Abstract
The food allergy epidemic of recent years has led to the search for safe and effective methods of immunotherapy for foods. Studies of epicutaneous immunotherapy (EPIT) in mice have shown promising safety and efficacy data. Murine models have also identified probable mechanisms for the development of tolerance to food allergens, including the induction of regulatory T cells. Clinical data is lacking, but relatively small and early studies among peanut and cow’s milk allergic subjects suggest that EPIT has an excellent safety profile, particularly compared to other methods of specific allergen immunotherapy. Efficacy data are also promising for peanut allergy, among younger patients (ages 4–11 years of age), suggesting that a majority of young patients will experience an increase in reaction threshold with therapy. The goal of this therapy is the protection from accidental exposures to a known food allergen. Additional clinical data is needed to prove efficacy and further demonstrate the safety profile of EPIT for food allergy, prior to approval by the Food and Drug Administration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food allergy is increasing in prevalence worldwide, affecting up to 8% of children [1]. Peanut allergy affects more than 2% of US children, and is the most common life-threatening food allergy [2]. The current standard of care for food allergy remains strict avoidance of the allergen, reading product labels, education, and appropriately treating any accidental exposures [3]. This includes having epinephrine auto-injectors available at all times. The need for strict vigilance understandably causes considerable stress in daily life for patients and families living with food allergies, leading to reduced quality of life [4]. The goal has long been to develop safe and effective methods of immunotherapy to induce tolerance to food allergens. While this process has taken longer than many had anticipated or hoped, options are on the horizon, but none yet are approved by the Food and Drug Administration (FDA). Peanut has been the most studied allergen for immunotherapy as peanut allergy is often lifelong. Epicutaneous immunotherapy (EPIT) offers an appealing option to induce tolerance while having minimal side effects. However, peer-reviewed, published clinical data is lacking, but preliminary data supports both safety and efficacy, with mouse models supporting its theoretical basis.
EPIT was first investigated as a method to treat allergic rhinitis. In 1921, its first clinical use was reported on scarified skin to treat horse allergy [5]. In 1957, EPIT was investigated for the treatment of house dust mite and pollen allergies [6]. Following these initial reports, EPIT continued to be investigated for the treatment of aeroallergen sensitivity. More recently, given the epidemic increase in food allergy, this method has been studied in both mice and humans for the treatment of peanut, cow’s milk, and hen’s egg.
This review will present the experimental data supporting the use of EPIT, providing initial safety and efficacy data, as well as important insights into the possible immunologic mechanisms by which EPIT is likely inducing tolerance. The limited clinical human data will also be presented in detail to provide a comprehensive review of the current state of EPIT for food allergy, giving consideration to the limitations of these studies and need for additional research.
The Viaskin Epicutaneous Delivery System
In all clinical trials, and most murine models discussed here, EPIT is performed using the proprietary Viaskin® epicutaneous delivery system (VEDS, DBV Technologies, Paris, France). The VEDS consists of a transparent, overlying, adhesive layer that covers a central, translucent polyethylene membrane (11 mm in diameter). Dry powder allergen is applied by spraying onto the membrane and is maintained by electrostatic forces. When the patch is applied to skin, the occlusive adhesive layer induces moisture production, solubilizing the dried allergen, which facilitates absorption of allergen into the epidermis primarily, but also minimally into the dermis [7]. See Fig. 1 for a depiction of the layers of the VEDS and an image of the patch placed on intact skin in a human subject. The VEDS patch is applied to one of six rotating sites on the upper arm in older subjects, and the interscapular space in younger children [8]. Table 1 includes a sample up-dosing schedule used in EPIT trials.
a Graphical representation of the Viaskin epicutaneous delivery system (VEDS, DBV Technologies, Paris, France). b Image of patch application on the upper arm of a human subject. Reprinted with permission from DBV [https://www.dbv-technologies.com/en/viaskin-technology/viaskin-patch]
Immune Mechanism of EPIT-induced Tolerance in Murine Models
Several initial studies utilizing the VEDS on intact skin in mice have demonstrated consistent findings using different allergens (OVA and peanut). Within the epidermis and dermis, allergen is primarily taken up by dendritic cells (DCs) [7, 9, 10]. This uptake occurs more quickly and efficiently in mice who are sensitized to the allergen as compared to naïve mice [7] (Fig 2). These skin DCs then migrate through the dermis to local draining lymph nodes (LNs) with subsequent systemic effects. With continued application of EPIT, a significant decrease in specific IgE and increase in specific IgG2a (akin to IgG4 in humans) were observed [7, 9, 10]. One study compared EPIT to sublingual immunotherapy (SLIT) for pollen, and found that EPIT results in a significantly greater increase in IgG2a levels compared to SLIT [11]. Further, serum TH2 cytokines (IL-4, IL-5 and IL-13) are seen to decrease with EPIT, significantly more than with SLIT [7, 10, 11]. There was also a trend towards a significant increase in serum TGF-Β [11]. Together, these changes all suggest a switch in the immune response from a predominantly TH2 profile, to a more balanced TH2/TH1 profile. Additionally, EPIT-treated, sensitized mice demonstrated reduced reactivity to food allergen via a variety of models [9,10,11]. Other immunologic changes have included a reduction in serum food-specific IgE, with increases in IgG2a. However, these are more likely markers of tolerance rather than mediators acting as blocking antibodies.
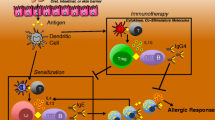
Proposed mechanism of tolerance induction using epicutaneous immunotherapy (patch) for food. Food protein allergen is absorbed from the patch through the epidermis and dermis, where it is processed by dendritic cells (DCs). DCs migrate to local draining lymph nodes where LAP+ regulatory T cells are produced. These cells have surface markers for gut-homing (CCR 6 and CCR9) and skin-homing (CCR4), where they produce TGF-B, which directly suppresses mast cell activation. Reprinted with permission from [19]
The development of tolerance is believed to be mediated by antigen-specific Foxp3+ regulatory T cells (Treg) that may directly suppress mast cell reactivity [12]. Several subsets of inducible Tregs have been identified, including IL-10 producing Tr1 cells, TGF-B-inducing Th3 cells, and CD4+CD25+ Foxp3+ Tregs [13]. Indeed, EPIT increases peripheral CD4+CD25+ Foxp3+ Tregs, but not IL10+ Tr1 cells [14]. When Tregs were depleted with anti-CD25 antibody, the tolerance induced by EPIT was lost. However, anti-IL-10 antibodies did not have any effect on tolerance. This study also found that in mice, the population of Tregs stimulated by EPIT are long-lasting, and suggest that there may be a protective effect even after stopping therapy. Additional studies have found that in Langerhans cell-depleted mice, there is no protection from anaphylaxis in sensitized mice, and no increase in Foxp3+ Tregs [15]. There is also evidence to suggest that treatment with EPIT could protect against the development of sensitization to other allergens, as has been shown with environmental subcutaneous immunotherapy (SCIT) [16, 17]. Mondoulet, et al., showed that monosensitized mice (to either cow’s milk or house dust mite) treated with EPIT for that allergen, did not develop sensitization to a second, unrelated allergen (pollen, house dust mite, or peanut) [18]. While the mechanism is not fully elucidated, it appears again to be Treg-dependent, and also long-lasting.
Despite a seemingly clear and important role for Tregs in inducing tolerance after treatment with EPIT, the question has been raised how these skin-derived DCs, which take up allergen via EPIT, could have a systemic effect and specifically be tolerogenic in the gut. A recent study by Tordesillas et al. identified a significant expansion of latency-associated peptide (LAP+) Foxp3− Treg cells in the mesenteric LNs of OVA-sensitized mice treated with EPIT [19]. LAP+ Tregs were not found in other LNs, and these cells appear to have a unique imprinting for gut-homing. It had previously been believed that surface markers on skin DC-derived Tregs would maintain skin-homing. However, surface markers on these LAP+ Tregs include CCR6 and CCR9, allowing them to home to the gut, in addition to the skin-homing surface marker CCR4. These cells produce TGF-B and IL-10. It is believed that the production of TGF-B, at least in part, directly suppresses mast cell activation and protects against anaphylaxis. Similar to prior studies, this study again demonstrated a long-lasting effect of EPIT, protecting EPIT-treated, peanut-sensitized mice from anaphylaxis after 4 weeks off therapy, while oral immunotherapy (OIT)-treated mice experienced anaphylaxis off therapy. This effect was maintained regardless of the route by which the mice were sensitized (epicutaneous or oral). A recent study presented in abstract form demonstrated similar efficacy for EPIT to egg, in sensitized mice [20]. They demonstrated an increase in OVA-specific IgG2a, a decrease in ex vivo splenocyte secretion of Th2 cytokines, and protection from anaphylaxis in the majority of mice, compared to placebo.
The Skin Barrier and Adverse Events in Murine Models
Initial studies examined EPIT on a disrupted skin barrier induced by scarification using needles, tape-stripping, or other methods. Trials investigating the use of EPIT as a method of vaccination against Japanese encephalitis and enterotoxogenic Escherichia coli found a direct relationship between the amount of skin barrier disruption and the level of antibody response [21, 22]. However, mouse and then human studies revealed that using the VEDS for allergens is only successful on intact skin. When the VEDS is applied to scarified skin in sensitized mice, allergen can be measured in the serum, while when applied to intact skin, no protein is measured in the serum [23]. Systemic symptoms were observed in sensitized mice treated with EPIT on scarified skin, but not in those with intact skin. Immunologic processing also differs between these groups. EPIT in unsensitized mice with scarified skin induced switching from a Th1 to Th2 response, suggesting that tape-stripping acts as an adjuvant. However, with an intact skin barrier, allergen is found in the epidermis and dermis in greater quantities than with scarified skin, and this leads to local allergen uptake and processing by DCs migrating to LNs. Importantly, this is not believed to be a passive process, but rather one that is actively mediated by immune capture in the skin and immune modulation in local lymphatics [7]. This leads to a significant decrease in specific IgE and increase in specific IgG2a among sensitized mice treated with EPIT on intact skin [23]. However, when EPIT is applied to the stripped skin of sensitized mice, an increase in specific IgE is seen, but there is no change in IgG2a. Histamine release after oral food challenge (OFC) is the same in this group as in placebo, while the treatment group with intact skin demonstrates tolerance to allergen. When studied in filaggrin deficient mice (FLG−/−), EPIT remained effective and safe compared to wild-type mice [24].
While mouse models make the distinction between intact and scarified skin seemingly very clear, human studies have not identified the mechanism as clearly. However, they do demonstrate that the level of skin disruption induced by various scarification methods, as measured by transepidermal water loss, correlates with human subjects experiencing systemic reactions [25]. Clinically, this raises the concern that the application of VEDS on skin in patients with active atopic dermatitis could carry an increased risk of systemic reactions, though this has not been reported in humans to date.
The concern has been raised by one study in mice, that EPIT (not using the VEDS) could induce eosinophilic esophagitis (EoE) [26]. It has been shown in mice that treatment with EPIT initially induces the massive recruitment of eosinophils to the epidermis and dermis [7, 26]. However, EPIT alone was not enough to induce esophageal eosinophilia in sensitized mice [26]. Only after EPIT and one intranasal exposure to allergen (OVA) was EoE induced. In contrast, a more recent study using VEDS EPIT found that EPIT could actually protect against the eosinophilic infiltration of the esophagus [27]. Similar immunologic changes were seen in treated mice, as in prior EPIT studies, but there was no increase in esophageal eosinophilia. Notably, these two studies employed different methodologies, with the latter using peanut-sensitized mice with oral exposure to peanut after EPIT, as opposed to intranasal exposure as in the former study. Given that EoE has been observed in some patients undergoing OIT, further study in mice is warranted regarding the possible association between EPIT and EoE, and human subjects in EPIT trials should be monitored for the development of EoE symptoms [28].
Human Trials of EPIT for Food Allergy
Peer-reviewed publications regarding the safety and efficacy of EPIT are limited. As such, abstracts presented at national and international allergy meetings are cited. Table 2 includes a list of completed clinical trials of EPIT with pertinent characteristics, including study design, and the use of double-blind, placebo-controlled (DBPC) OFCs at entry. Table 3 includes a list of ongoing clinical trials using EPIT. Any available results of safety and efficacy from these studies will be discussed in the following sections.
Safety
The first study to demonstrate the safety of EPIT in human subjects with food allergy was published in 2010 [29]. Among the 19 children (ages 3 months to 15 years) with IgE-mediated cow’s milk allergy enrolled in this bi-center, DBPC study, no episodes of anaphylaxis were observed with treatment. However, the active group did experience higher rates of local eczema with patch application and more frequent complaints of pruritus and discomfort that did not affect the tolerability of treatment. This initial study also demonstrated that children in the active treatment group did not experience an increase in sensitization to milk, which had been an early concern with EPIT. More recently, the safety of milk EPIT was published in abstract form [30]. Eighteen subjects enrolled in an ongoing study of children ages 2–17 years did not experience any serious adverse events with milk EPIT at three different doses. Drug-related adverse events including mild or moderate skin reactions were reported; however, these did not result in any subjects withdrawing treatment.
Similar safety results have been seen with peanut EPIT performed in several studies in children and adults. In total, these published studies have included 391 subjects with IgE-mediated peanut allergy [8, 31,32,33]. Adherence to therapy was excellent, at 94.8 to 97.1% of expected patch administrations, and did not vary significantly by age group. The use of both topical corticosteroids and oral antihistamines was found to increase similarly from baseline in both active treatment and placebo groups [8]. Again, no serious treatment associated adverse events (TAAEs) were observed; however, unlike in the milk EPIT studies, systemic reactions were observed with peanut EPIT. Two subjects experienced systemic symptoms that were likely related to transfer of allergen from active patch to the mucosal surfaces of peanut-allergic subjects, and two other subjects withdrew from the study due to non-severe TAAEs. A single subject on active therapy did experience systemic hives on one occasion [31]. Roughly, 50% of both active and placebo groups did not report any TAAEs. Local reactions were common, while reactions extending beyond the patch site were exceedingly rare in only 0.1 to 0.2% of doses. Typically, local reactions are characterized by localized pruritis, erythema, angioedema, and sometimes papules or vesicles in more severe reactions. Statistically significant differences were seen between low VP100 (100 mcg of peanut protein) and medium VP250 (250 mcg of peanut protein) patch doses for any dosing reaction, and number and severity of patch-site reactions [8]. Overall, EPIT appears to have an excellent safety profile and is well-tolerated, despite frequent, mostly mild or moderate, local skin reactions. This is in contrast to significant side effects (primarily GI) experienced in OIT studies, including systemic reactions sometimes requiring epinephrine, which have led to approximately 10 to 20% of patients withdrawing from trials [34]. OIT with peanut may also have the potential to unmask or induce EoE in some patients, which has not been seen in EPIT trials to date [35].
Efficacy in Human Studies
The efficacy of milk EPIT has only been presented as an abstract at the 2009 American Academy of Allergy and Immunology Annual Meeting [36]. This study by Dupont, et al., included 19 children, and showed a nonsignificant change in cumulative tolerated dose (CTD) of milk. A significant increase in CTD was seen with three additional months of therapy after the initial 3 months. However, there was no clear profile of changes in immunologic parameters seen in this trial, though some subjects did have individual changes in various markers. Notably, this study methodology differs from current peanut EPIT studies which use daily patch application as shown in Table 1, while the milk patch was applied every other day.
The efficacy of peanut EPIT has been published in one peer-reviewed publication. This multicenter, DBPC study evaluated peanut EPIT at the VP100 and VP250 doses in 74 subjects (ages 4–25 years) was performed by the NIH/NIAID sponsored Consortium of Food Allergy Research [8]. No statistically significant difference was observed between active doses, but both treatment groups did experience a significant increase in successfully consumed dose (SCD) compared to the placebo group. In the VP100 and VP250 groups, 45.8 and 48% of subjects, respectively, met the primary endpoint of either tolerating 5044 mg of peanut protein (compared to 200 mg of peanut protein in one peanut or about 7000 mg in two tablespoons of peanut butter) or at least a 10-fold increase in SCD after 52 weeks of treatment, compared to 12% in the placebo group. Of great interest, exploratory analysis found a statistically significant age-by-treatment interaction, with younger children (≤ 11 year age group) experiencing a more favorable outcome. Among children between 4 and 11 years of age, treated with VP250, 61% met the primary endpoint. Logistic regression also identified that subjects with an SCD < 44 mg of peanut protein at baseline was associated with a statistically significant successful outcome. This increase in tolerated peanut dose was accompanied by significant changes in peanut IgG4 levels and the IgG4:IgE ratio, despite non-significant changes in peanut-specific IgE. SPT size was also observed to decrease, only in the VP250 group. Basophil activation to peanut was not completely lost in EPIT-treated subjects, but rather the threshold of reactivity shifted towards reactivity at higher concentrations. More recently, post hoc analysis of patients in the VIPES study demonstrated that the increase in IgG4 was specific to Ara h 2 and 6 predominantly, along with a less substantial increase in IgG4 to Ara h 1 and 3 [37]. Ara h 1, 2, 3 and 6 are the most important peanut allergens and are associated with systemic reactions. Ara h 8 and 9 are often associated with mild symptoms associated with oral allergy syndrome, and in the VIPES study, there was no significant increase in IgG4 to Ara h 8 or 9.
Additional efficacy data with peanut EPIT has been presented in abstract form with similar results. These studies have also shown greater efficacy in a younger age group, with a treatment response in 40 to 80% of subjects, and saw similar changes in immunologic parameters [32, 33, 38]. Among the 207 subjects completing the initial 12 month VIPES randomized, DBPC trial and the open-label extension (OLFUS-VIPES), greater increases in SCD were seen with a longer duration of therapy. Treatment success was observed in 53.6% of children 6–11 years after 12 months of treatment with VP250, while this increased to 80% after 24 months [32]. Collectively, the safety and efficacy data from these studies have identified the VP250 dose as the optimum treatment dose (a VP500 dose was only studied in adults), and suggest that long-term use will also lead to more significant results [31].
Future Directions and Unanswered Questions
Initial efficacy data is promising for EPIT among subjects in a younger age group, but additional data from an ongoing phase 3 efficacy study (REALISE) is necessary to elucidate optimal patients for treatment with EPIT, necessary duration of treatment, and clarify the effect of treatment on immunologic parameters. At least some of these questions must be answered before approval will be granted by the FDA. However, it is promising that this therapy has been granted breakthrough therapy designation status by the FDA, and approval is anticipated within the next few years [35]. It is also important to note that many of the researchers on the studies cited in this review are employees of DBV Technologies, the company that produces the VEDS, and many others have conflicts of interest related to the company. In the future, independent clinical trials utilizing the VEDS should be performed.
A biomarker should be evaluated to accurately identify patients who are likely to have a successful response to EPIT, without the need to conduct OFCs before and during therapy, to confirm a meaningful clinical response. The mechanisms by which EPIT induces desensitization must also be elucidated. Importantly, no studies have been published that address the effect EPIT has on quality of life in subjects with food allergy, which is an important consideration. Additionally, this therapy must be studied in children under 4 years of age, including infants. Those infants who are unable to safely initiate regular consumption of peanut between 6 and 11 months of age based on a SPT to peanut ≥ 8 mm, convincing reaction, or failed OFC (the so-called “LEAP failures”), may be the ideal candidate for EPIT [39].
Natural history and other studies have found that about 20% of children with a peanut allergy will naturally develop durable tolerance, or outgrow their food allergy [40]. This tolerance is in contrast to desensitization, which is a reversible state induced by short-term exposure to an allergen. The previous level of clinical reactivity will return once the allergen exposure is stopped. Given ethical and safety concerns with studying the development of durable tolerance after stopping allergen administration for an extended period of time, sustained unresponsive (SU) has been used as a surrogate endpoint in some other studies. SU is defined as the elimination of reactivity to an allergen while off therapy. This can be assessed after a period of maintenance food allergen immunotherapy, which is then withheld for 1–4 weeks, at which point another OFC is performed. Notably, no EPIT studies have evaluated SU or durable tolerance with EPIT in humans to date, but this should be studied.
While other reviews in this edition have reviewed other methods of allergen immunotherapy in detail, it is important to compare these methods as much as is possible, despite a lack of head-to-head studies. Prior to the use of EPIT, there were also early attempts at achieving desensitization to food using OIT, as early as 1905 for milk [41]. OIT and SLIT have continued to be investigated largely for peanut, milk and egg allergies, with OIT having the same FDA breakthrough therapy designation status [35]. SCIT for peanut allergy was also investigated initially, but has all but been abandoned given the significant rate of severe, systemic reactions [42]. OIT also carries a significantly greater risk of TAAEs compared to SLIT and EPIT [35]. The majority of these TAAEs occur during initial escalation and up-dosing, which is performed under observation in clinic, unlike with EPIT. However, systemic TAAEs are also possible with OIT, including a risk for anaphylaxis, which is increased with a variety of factors such as illness, exercise, or poorly controlled asthma. Most notably, a significant percentage of patients on OIT experience dose-limiting gastrointestinal side effects, including abdominal pain and nausea that have led some of these subjects to discontinue therapy. As noted previously, EoE has also been observed in patients on OIT, but not EPIT or SLIT for food [28]. If therapy is tolerated, OIT likely results in a greater increase in allergen threshold, compared to both SLIT and EPIT [35]. SLIT has a similar safety profile compared to EPIT, with primarily local TAAEs, consisting of oral pruritus.
Conclusion
EPIT remains a promising method to achieve desensitization to at least some foods in children with food allergy, pending FDA approval. Mouse models have provided insight into the mechanism of action, but further work is still needed to confirm the role of Tregs and prove this mechanism in humans. Preliminary human clinical trial results are also promising, that at least a modest level of desensitization can be achieved in a majority of subjects, if EPIT is initiated early enough in life. The potential effect of active atopic dermatitis and concomitant S. aureus colonization, a known inhibitor of T cell tolerance and cause of skin barrier dysfunction, remains to be elucidated. While efficacy data is still lacking, EPIT clearly has a very good safety profile, and is well-tolerated compared to other forms of immunotherapy in food allergy. Its efficacy is similar to that of SLIT, but is better tolerated than OIT. EPIT must be studied to assess for SU, and validate the murine models that showed a long-lasting or durable effect.
Abbreviations
- EPIT:
-
epicutaneous immunotherapy
- OIT:
-
oral immunotherapy
- SLIT:
-
sublingual immunotherapy
- SU:
-
sustained unresponsiveness
- OFC:
-
oral food challenge
- DBPC:
-
double-blind, placebo-controlled
- TAAEs:
-
treatment associated adverse events
- CTD:
-
cumulative tolerated dose
- SCD:
-
successfully consumed dose
- EDS:
-
epicutaneous delivery system
- DCs:
-
dendritic cells
- LN:
-
lymph node
- EoE:
-
eosinophilic esophagitis
- VEDS:
-
Viaskin® epicutaneous delivery system
- SCIT:
-
subcutaneous immunotherapy
- FDA:
-
Food and Drug Administration
- RCT:
-
Randomized controlled trial
References
Gupta RS et al (2011) The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 128(1):e9–17
Sicherer SH et al (2010) US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol 125(6):1322–1326
Sampson HA et al (2014) Food allergy: a practice parameter update-2014. J Allergy Clin Immunol 134(5):1016–25.e43
Fong AT, Katelaris CH, Wainstein B (2017) Bullying and quality of life in children and adolescents with food allergy. J Paediatr Child Health 53(7):630–635
Vallery-Radot P, Hangenau J (1921) Essai de désensibilisation par des cutiréactions répétées. Bull Soc Méd Hôp Paris 45:1251–1260
Pautrizel R et al (1957) Allergenic group specificity & therapeutic consequences in asthma; specific desensitization method by epicutaneous route. Sem Hop 33(22):1394–1403
Dioszeghy V et al (2011) Epicutaneous immunotherapy results in rapid allergen uptake by dendritic cells through intact skin and downregulates the allergen-specific response in sensitized mice. J Immunol 186(10):5629–5637
Jones SM et al (2017) Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. J Allergy Clin Immunol 139(4):1242–1252.e9
Mondoulet L et al (2010) Epicutaneous immunotherapy on intact skin using a new delivery system in a murine model of allergy. Clin Exp Allergy 40(4):659–667
Mondoulet L et al (2011) Epicutaneous immunotherapy using a new epicutaneous delivery system in mice sensitized to peanuts. Int Arch Allergy Immunol 154(4):299–309
Mondoulet L et al (2012) Epicutaneous immunotherapy compared with sublingual immunotherapy in mice sensitized to pollen (Phleum pratense). ISRN Allergy 2012:375735
Hadis U et al (2011) Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 34(2):237–246
Akdis CA, Akdis M (2009) Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol 123(4):735–746 quiz 747-8
Dioszeghy V et al (2014) The regulatory T cells induction by epicutaneous immunotherapy is sustained and mediates long-term protection from eosinophilic disorders in peanut-sensitized mice. Clin Exp Allergy 44(6):867–881
Dioszeghy V et al (2017) Crucial role of Langerhans cells in epicutaneous immunotherapy. Allergy 72(S103):155
Pajno GB et al (2001) Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin Exp Allergy 31(9):1392–1397
Purello-D'Ambrosio F et al (2001) Prevention of new sensitizations in monosensitized subjects submitted to specific immunotherapy or not. A retrospective study. Clin Exp Allergy 31(8):1295–1302
Mondoulet L et al (2015) Specific epicutaneous immunotherapy prevents sensitization to new allergens in a murine model. J Allergy Clin Immunol 135(6):1546–1557 e4
Tordesillas L et al (2017) Epicutaneous immunotherapy induces gastrointestinal LAP(+) regulatory T cells and prevents food-induced anaphylaxis. J Allergy Clin Immunol 139(1):189–201.e4
Wavrin S et al (2017) Epicutaneous immunotherapy (EPIT) prevents anaphylaxis to egg in sensitized mice. Allergy 72(S103):156
Glenn GM et al (2007) Safety and immunogenicity of an enterotoxigenic Escherichia coli vaccine patch containing heat-labile toxin: use of skin pretreatment to disrupt the stratum corneum. Infect Immun 75(5):2163–2170
Frerichs DM et al (2008) Controlled, single-step, stratum corneum disruption as a pretreatment for immunization via a patch. Vaccine 26(22):2782–2787
Mondoulet L et al (2012) Intact skin and not stripped skin is crucial for the safety and efficacy of peanut epicutaneous immunotherapy (EPIT) in mice. Clin Transl Allergy 2(1):22
Wavrin S et al (2016) No impact of filaggrin deficiency on Epit efficacy in a murine model. J Allergy Clin Immunol 137(2S):AB133
von Moos S et al (2014) Comparing safety of abrasion and tape-stripping as skin preparation in allergen-specific epicutaneous immunotherapy. J Allergy Clin Immunol 134(4):965–7.e4
Akei HS et al (2005) Epicutaneous antigen exposure primes for experimental eosinophilic esophagitis in mice. Gastroenterology 129(3):985–994
Mondoulet L et al (2012) Epicutaneous immunotherapy (EPIT) blocks the allergic esophago-gastro-enteropathy induced by sustained oral exposure to peanuts in sensitized mice. PLoS One 7(2):e31967
Lucendo AJ, Arias A, Tenias JM (2014) Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Ann Allergy Asthma Immunol 113(6):624–629
Dupont C et al (2010) Cow’s milk epicutaneous immunotherapy in children: a pilot trial of safety, acceptability, and impact on allergic reactivity. J Allergy Clin Immunol 125(5):1165–1167
Karine, R., et al., Safety of Viaskin milk epicutaneous immunotherapy (EPIT) in IgE-mediated cow’s milk allergy (CMA) in children (MILES Study). 2016. 137(2, Supplement): p. AB132
Jones SM et al (2016) Safety of epicutaneous immunotherapy for the treatment of peanut allergy: A phase 1 study using the Viaskin patch. J Allergy Clin Immunol 137(4):1258–61.e1–10
Sampson HA et al (2016) Enhanced efficacy and confirmed safety of a two-year epicutaneous immunotherapy (EPIT) treatment of peanut allergy with Viaskin peanut: the continuation of the Vipes phase IIb randomized controlled trial (RCT). J Allergy Clin Immunol 137(2S):AB408
Sampson HA et al (2015) Epicutaneous immunotherapy (EPIT) is effective and safe to treat peanut allergy: a multi-national double-blind placebo-controlled randomized phase IIb trial. J Allergy Clin Immunol 135(2S):AB390
Wood RA (2016) Food allergen immunotherapy: current status and prospects for the future. J Allergy Clin Immunol 137(4):973–982
Gernez Y, Nowak-Wegrzyn A (2017) Immunotherapy for food allergy: are we there yet? J Allergy Clin Immunol Pract 5(2):250–272
Dupont C et al (2009) Epicutaneous immunotherapy in severe cow milk allergy: a double blind pilot trial. J Allergy Clin Immunol 123(2S):S183
Koppelman S et al (2017) Epicutaneous immunotherapy (EPIT) for peanut allergy modifies IgG4 responses to major peanut allergens. Allergy 72(S103):737
Dupont C et al (2014) Peanut epicutaneous immunotherapy (EPIT) in peanut-allergic children: 18 months treatment in the Arachild Study. J Allergy Clin Immunol 133(2S):AB102
Togias A et al (2017) Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol 139(1):29–44
Peters RL et al (2014) The natural history and clinical predictors of egg allergy in the first 2 years of life: a prospective, population-based cohort study. J Allergy Clin Immunol 133(2):485–491
Freier S, Kletter B (1970) Milk allergy in infants and young children. Current knowledge. Clin Pediatr (Phila) 9(8):449–454
Nelson HS et al (1997) Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol 99(6 Pt 1):744–751
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
BJL and DYML have received research support from DBV Technologies, and participate in CoFAR, funded by NIAID/NIH. BJL has received research support from AImmune Therapeutics, has formerly received a speaker honorarium from Mylan, and served as a consultant to AImmune Therapeutics. DYML is the chair of the AImmune Therapeutics DSMB for phase 3 clinical trials.
Rights and permissions
About this article
Cite this article
Lanser, B.J., Leung, D.Y.M. The Current State of Epicutaneous Immunotherapy for Food Allergy: a Comprehensive Review. Clinic Rev Allerg Immunol 55, 153–161 (2018). https://doi.org/10.1007/s12016-017-8650-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-017-8650-3