitis. Clin Exp Allergy 32:1160–1165
Bergmann KC, Demoly P, Worm M, Fokkens WJ, Carrillo T, Tabar AI, Nguyen H, Montagut A, Zeldin RK (2014) Efficacy and safety of sublingual tablets of house dust mite allergen extracts in adults with allergic rhinitis. J Allergy Clin Immunol 133:1608–14 e6
Roux M, Devillier P, Yang WH, Montagut A, Abiteboul K, Viatte A, Zeldin RK (2016) Efficacy and safety of sublingual tablets of house dust mite allergen extracts: results of a dose-ranging study in an environmental exposure chamber. J Allergy Clin Immunol 138:451–458.e5
Ryu JH, Yoo JY, Kim MJ, Hwang SG, Ahn KC, Ryu JC, Choi MK, Joo JH, Kim CH, Lee SN, Lee WJ, Kim J, Shin DM, Kweon MN, Bae YS, Yoon JH (2013) Distinct TLR-mediated pathways regulate house dust mite-induced allergic disease in the upper and lower airways. J Allergy Clin Immunol 131:549–561
Klossek JM, Annesi-Maesano I, Pribil C, Didier A (2012) The burden associated with ocular symptoms in allergic rhinitis. Int Arch Allergy Immunol 158:411–417
Ciprandi G, Buscaglia S, Pesce G, Pronzato C, Ricca V, Parmiani S, Bagnasco M, Canonica GW (1995) Minimal persistent inflammation is present at mucosal level in patients with asymptomatic rhinitis and mite allergy. J Allergy Clin Immunol 96:971–979
Mumcuoglu YK, Zavaro A, Samra Z, Lazarowitz Z (1988) House dust mites and vernal keratoconjunctivitis. Ophthalmologica 196:175–181
Beck HI, Korsgaard J (1989) Atopic dermatitis and house dust mites. Br J Dermatol 120:245–251
Ring J, Darsow U, Gfesser M, Vieluf D (1997) The ‘atopy patch test’ in evaluating the role of aeroallergens in atopic eczema. Int Arch Allergy Immunol 113:379–383
Scalabrin DM, Bavbek S, Perzanowski MS, Wilson BB, Platts-Mills TA, Wheatley LM (1999) Use of specific IgE in assessing the relevance of fungal and dust mite allergens to atopic dermatitis: a comparison with asthmatic and nonasthmatic control subjects. J Allergy Clin Immunol 104:1273–1279
Teplitsky V, Mumcuoglu KY, Babai I, Dalal I, Cohen R, Tanay A (2008) House dust mites on skin, clothes, and bedding of atopic dermatitis patients. Int J Dermatol 47:790–795
Boralevi F, Hubiche T, Leaute-Labreze C et al (2008) Epicutaneous aeroallergen sensitization in atopic dermatitis infants—determining the role of epidermal barrier impairment. Allergy 63:205–210
Arlian LG, Morgan MS (2011) Immunomodulation of skin cytokine secretion by house dust mite extracts. Int Arch Allergy Immunol 156:171–178
Jang YH, Choi JK, Jin M, Choi YA, Ryoo ZY, Lee HS, Park PH, Kim SU, Kwon TK, Jang MH, Im SH, Moon SY, Lee WJ, Lee SJ, Kim DW, Kim SH (2017) House dust mite increases pro-Th2 cytokines IL-25 and IL-33 via the activation of TLR1/6 signaling. J Invest Dermatol 137:2354–2361
Dai X, Sayama K, Tohyama M, Shirakata Y, Hanakawa Y, Tokumaru S, Yang L, Hirakawa S, Hashimoto K (2011) Mite allergen is a danger signal for the skin via activation of inflammasome in keratinocytes. J Allergy Clin Immunol 127:806–814.e4
Koller B, Muller-Wiefel AS, Rupec R, Korting HC, Ruzicka T (2011) Chitin modulates innate immune responses of keratinocytes. PLoS One 6:e16594
Landheer J, Giovannone B, Mattson JD, Tjabringa S, Bruijnzeel-Koomen CAFM, McClanahan T, de Waal Malefyt R, Knol E, Hijnen DJ (2013) Epicutaneous application of house dust mite induces thymic stromal lymphopoietin in nonlesional skin of patients with atopic dermatitis. J Allergy Clin Immunol 132:1252–1254
van Ree R, Antonicelli L, Akkerdaas JH et al (1996) Asthma after consumption of snails in house-dust-mite-allergic patients: a case of IgE cross-reactivity. Allergy 51:387–393
Sidenius KE, Hallas TE, Poulsen LK, Mosbech H (2001) Allergen cross-reactivity between house-dust mites and other invertebrates. Allergy 56:723–733
Ayuso R, Reese G, Leong-Kee S, Plante M, Lehrer SB (2002) Molecular basis of arthropod cross-reactivity: IgE-binding cross-reactive epitopes of shrimp, house dust mite and cockroach tropomyosins. Int Arch Allergy Immunol 129:38–48
Bessot JC, Metz-Favre C, Rame JM, De Blay F, Pauli G (2010) Tropomyosin or not tropomyosin, what is the relevant allergen in house dust mite and snail cross allergies? Eur Ann Allergy Clin Immunol 42:3–10
Villalta D, Tonutti E, Visentini D, Bizzaro N, Roncarolo D, Amato S, Mistrello G (2010) Detection of a novel 20 kDa shrimp allergen showing cross-reactivity to house dust mites. Eur Ann Allergy Clin Immunol 42:20–24
Gamez C, Zafra M, Boquete M et al (2014) New shrimp IgE-binding proteins involved in mite-seafood cross-reactivity. Mol Nutr Food Res 58:1915–1925
Fernandes J, Reshef A, Patton L, Ayuso R, Reese G, Lehrer SB (2003) Immunoglobulin E antibody reactivity to the major shrimp allergen, tropomyosin, in unexposed orthodox Jews. Clin Exp Allergy 33:956–961
Adalsteinsdottir B, Sigurdardottir ST, Gislason T, Kristensen B, Gislason D (2007) What characterizes house dust mite sensitive individuals in a house dust mite free community in Reykjavik, Iceland? Allergol Int 56:51–56
Wang J, Calatroni A, Visness CM, Sampson HA (2011) Correlation of specific IgE to shrimp with cockroach and dust mite exposure and sensitization in an inner-city population. J Allergy Clin Immunol 128:834–837
van Ree R, Antonicelli L, Akkerdaas JH, Garritani MS, Aalberse RC, Bonifazi F (1996) Possible induction of food allergy during mite immunotherapy. Allergy 51:108–113
Peroni DG, Piacentini GL, Bodini A, Boner AL (2000) Snail anaphylaxis during house dust mite immunotherapy. Pediatr Allergy Immunol 11:260–261
Pajno GB, La Grutta S, Barberio G, Canonica GW, Passalacqua G (2002) Harmful effect of immunotherapy in children with combined snail and mite allergy. J Allergy Clin Immunol 109:627–629
Cortellini G, Spadolini I, Santucci A, Cova V, Conti C, Corvetta A, Passalacqua G (2011) Improvement of shrimp allergy after sublingual immunotherapy for house dust mites: a case report. Eur Ann Allergy Clin Immunol 43:162–164
Sokol WN, Wunschmann S, Agah S (2017) Grasshopper anaphylaxis in patients allergic to dust mite, cockroach, and crustaceans: Is tropomyosin the cause? Ann Allergy Asthma Immunol 119:91–92
Erben AM, Rodriguez JL, McCullough J, Ownby DR (1993) Anaphylaxis after ingestion of beignets contaminated with Dermatophagoides farinae. J Allergy Clin Immunol 92:846–849
Wen DC, Shyur SD, Ho CM, Chiang YC, Huang LH, Lin MT, Yang HC, Liang PH (2005) Systemic anaphylaxis after the ingestion of pancake contaminated with the storage mite Blomia freemani. Ann Allergy Asthma Immunol 95:612–614
Blanco C, Quiralte J, Castillo R et al (1997) Anaphylaxis after ingestion of wheat flour contaminated with mites. J Allergy Clin Immunol 99:308–313
Guerra Bernd LA, Arruda LK, Barros Antunes HB (2001) Oral anaphylaxis to mites. Allergy 56:83–84
Posthumus J, Borish L (2012) A 71-year-old man with anaphylaxis after eating grits. Allergy Asthma Proc 33:110–113
Sanchez-Borges M, Suarez Chacon R, Capriles-Hulett A, Caballero-Fonseca F, Fernandez-Caldas E (2013) Anaphylaxis from ingestion of mites: pancake anaphylaxis. J Allergy Clin Immunol 131:31–35
Hannaway PJ, Miller JD (2008) The pancake syndrome (oral mite anaphylaxis) by ingestion and inhalation in a 52-year-old woman in the northeastern United States. Ann Allergy Asthma Immunol 100:397–398
Cain G, Elderfield AJ, Green R, Smillie FI, Chapman MD, Custovic A, Woodcock A (1998) The effect of dry heat on mite, cat, and dog allergens. Allergy 53:1213–1215
Sanchez-Borges M, Capriles-Hulett A, Caballero-Fonesca F (2006) Oral mite anaphylaxis (pancake syndrome) also observed in children. Ann Allergy Asthma Immunol 96:755–756
Sanchez-Borges M, Capriles-Hulett A, Fernandez-Caldas E et al (1997) Mite-contaminated foods as a cause of anaphylaxis. J Allergy Clin Immunol 99:738–743
Sanchez-Borges M, Iraola V, Fernandez-Caldas E, Capriles-Hulett A, Caballero-Fonseca F (2007) Dust mite ingestion-associated, exercise-induced anaphylaxis. J Allergy Clin Immunol 120:714–716
Portnoy J, Miller JD, Williams PB, Chew GL, Miller JD, Zaitoun F, Phipatanakul W, Kennedy K, Barnes C, Grimes C, Larenas-Linnemann D, Sublett J, Bernstein D, Blessing-Moore J, Khan D, Lang D, Nicklas R, Oppenheimer J, Randolph C, Schuller D, Spector S, Tilles SA, Wallace D, Joint Taskforce on Practice Parameters, Practice Parameter Workgroup (2013) Environmental assessment and exposure control of dust mites: a practice parameter. Ann Allergy Asthma Immunol 111:465–507
Dust mites in pancake flour. 2018. Accessed 1/14/18, at https://youtu.be/goKCIOShO3w.
Ramirez RM, Jacobs RL (2013) Eosinophilic esophagitis treated with immunotherapy to dust mites. J Allergy Clin Immunol 132:503–504
Bene J, Ley D, Roboubi R, Gottrand F, Gautier S (2016) Eosinophilic esophagitis after desensitization to dust mites with sublingual immunotherapy. Ann Allergy Asthma Immunol 116:583–584
Wildenberg ME, van den Brink GR (2016) House dust mite: a new player in intestinal inflammation? Gut 65:727–728
Carnes J, Iraola V, Cho SH, Esch RE (2017) Mite allergen extracts and clinical practice. Ann Allergy Asthma Immunol 118:249–256
Wilson JM, Platts-Mills TAE (2018) Home environmental interventions for house dust mite. J Allergy Clin Immunol Pract 6:1–7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
Dr. Miller is the owner and CEO of Mission: Allergy, Inc.
Rights and permissions
About this article
Cite this article
Miller, J.D. The Role of Dust Mites in Allergy. Clinic Rev Allerg Immunol 57, 312–329 (2019). https://doi.org/10.1007/s12016-018-8693-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-018-8693-0