Abstract
Annually chronic liver diseases cause two million death worldwide. Although liver transplantation (LT) is still considered the best therapeutic option, the limited number of donated livers and lifelong side effects of LT has led researchers to seek alternative therapies. Tissue engineering (TE) as a promising method is considered for liver repair and regeneration. TE uses natural or synthetic scaffolds, functional somatic cells, multipotent stem cells, and growth factors to develop new organs. Biological scaffolds are notable in TE because of their capacity to mimic extracellular matrices, biodegradability, and biocompatibility. Moreover, natural scaffolds are classified based on their source and function in three separate groups. Hemostat-based scaffolds as the first group were reviewed for their application in coagulation in liver injury or surgery. Furthermore, recent studies showed improvement in the function of biological hydrogels in liver regeneration and vascularity. In addition, different applications of natural scaffolds were discussed and compared with synthetic scaffolds. Finally, we focused on the efforts to improve the performance of decellularized extracellular matrixes for liver implantation.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The liver is a vital organ in the human body that plays a crucial role in metabolic homeostasis, xenobiotic metabolism, detoxification, and immune regulation [90]. The total number of chronic liver disease cases is estimated at 1.5 billion worldwide, including nonalcoholic fatty liver disease (59%), hepatitis B virus (29%), hepatitis C virus (9%), and alcoholic liver disease (2%) [14]. The hepatitis B and C viruses are the most common etiologic factors for liver cirrhosis and liver carcinoma [5]. The World Health Organization's statistics reported that 354 million people are infected with these viruses in the world [84] . Annually, viral hepatitis, hepatocellular carcinoma, and complications of liver cirrhosis are responsible for approximately two million death worldwide. Hepatocellular carcinoma and cirrhosis were known 11th and 16th-leading causes of death in 2019, respectively [8].
The liver has a unique regeneration capacity, and it can restore mass and function [86]. In critical clinical conditions that the liver couldn't regenerate itself, orthotopic liver transplant (OLT) is the gold standard and known best treatment method for liver failure. However, the limited number of donated livers is one of the most significant obstacles to the broad application of this therapy. Moreover, the use of immunosuppressive drugs causes unwanted side effects and severe microbial infections in these patients.
On the other hand, this surgical procedure causes a high financial burden to the health system and the patient [2]. Another approach is to use extracorporeal liver support devices, which are used for patients on waiting lists for liver transplantation or during the early treatment stages after liver transplantation. The limitations of these devices can refer to restriction in patient's movement, limited processing volumes, the optimum supply of oxygen to cell compounds, and removal of the bile [104]. Tissue engineering could be considered the third modality to reduce the mortality rate due to liver diseases, which does not have these restrictions and is more accessible.
Nowadays, tissue engineering is known promising technology in biomedicine that is developing by using engineering science and biology to repair damaged tissues [4, 48]. TE is the knowledge of new tissue or organ formation from cells, scaffolds, and growth factors. These scaffolds are three-dimensional structures that provide cell growth and a supportive microenvironment for the differentiation and maintenance of cells in ECM [43]. Researchers in tissue engineering science investigate cell implantation by suitable scaffolds, microenvironmental conditions for cell survival, and finally, controlling all factors that contribute to the development of new tissue [34]. In this review, we highlighted natural scaffolds in liver regeneration. In addition, we compared biological scaffolds with synthetic ones used in tissue engineering.
Natural biomaterials
An ideal scaffold should have the following characteristics: biocompatibility, biodegradability with controllable degradation rate, highly porous three-dimensional structure for cell connection, penetration and division to simulate extracellular matrices, suitable mechanical strength to support regeneration, and finally, surface chemistry and topography to improve cellular interactions and tissue growth [67]. Scaffolds are divided into two general groups: synthetic and biological scaffolds. Synthetic polymers are hydrophobic materials with a slow degradation rate in biological conditions due to their high mechanical strength [39]. Many synthetic polymers are used as scaffolds in tissue engineering, and several cases have been specifically designed for skeletal muscle regeneration. Control of physical and chemical properties distinguishes these polymers from natural polymers [115]. These polymers have limitations such as hydrophobicity, low degradation rate, low cell affinity, weak cellular response, acidic byproduct, and unsuitable conditions for drug delivery (H.-S. [62, 64]. The biological scaffolds offer suitable biocompatibility dependent on the material source [44, 83] and do not stimulate the immunity system [3, 103]. These scaffolds can provide environmental conditions similar to extracellular matrices (ECM).
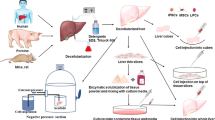
Biological scaffolds are in the spotlight due to the appropriate immune response, mild antigenic properties, angiogenesis, improvement of cellular adherence, and hemostasis [71]. However, natural scaffolds don't support the required mechanical properties of an ideal scaffold for tissue repairing and require modification with other compounds e.g. galactose [33]. Natural scaffolds are usually fabricated in a form such as fiber, powder, 3D printed structures, and sponges. Decellularizing whole tissue or organs also leads to potential natural scaffolds for tissue repair (Fig. 1). In this article, we reviewed the natural platforms used in liver regeneration in three groups: hemostats, hydrogels, and decellularized extracellular matrices (dECM) (Fig. 2).
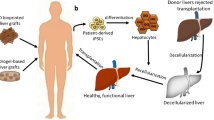
Comparison of liver reconstruction strategies: A) Hemostat scaffolds inhibit bleeding at the injury or surgery site by absorption and coagulation of blood. B and C) Hepatocytes and endothelial cells are cultured in vitro in the presence or absence of scaffolding, then used in liver regeneration. D) Liver tissue originated from humans or animals is decellularized. Hepatocyte and vascular endothelial cells are perfused into the decellularized scaffold. The repopulated scaffold is placed in a bioreactor to prepare whole tissue and complete the vascular network
Hemostat-based scaffolds
In recent years, some studies have focused on the integration of hemostatic properties in the studied scaffolds in tissue regeneration. Because these scaffolds, while preventing bleeding at the injuried site, provide conditions for cell proliferation and differentiation. Therefore, in this review article, homeostatic scaffolds are also considered as an independent section.
High blood loss during liver surgeries is a concerning problem [54]. The hemostat scaffolds have a porous structure that provides conditions for fluid crossing from hemostats. Depleting free water stabilizes the hemostat at the injury site and allows tissue repair by absorbing fluids [61, 126]. The high porosity is an ideal property of homeostatic polymer, which is converted to a unique scaffold in tissue engineering. These structures are usually biocompatible, available, biodegradable, lightweight, and low cost [7, 9, 118, 126]. Biodegradability is one of the main parameters that should consider. Otherwise, if the polymers don't decompose on the target site, they must drain from the wound, which can be painful for the patient [61]. Sometimes designed hemostat polymers don't possess highly interconnected porous structures that lead to poor absorption of blood, so inefficient in controlling bleeding [118].
Chitosan is an excellent candidate for hemostat scaffold synthesis due to with antibacterial and hemostasis properties. Some studies have shown that adding a hydrophobic group to chitosan leads to solid hydrophobic interactions with the membrane of platelet, red blood cells, and bacteria that improve its anti-infective and hemostasis strength [16, 25, 26]. Chitosan is not much soluble in physiological conditions. Haddad-Mashadrizeh et al. increased its solubility at neutral pH by blending it with sodium glycerol phosphate [40]. Du et al. designed the alkylated chitosan sponge with microchannels (MACS) that absorbed water and blood. Its shape can be rapidly recovered. MACS has a higher capacity in hemostasis and procoagulant properties than commercial hemostats. In situ experimental results indicated successful vascularization, tissue integration, and infiltration of the liver parenchymal cell (Table 1)[27].
Gelatin has benefits similar to other natural materials (Q. [52, 53]. Its most notable feature is activating platelet aggregation that converted it to favorable hemostatic material [66]. Gelatin has a high capacity for blood absorption, which leads to in situ concentrated blood proteins and improved blood clotting [49]. Swelling of gelatin stops bleeding by inducing pressure on the injury site [54]. In recent years, hemostats have been fabricated into forms of sponges, films, webs, silks, and powders [101]. The powder hemostats have always been considered for increasing the contact surface with blood cells [68]. Porous gelatin microspheres (PGMs) showed better hemostatic performance than commercial samples [66]. Fabricated hemostats from gelatin matrix-thrombin, and microbial transglutaminase cross-linked thrombin (TB) loaded Gelatin (mTG/TB/gelatin) reduced clotting time to about one minute [18, 22]. Mushroom tyrosinase and microbial transglutaminase (mTG) were used to crosslink silk fibroin/gelatin that showed a similar result [107]. Three-dimensional gelatin sponges comprised a porous structure with low density, extremely high capacity for absorption of blood and tissue secretions, high surface area, and compressibility, which showed its superiority compared with commercial hemostats, medicine gauze, and gelatin nanofiber membrane (details are shown in Table 1) [116].
Nano or cellulose microfibers have been used widely in tissue engineering [85] and wound healing [108]. Cellulose has a high potential for surface chemical modifications [76] as carboxylation, which improved its absorption and degradation in biological conditions [122]. The blending of oxidized cellulose to the chitosan (CS)/gelatin sponge has improved the hemostatic activity of CS/gelatin (Table 1)[93].
Hydrogel-based scaffolds
Hydrogels are the hydrophobic structure that is insoluble in water. Hydrogels are similar to natural tissue because of their high potential in absorbing a massive amount of biological liquids. In addition, the other advantages of these scaffolds could be referred to as suitable mechanical strength and long life. They have a high potential for carrying cells, drugs, and bioactive compounds. In addition, hydrogels provide an environment for remodeling, migration, and adhesion [79, 99]. Hydrogels keep the wound environment moist and cool and allow oxygen to penetrate, which reduces pain in the patient [124]. Antioxidation, adhesiveness, hemostasis, anti-infection, and drug encapsulation are ideal properties of wound healing hydrogels [36]. Hydrogels structure is impacted by polymer concentration, temperature, pH, cross-linking degree, and salt concentration. These polymers were fabricated by polysaccharides, proteins, synthetic polymers, and their hybrid [79, 99]. Despite the high potential of hydrogels for tissue engineering, their application in medicine has limitations. These limitations include pore size-dependent release rate, limited drug loading capacity, the toxicity of some cross-linker, sensitivity to temperature and pH, low survival of encapsulated cells, and cell damage during photopolymerization [79]. The hydrogels are usually applied for one of the wound types and have limited applicated features [56].
The prepared hybrid hydrogel by Chen et al. significantly improved tissue adhesion, coagulation, hemostasis, and anti-infection in the skin and liver injuries. In addition, the controllable release of VEGF leads to the improvement of cellular proliferation and tissue regeneration (Table 2)[16]. The growth factors have a high affinity for binding to the glycosaminoglycans like heparin, which regulate many of their activities and provide biological stability against proteases activity. The chitosan/galactosylated hyaluronic acid /heparin sponge's ability to carry EGF could improve cell maintenance microenvironment and prolong hepatocyte activities for a long time (Table 2)[32].
Pore size is a limiting factor for cell growth, proliferation, and migration. The photo-cross-linkable gelatin-glycidyl methacrylate was developed from the porous alginate [28] and gelatin methacryloyl (GMA) hydrogels [63], resulting in improving pore size by maintaining mechanical strength (Table 2) [102]. Protease cleavage sites of gelatin lead to its biodegradation during the replacement of new ECM [12]. In addition, the presence of Arg-Gly-Asp sequences gives it the ability to connect to membrane proteins like integrin [70]. However, gelatin-based hydrogels possess weak mechanical and thermal strength as their main limitation [30]. Methylation of lysine residues causes 3D cross-linked polymer formation that is more resistant to temperature [72]. ECM compounds such as hyaluronic acid (HA) can compensate for the poor mechanical strength of GMA [111]. Hyaluronic acid is one of the central compounds of ECM in many tissues. It is responsible for tissue formation, wound healing, and morphogenesis. These linear polysaccharides have a high affinity for adhesive receptors, responsible for poor cell adhesiveness [72, 73]. Cross-linked methacrylic HA (HAMA) improved mechanical and chemical properties. Enhance resistance to enzymatic degradation while maintaining biocompatibility. So far, various hybrids of GMA/HAMA have been made [12, 45, 60], but the combination of these two polymers in a ratio of 4:1 had a significant effect on improving its properties [111]. Chitosan/galactosylated HA (GHA) improved hepatocyte cells adhesion while maintaining liver functions. Added hyaluronic acid to gelatin cryogel increased the elastic modulus while decreasing the scaffold toughness, compressive strength, cell growth, and proliferation (Table 2) [55].
Three-dimensional bioprinting is a high-potential approach for the simulation of ECM in tissue engineering via the building of scaffolds. Hydrogels due to the significant similarity to ECM used as bio-inks [78, 94, 97]. The hydrogels such as elastin, agarose, collagen, chitosan, alginate, gelatin, and hyaluronic acid were used in 3D bioprinting. However, they indicated poor printability [51]. Collagen is the most common compound of ECM used in many bio-scaffolds structures (A. [62, 64]. Despite the benefits of this biomaterial, such as low mechanical strength, high degradation rate, variable elastic modulus, and viscosity during processing, its usage in bioprinting is limited [74]. A slower degradation rate of chitosan than collagen [23] leads to their simultaneous use as bio-inks in bioprinting, improving their degradation rate and mechanical strength [105, 127]. Sue et al. improved the printability of hybrid collagen/chitosan via the formation of hydrogen bonds and careful temperature control during bioprinting. By changing the presence ratio of each, the bioscaffolds properties could benefit different tissue engineering applications (Table 2) [106]. Chang et al. fabricated volvox spheres with alginate and collagen polymers and encapsulated them with MSCs and AML12 hepatocytes. These 3D scaffolds improved liver repair and regeneration in necrosis liver (Table 2) [13]. Reports indicate the unique role of silk-gelatin bio-ink in cell signalings such as Wnt/β-catenin, bone morphogenetic pathways, and Indian hedgehog play an essential role in liver regeneration [98]. Studies have been shown the successful cultivation of hepatocytes into silk scaffolds. Chitosan presence in these structures reduced inflammatory response [100], while RGD peptide improved gene expression [50]. Co-cultivation of hepatocyte and stellate cells in silk scaffolds promoted hepatic functions [114]. Bioengineering porous silk/extracellular protein scaffold provided conditions for co-cultivation of non-parenchymal cells and primary human hepatocytes [59].
Nano-fibrous structures could act as cellular scaffolds due to are their similarity to ECM [113]. Studies have confirmed that nanofibers and the chemical nature of their structure have significant positive effects on cellular proliferation, growth, migration, and adhesion [15]. However, the application of chitosan nanofibers has been limited due to their high degradation rate and edema [21, 123]. The presence of one synthetic polymer such as polycaprolactone (PCL) [41] or polyethylene oxide (PEO) [20] into chitosan nanofibers leads to improved mechanical strength and processability. However, the synthetic polymers, due to high hydrophobicity properties, reduced cell growth and adhesion [10]. Increased hydrophilicity of scaffolds enhances cell growth and proliferation [120]. Studies have indicated that adding hydrophilic compounds like Aloe vera [120], Gum [92], and galactose [37] can enhance hydrophilic groups of scaffolds surface and improve cell adhesion [91].
The importance of 3D shape controlling cellular constructs in tissue engineering led to producing a hepatic lobule-shaped microtissue. These structures were fabricated from capsulated rat liver cells with poly-L-lysine-alginate and have higher hepatic function than spheroids; thus, they could be used as blocks in the bioengineering of liver scaffolds (Table 2) [69]. Yajima et al. simulated a hepatic lobule through assembled cell-laden hydrogel microfiber coated with vascular endothelial cells for liver cells perfusion (Table 2) [117].
Decellularized extracellular matrices-based scaffolds
The biological ECM is prepared from the decellularization of part of the organ or its whole, which its remaining cells made the specific ECM and containing ideal scaffold properties such as complexity, vascular networks, and tissue-specific construction [80, 109]. Stem cells are differentiated based on environmental conditions, which usually don't consider in tissue engineering and tissue repair. Stem cells are often delivered directly into damaged tissue and are ignore tissue biochemistry, needed complexity for 3D scaffold, and vascularity. While tissue repair usually occurs at the wound border, environmental conditions are similar to host tissue [109]. Decellularized ECM (dECM) has tensile strength similar to native tissue and maintains structural integrity. Lack of proper vascular network and primary engraftment transplantation is the most significant limitation in successful tissue engineering [6]. These structures often carry the main vascular branches of origin tissue, which could proliferate and differentiate into the new tissue. Sometimes, they repopulate with endothelial cells or stem cells for new vascular formation [95].
Liver dECM has been studied (Table 3) as a scaffold for proliferation and differentiation of stem cells, endothelial cells, and hepatocytes for the new liver formation and, finally, its transplant (Table 2). Another application of these scaffolds is their use as a liver disease model, which has led to a better understanding of its role in liver fibrosis and discovering new methods for fibrosis therapy by stem cells [57].
The liver-derived ECM was used for liver injuries treatment, but this type of ECM has low mechanical strength [42]. Therefore, the gelatin/PCL/ECM [10] could be a good scaffold in tissue engineering due to the high potential of gelatin in tissue regeneration [128]and the mechanical strength of PCL [77]. Collagen identifies as a central compound of dECM, which leads to in situ clotting. Collagen-coated with heparin in the dECM scaffold was able to give it anticoagulant properties [6]. Also, the heparin-gelatin mixture has been effective in angiogenesis [47]. Anti-endothelial cell antibodies conjugation in dECM causes the proliferation of vascular cells, and it can develop the vascular network in the scaffold structure [58].
Conclusion
Liver transplantation is yet the best option for treating liver failure. However, due to the limited number of liver donors, an alternative method to diminish liver disease mortality is necessary. Over the past few decades, research in the area of xenotransplantation has progressed dramatically. However, cross-species pathogen infectivity and immunological response to the transplanted tissue are the main problems of this type of transplant [19, 35]. Also, traditional cell therapies perfused stem cells directly in vivo, which has led to poor cell engraftment and a low cell viability rate. Hepatocytes function and survival are dependent on cell–cell interaction and connection to ECM compositions. Natural scaffolds can be used as a supportive platform for cell growth, proliferation, and differentiation. Biological scaffolds are biodegradable, decomposing in physiologic conditions, and are replaced with new extracellular matrices. These structures can also simulate cell physiological conditions, which lead to cell growth and differentiation, and finally, liver regeneration. Studies indicated that these scaffolds could simultaneously cultivate two cells type, hepatocytes, and vascular endothelial cells, which caused vascular network formation and finally provided necessary nutrients and oxygen for newly regenerated tissue. Due to their ability to carry drugs and growth factors, they also play a role in regeneration and rescue the liver. Some of them are in a clinical trial for liver disease treatment (Table 4).
Abbreviations
- OLT:
-
Orthotopic Liver Transplant
- MSCs:
-
Mesenchymal Stem Cells
- ESCs:
-
Embryonic Stem Cells
- iPSCs:
-
Induced Pluripotent Stem Cells
- ECM:
-
Extracellular Matrices
- dECM:
-
Decellularized Extracellular Matrices
- MACS:
-
Microchanneled Alkylated Chitosan Sponge
- PGMs:
-
Porous Gelatin Microspheres
- mTG:
-
Microbial Transglutaminase Enzyme
- GMA:
-
Gelatin Methacryloyl
- HA:
-
Hyaluronic Acid
- HAMA:
-
Cross-Linked Methacrylated HA
- GHA:
-
Galactosylated HA
- EGF:
-
Epidermal Growth Factor
- VEGF:
-
Vascular Endothelial Growth Factor
- PCL:
-
Polycaprolactone
- PEO:
-
Polyethylene Oxide
References
Acun, A., Oganesyan, R., Uygun, K., Yeh, H., Yarmush, M. L., & Uygun, B. E. (2021). Liver donor age affects hepatocyte function through age-dependent changes in decellularized liver matrix. Biomaterials, 270, 120689. https://doi.org/10.1016/j.biomaterials.2021.120689
Agarwal, T., Subramanian, B., & Maiti, T. K. (2019). Liver tissue engineering: Challenges and opportunities. Acs Biomaterials Science & Engineering, 5, 4167–4182. https://doi.org/10.1515/biolog-2016-0056
Alaribe, F. N., Manoto, S. L., & Motaung, S. C. (2016). Scaffolds from biomaterials: Advantages and limitations in bone and tissue engineering. Biologia, 71, 353–366. https://doi.org/10.1515/biolog-2016-0056
Armstrong, J. P., & Stevens, M. M. (2019). Emerging technologies for tissue engineering: From gene editing to personalized medicine. Tissue Engineering Part A, 25, 688–692. https://doi.org/10.1089/ten.tea.2019.0026
Asrani, S. K., Devarbhavi, H., Eaton, J., & Kamath, P. S. (2019). Burden of liver diseases in the world. Journal of hepatology, 70, 151–171. https://doi.org/10.1016/j.jhep.2018.09.014
Bao, J., Shi, Y., Sun, H., Yin, X., Yang, R., Li, L., Chen, X., & Bu, H. (2011). Construction of a portal implantable functional tissue-engineered liver using perfusion-decellularized matrix and hepatocytes in rats. Cell transplantation, 20, 753–766. https://doi.org/10.3727/096368910X536572
Boerman, M. A., Roozen, E., Sánchez-Fernández, M. J., Keereweer, A. R., Félix Lanao, R. P., Bender, J. C., Hoogenboom, R., Leeuwenburgh, S. C., Jansen, J. A., & Van Goor, H. (2017). Next generation hemostatic materials based on NHS-ester functionalized poly (2-oxazoline) s. Biomacromolecules, 18, 2529–2538. https://doi.org/10.1021/acs.biomac.7b00683
Bram, Y., Nguyen, D.-H. T., Gupta, V., Park, J., Richardson, C., & Schwartz, R. E. (2021). Cell and Tissue Therapy for the Treatment of Chronic Liver Disease. Annual Review of Biomedical Engineering, 23. https://doi.org/10.1146/annurev-bioeng-112619-044026
Bu, Y., Zhang, L., Liu, J., Zhang, L., Li, T., Shen, H., Wang, X., Yang, F., Tang, P., & Wu, D. (2016). Synthesis and properties of hemostatic and bacteria-responsive in situ hydrogels for emergency treatment in critical situations. ACS applied materials & interfaces, 8, 12674–12683. https://doi.org/10.1021/acsami.6b03235
Bual, R., Kimura, H., Ikegami, Y., Shirakigawa, N., & Ijima, H. (2018). Fabrication of liver-derived extracellular matrix nanofibers and functional evaluation in in vitro culture using primary hepatocytes. Materialia, 4, 518–528. https://doi.org/10.1016/j.mtla.2018.11.014
Caires-Júnior, L. C., Goulart, E., Telles-Silva, K. A., Araujo, B. H. S., Musso, C. M., Kobayashi, G., Oliveira, D., Assoni, A., Carvalho, V. M., & Ribeiro-Jr, A. F. (2021). Pre-coating decellularized liver with HepG2-conditioned medium improves hepatic recellularization. Materials Science and Engineering: C, 121, 111862. https://doi.org/10.1016/j.msec.2020.111862
Camci-Unal, G., Cuttica, D., Annabi, N., Demarchi, D., & Khademhosseini, A. (2013). Synthesis and characterization of hybrid hyaluronic acid-gelatin hydrogels. Biomacromolecules, 14, 1085–1092. https://doi.org/10.1021/bm3019856
Chang, S. H., Huang, H. H., Kang, P. L., Wu, Y. C., Chang, M.-H., & Kuo, S. M. (2017). In vitro and in vivo study of the application of volvox spheres to co-culture vehicles in liver tissue engineering. Acta Biomaterialia, 63, 261–273. https://doi.org/10.1016/j.actbio.2017.09.028
Cheemerla, S., & Balakrishnan, M. (2021). Global epidemiology of Chronic liver Disease. Clinical Liver Disease, 17, 365. https://doi.org/10.1002/cld.1061
Chen, F., Tian, M., Zhang, D., Wang, J., Wang, Q., Yu, X., Zhang, X., & Wan, C. (2012). Preparation and characterization of oxidized alginate covalently cross-linked galactosylated chitosan scaffold for liver tissue engineering. Materials Science and Engineering: C, 32, 310–320. https://doi.org/10.1016/j.msec.2011.10.034
Chen, G., Yu, Y., Wu, X., Wang, G., Ren, J., & Zhao, Y. (2018). Bioinspired multifunctional hybrid hydrogel promotes wound healing. Advanced Functional Materials, 28, 1801386. https://doi.org/10.1002/adfm.201801386
Chu, T. L., Tripathi, G., Bae, S. H., & Lee, B.-T. (2021). In-vitro and in-vivo hemostat evaluation of decellularized liver extra cellular matrix loaded chitosan/gelatin spongy scaffolds for liver injury. International Journal of Biological Macromolecules, 193, 638–646. https://doi.org/10.1016/j.ijbiomac.2021.10.128
Chu, T. L., Tripathi, G., Bae, S. H., & Lee, B.-T. (2021). Physico-mechanical and biological evaluation of an injectable m-TG cross-linked thrombin loaded amended gelatin hemostat to heal liver trauma. International Journal of Biological Macromolecules, 181, 339–348. https://doi.org/10.1016/j.ijbiomac.2021.03.114
Cooper, D. K., Dou, K.-F., Tao, K.-S., Yang, Z.-X., Tector, A. J., & Ekser, B. (2016). Pig liver xenotransplantation: A review of progress towards the clinic. Transplantation, 100, 2039. https://doi.org/10.1097/TP.0000000000001319
Deineka, V., Sulaieva, O., Pernakov, M., Korniienko, V., Husak, Y., Yanovska, A., Yusupova, A., Tkachenko, Y., Kalinkevich, O., & Zlatska, A. (2021). Hemostatic and Tissue Regeneration Performance of Novel Electrospun Chitosan-Based Materials. Biomedicines, 9, 588. https://doi.org/10.3390/biomedicines9060588
Di Filippo, M. F., Panzavolta, S., Albertini, B., Bonvicini, F., Gentilomi, G. A., Orlacchio, R., Passerini, N., Bigi, A., & Dolci, L. S. (2020). Functional properties of chitosan films modified by snail mucus extract. International Journal of Biological Macromolecules, 143, 126–135. https://doi.org/10.1016/j.ijbiomac.2019.11.230
Dimitroulis, D., Antoniou, E., Karidis, N. P., Kontzoglou, K., & Kouraklis, G. (2012). Surgical control of life-threatening post-ERCP bleeding with a gelatin matrix-thrombin hemostatic agent. International journal of surgery case reports, 3, 471–473. https://doi.org/10.1016/j.ijscr.2012.05.014
Ding, C.-M., Zhou, Y., He, Y.-N., & Tan, W.-S. (2008). Perfusion seeding of collagen–chitosan sponges for dermal tissue engineering. Process Biochemistry, 43, 287–296. https://doi.org/10.1016/j.procbio.2007.12.005
Du, C., Narayanan, K., Leong, M. F., & Wan, A. C. (2014). Induced pluripotent stem cell-derived hepatocytes and endothelial cells in multi-component hydrogel fibers for liver tissue engineering. Biomaterials, 35, 6006–6014. https://doi.org/10.1016/j.biomaterials.2014.04.011
Du, X., Liu, Y., Wang, X., Yan, H., Wang, L., Qu, L., Kong, D., Qiao, M., & Wang, L. (2019). Injectable hydrogel composed of hydrophobically modified chitosan/oxidized-dextran for wound healing. Materials Science and Engineering: C, 104, 109930. https://doi.org/10.1016/j.msec.2019.109930
Du, X., Liu, Y., Yan, H., Rafique, M., Li, S., Shan, X., Wu, L., Qiao, M., Kong, D., & Wang, L. (2020). Anti-infective and pro-coagulant chitosan-based hydrogel tissue adhesive for sutureless wound closure. Biomacromolecules, 21, 1243–1253. https://doi.org/10.1021/acs.biomac.9b01707
Du, X., Wu, L., Yan, H., Jiang, Z., Li, S., Li, W., Bai, Y., Wang, H., Cheng, Z., & Kong, D. (2021). Microchannelled alkylated chitosan sponge to treat noncompressible hemorrhages and facilitate wound healing. Nature communications, 12, 1–16. https://doi.org/10.1038/s41467-021-24972-2
Dvir-Ginzberg, M., Gamlieli-Bonshtein, I., Agbaria, R., & Cohen, S. (2003). Liver tissue engineering within alginate scaffolds: Effects of cell-seeding density on hepatocyte viability, morphology, and function. Tissue engineering, 9, 757–766. https://doi.org/10.1089/107632703768247430
Ebrahim, N., Badr, O. A., Yousef, M. M., Hassouna, A., Sabry, D., Farid, A. S., Mostafa, O., Saihati, H. A. A., Seleem, Y., & Abd El Aziz, E. (2021). Functional Recellularization of Acellular Rat Liver Scaffold by Induced Pluripotent Stem Cells: Molecular Evidence for Wnt/B-Catenin Upregulation. Cells, 10, 2819. https://doi.org/10.3390/cells10112819
Eke, G., Mangir, N., Hasirci, N., MacNeil, S., & Hasirci, V. (2017). Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering. Biomaterials, 129, 188–198. https://doi.org/10.1016/j.biomaterials.2017.03.021
Erro, E., Bundy, J., Massie, I., Chalmers, S.-A., Gautier, A., Gerontas, S., Hoare, M., Sharratt, P., Choudhury, S., & Lubowiecki, M. (2013). Bioengineering the liver: Scale-up and cool chain delivery of the liver cell biomass for clinical targeting in a bioartificial liver support system. BioResearch Open Access, 2, 1–11. https://doi.org/10.1089/biores.2012.0286
Fan, J., & Yang, J. (2017). Preparation and characterization of a chitosan/galactosylated hyaluronic acid/heparin scaffold for hepatic tissue engineering. Journal of Biomaterials science, Polymer edition, 28(6), 569–581. https://doi.org/10.1080/09205063.2017.1288076
Feng, Z.-Q., Chu, X., Huang, N.-P., Wang, T., Wang, Y., Shi, X., Ding, Y., & Gu, Z.-Z. (2009). The effect of nanofibrous galactosylated chitosan scaffolds on the formation of rat primary hepatocyte aggregates and the maintenance of liver function. Biomaterials, 30, 2753–2763. https://doi.org/10.1016/j.biomaterials.2009.01.053
Fisher, J. P., Mikos, A. G., Bronzino, J. D., & Peterson, D. R. (2012). Tissue engineering: Principles and practices. CRC Press.
Fodor, W. L. (2003). Tissue engineering and cell based therapies, from the bench to the clinic: The potential to replace, repair and regenerate. Reproductive Biology and Endocrinology, 1, 1–6. https://doi.org/10.3390/cells10112819
Fu, F., Chen, Z., Zhao, Z., Wang, H., Shang, L., Gu, Z., & Zhao, Y. (2017). Bio-inspired self-healing structural color hydrogel. Proceedings of the National Academy of Sciences, 114, 5900–5905. https://doi.org/10.1073/pnas.1703616114
Ghahremanzadeh, F., Alihosseini, F., & Semnani, D. (2021). Investigation and comparison of new galactosylation methods on PCL/chitosan scaffolds for enhanced liver tissue engineering. International Journal of Biological Macromolecules, 174, 278–288. https://doi.org/10.1016/j.ijbiomac.2021.01.158
Grigoryan, B., Paulsen, S. J., Corbett, D. C., Sazer, D. W., Fortin, C. L., Zaita, A. J., Greenfield, P. T., Calafat, N. J., Gounley, J. P., & Ta, A. H. (2019). Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science, 364(6439), 458–464. https://doi.org/10.1126/science.aav9750
Gyles, D. A., Castro, L. D., Silva, J. O. C., Jr., & Ribeiro-Costa, R. M. (2017). A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. European Polymer Journal, 88, 373–392. https://doi.org/10.1016/j.eurpolymj.2017.01.027
Haddad-Mashadrizeh, A., Matin, M. M., Shahabipour, F., Ensandost, S., Zomorodipour, A., & Bahrami, A. R. (2021). Effects of chitosan-glycerol phosphate hydrogel on the maintenance and homing of hAd-MSCs after xenotransplantation into the rat liver. Emergent Materials, 1-10. https://doi.org/10.1007/s42247-021-00167-9
Hajilou, H., Farahpour, M. R., & Hamishehkar, H. (2020). Polycaprolactone nanofiber coated with chitosan and Gamma oryzanol functionalized as a novel wound dressing for healing infected wounds. International Journal of Biological Macromolecules, 164, 2358–2369. https://doi.org/10.1016/j.ijbiomac.2020.08.079
Hammond, J. S., Beckingham, I. J., & Shakesheff, K. M. (2006). Scaffolds for liver tissue engineering. Expert review of medical devices, 3, 21–27. https://doi.org/10.1016/j.ijbiomac.2020.08.079
Hasan, A., Morshed, M., Memic, A., Hassan, S., Webster, T. J., & Marei, H.E.-S. (2018). Nanoparticles in tissue engineering: Applications, challenges and prospects. International Journal of Nanomedicine, 13, 5637. https://doi.org/10.2147/IJN.S153758
He, L., Liu, B., Xipeng, G., Xie, G., Liao, S., Quan, D., Cai, D., Lu, J., & Ramakrishna, S. (2009). Microstructure and properties of nano-fibrous PCL-b-PLLA scaffolds for cartilage tissue engineering. European cells & materials, 18, 63–74. https://doi.org/10.22203/ecm.v018a06
Hjortnaes, J., Camci-Unal, G., Hutcheson, J. D., Jung, S. M., Schoen, F. J., Kluin, J., Aikawa, E., & Khademhosseini, A. (2015). Directing valvular interstitial cell myofibroblast-like differentiation in a hybrid hydrogel platform. Advanced Healthcare Materials, 4, 121–130. https://doi.org/10.1002/adhm.201400029
Huang, Y., Zhao, X., Zhang, Z., Liang, Y., Yin, Z., Chen, B., Bai, L., Han, Y., & Guo, B. (2020). Degradable gelatin-based IPN cryogel hemostat for rapidly stopping deep noncompressible hemorrhage and simultaneously improving wound healing. Chemistry of Materials, 32, 6595–6610. https://doi.org/10.1021/acs.chemmater.0c02030
Hussein, K. H., Park, K.-M., Kang, K.-S., & Woo, H.-M. (2016). Heparin-gelatin mixture improves vascular reconstruction efficiency and hepatic function in bioengineered livers. Acta biomaterialia, 38, 82–93. https://doi.org/10.1016/j.actbio.2016.04.042
Ikada, Y. (2006). Challenges in tissue engineering. Journal of the Royal Society Interface, 3, 589–601. https://doi.org/10.1098/rsif.2006.0124
Imani, R., Rafienia, M., & Hojjati Emami, S. (2013). Synthesis and characterization of glutaraldehyde-based crosslinked gelatin as a local hemostat sponge in surgery: An in vitro study. Bio-medical materials and engineering, 23, 211–224. https://doi.org/10.3233/BME-130745
Janani, G., Nandi, S. K., & Mandal, B. B. (2018). Functional hepatocyte clusters on bioactive blend silk matrices towards generating bioartificial liver constructs. Acta biomaterialia, 67, 167–182. https://doi.org/10.1016/j.actbio.2017.11.053
Ji, S., & Guvendiren, M. (2017). Recent advances in bioink design for 3D bioprinting of tissues and organs. Frontiers in bioengineering and biotechnology, 5, 23. https://doi.org/10.3389/fbioe.2017.00023
Jiang, Q., Xu, J., Li, T., Qiao, C., & Li, Y. (2014). Synthesis and antibacterial activities of quaternary ammonium salt of gelatin. Journal of Macromolecular Science, Part B, 53, 133–141. https://doi.org/10.1080/00222348.2013.808518
Jiang, W.-C., Cheng, Y.-H., Yen, M.-H., Chang, Y., Yang, V. W., & Lee, O. K. (2014). Cryo-chemical decellularization of the whole liver for mesenchymal stem cells-based functional hepatic tissue engineering. Biomaterials, 35, 3607–3617. https://doi.org/10.1016/j.biomaterials.2014.01.024
Kabiri, M., Emami, S. H., Rafinia, M., & Tahriri, M. (2011). Preparation and characterization of absorbable hemostat crosslinked gelatin sponges for surgical applications. Current Applied Physics, 11, 457–461. https://doi.org/10.1016/j.cap.2010.08.031
Kao, H.-H., Kuo, C.-Y., Chen, K.-S., & Chen, J.-P. (2019). Preparation of gelatin and gelatin/hyaluronic acid cryogel scaffolds for the 3D culture of mesothelial cells and mesothelium tissue regeneration. International Journal of Molecular Sciences, 20, 4527. https://doi.org/10.3390/ijms20184527
Karin, M., & Clevers, H. (2016). Reparative inflammation takes charge of tissue regeneration. Nature, 529, 307–315. https://doi.org/10.1038/nature17039
Karsdal, M. A., Manon-Jensen, T., Genovese, F., Kristensen, J. H., Nielsen, M. J., Sand, J. M. B., Hansen, N.-U.B., Bay-Jensen, A.-C., Bager, C. L., & Krag, A. (2015). Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. American Journal of Physiology-Gastrointestinal and Liver Physiology, 308, G807–G830. https://doi.org/10.1152/ajpgi.00447.2014
Ko, I. K., Peng, L., Peloso, A., Smith, C. J., Dhal, A., Deegan, D. B., Zimmerman, C., Clouse, C., Zhao, W., & Shupe, T. D. (2015). Bioengineered transplantable porcine livers with re-endothelialized vasculature. Biomaterials, 40, 72–79. https://doi.org/10.1016/j.biomaterials.2014.11.027
Kukla, D. A., Stoppel, W. L., Kaplan, D. L., & Khetani, S. R. (2020). Assessing the compatibility of primary human hepatocyte culture within porous silk sponges. RSC Advances, 10, 37662–37674. https://doi.org/10.1039/D0RA04954A
Kuo, Y.-C., & Chen, Y.-C. (2016). Regeneration of neurite-like cells from induced pluripotent stem cells in self-assembled hyaluronic acid-gelatin microhydrogel. Journal of the Taiwan Institute of Chemical Engineers, 67, 74–87. https://doi.org/10.1016/j.jtice.2016.07.045
Landsman, T., Touchet, T., Hasan, S., Smith, C., Russell, B., Rivera, J., . . . Cosgriff-Hernandez, E. (2017). A shape memory foam composite with enhanced fluid uptake and bactericidal properties as a hemostatic agent. Acta Biomaterialia, 47, 91-99. https://doi.org/10.1016/j.actbio.2016.10.008
Lee, A., Hudson, A., Shiwarski, D., Tashman, J., Hinton, T., Yerneni, S., . . . Feinberg, A. (2019). 3D bioprinting of collagen to rebuild components of the human heart. Science, 365, 482-487. https://doi.org/10.1126/science.aav9051
Lee, B. H., Shirahama, H., Kim, M. H., Lee, J. H., Cho, N.-J., & Tan, L. P. (2017). Colloidal templating of highly ordered gelatin methacryloyl-based hydrogel platforms for three-dimensional tissue analogues. NPG Asia Materials, 9, e412–e412. https://doi.org/10.1038/am.2017.126
Lee, H.-S., Byun, S.-H., Cho, S.-W., & Yang, B.-E. (2019). Past, present, and future of regeneration therapy in oral and periodontal tissue: A review. Applied Sciences, 9, 1046. https://doi.org/10.3390/app9061046
León-Mancilla, B., Martínez-Castillo, M., Guerrero-Bustos, R., Montesinos, J., & Hernández-Estévez, E. (2021). Human Mesenchymal Stem Cells seeded in 3D Collagen Matrix Scaffolds as a Therapeutic Alternative in Tissue Regeneration. Journal of Regenerative Medicine, 10, 5–8.
Li, L., Du, Y., Yin, Z., Li, L., Peng, H., Zheng, H., . . . Lv, G. (2020). Preparation and the hemostatic property study of porous gelatin microspheres both in vitro and in vivo. Colloids and Surfaces B: Biointerfaces, 187, 110641. https://doi.org/10.1016/j.colsurfb.2019.110641
Liu, X., Holzwarth, J. M., & Ma, P. X. (2012). Functionalized synthetic biodegradable polymer scaffolds for tissue engineering. Macromolecular Bioscience, 12, 911–919. https://doi.org/10.1002/mabi.201100466
Liu, X. J., Li, H. Q., Lin, X. Y., Liu, H. Y., & Gao, G. H. (2015). Synthesis of siloxane-modified melamine-formaldehyde microsphere and its heavy metal ions adsorption by coordination effects. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 482, 491–499. https://doi.org/10.1016/j.colsurfa.2015.06.051
Liu, Z., Takeuchi, M., Nakajima, M., Hu, C., Hasegawa, Y., Huang, Q., & Fukuda, T. (2017). Three-dimensional hepatic lobule-like tissue constructs using cell-microcapsule technology. Acta Biomaterialia, 50, 178–187. https://doi.org/10.1016/j.actbio.2016.12.020
Loessner, D., Meinert, C., Kaemmerer, E., Martine, L. C., Yue, K., Levett, P. A., . . . Hutmacher, D. W. (2016). Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms. Natare Protocol, 11, 727-746. https://doi.org/10.1038/nprot.2016.037
Maghsoudlou, P., Georgiades, F., Smith, H., Milan, A., Shangaris, P., Urbani, L., . . . Hagen, C. (2016). Optimization of liver decellularization maintains extracellular matrix micro-architecture and composition predisposing to effective cell seeding. PloS One, 11, e0155324https://doi.org/10.1371/journal.pone.0155324
Masters, K. S., Shah, D. N., Leinwand, L. A., & Anseth, K. S. (2005). Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells. Biomaterials, 26, 2517–2525. https://doi.org/10.1016/j.biomaterials.2004.07.018
Matou-Nasri, S., Gaffney, J., Kumar, S., & Slevin, M. (2009). Oligosaccharides of hyaluronan induce angiogenesis through distinct CD44 and RHAMM-mediated signalling pathways involving Cdc2 and γ-adducin. International Journal of Oncology, 35, 761–773. https://doi.org/10.3892/ijo_00000389
Mazzocchi, A., Devarasetty, M., Huntwork, R., Soker, S., & Skardal, A. (2018). Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication, 11, 015003. https://doi.org/10.1088/1758-5090/aae543
Minami, T., Ishii, T., Yasuchika, K., Fukumitsu, K., Ogiso, S., Miyauchi, Y., . . . Oshima, Y. (2019). Novel hybrid three-dimensional artificial liver using human induced pluripotent stem cells and a rat decellularized liver scaffold. Regenerative Therapy, 10, 127-133. https://doi.org/10.1016/j.reth.2019.03.002
Mohammadkazemi, F., Barangenani, R. K., & Koosha, M. (2019). Development of organic–inorganic oxidized bacterial cellulose nanobiocomposites: Ternary complexes. Cellulose, 26, 6009–6022. https://doi.org/10.1007/s10570-019-02514-w
Mondal, D., Griffith, M., & Venkatraman, S. S. (2016). Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. International Journal of Polymeric Materials and Polymeric Biomaterials, 65, 255–265. https://doi.org/10.1080/00914037.2015.1103241
Murphy, S. V., & Atala, A. (2014). 3D bioprinting of tissues and organs. Nature Biotechnology, 32, 773–785. https://doi.org/10.1038/nbt.2958
Nadi, A., Moradi, L., Ai, J., & Asadpour, S. (2020). Stem Cells and Hydrogels for Liver Tissue Engineering: Synergistic Cure for Liver Regeneration. Stem Cell Reviews and Reports, 1-13. https://doi.org/10.1007/s12015-020-10060-3
Nakamura, S., & Ijima, H. (2013). Solubilized matrix derived from decellularized liver as a growth factor-immobilizable scaffold for hepatocyte culture. Journal of Bioscience and Bioengineering, 116, 746–753. https://doi.org/10.1016/j.jbiosc.2013.05.031
Navarro-Tableros V, Herrera Sanchez MB, Figliolini F, Romagnoli R, Tetta C, Camussi G. Recellularization of rat liver scaffolds by human liver stem cells. Tissue Engineering Part A. 2015 Jun 1;21(11-12):1929-39.
Ng, S. S., Saeb-Parsy, K., Blackford, S. J., Segal, J. M., Serra, M. P., Horcas-Lopez, M., . . . Cho, N. J. (2018). Human iPS derived progenitors bioengineered into liver organoids using an inverted colloidal crystal poly (ethylene glycol) scaffold. Biomaterials, 182, 299-311. https://doi.org/10.1016/j.biomaterials.2018.07.043
Nichols, J. E., Niles, J., Riddle, M., Vargas, G., Schilagard, T., Ma, L., . . . Vega, S. (2013). Production and assessment of decellularized pig and human lung scaffolds. Tissue Engineering Part A, 19, 2045-2062. https://doi.org/10.1089/ten.TEA.2012.0250
World Health Organization, (2021). Hepatitis, Retrieved from https://www.who.int/health-topics/hepatitis#tab=tab_1.
Osorio, M., Fernández-Morales, P., Gañán, P., Zuluaga, R., Kerguelen, H., Ortiz, I., & Castro, C. (2019). Development of novel three-dimensional scaffolds based on bacterial nanocellulose for tissue engineering and regenerative medicine: Effect of processing methods, pore size, and surface area. Journal of Biomedical Materials Research Part A, 107, 348–359. https://doi.org/10.1002/jbm.a.36532
Palakkan, A. A., Hay, D. C., PR, A. K., TV, K., & Ross, J. A. (2013). Liver tissue engineering and cell sources: issues and challenges. Liver International, 33, 666-676https://doi.org/10.1111/liv.12134
Pang, Y., Horimoto, Y., Sutoko, S., Montagne, K., Shinohara, M., Mathiue, D., . . . Sakai, Y. (2016). Novel integrative methodology for engineering large liver tissue equivalents based on three-dimensional scaffold fabrication and cellular aggregate assembly. Biofabrication, 8, 035016. https://doi.org/10.1088/1758-5090/8/3/035016
Pang, Y., Montagne, K., Shinohara, M., Komori, K., & Sakai, Y. (2012). Liver tissue engineering based on aggregate assembly: Efficient formation of endothelialized rat hepatocyte aggregates and their immobilization with biodegradable fibres. Biofabrication, 4, 045004. https://doi.org/10.1088/1758-5082/4/4/045004
Park, K.-M., Hussein, K. H., Hong, S.-H., Ahn, C., Yang, S.-R., Park, S.-M., . . . Woo, H.-M. (2016). Decellularized liver extracellular matrix as promising tools for transplantable bioengineered liver promotes hepatic lineage commitments of induced pluripotent stem cells. Tissue Engineering Part A, 22, 449-460. https://doi.org/10.1089/ten.TEA.2015.0313
Rajalekshmi, R., Shaji, A. K., Joseph, R., & Bhatt, A. (2021). Scaffold for liver tissue engineering: Exploring the potential of fibrin incorporated alginate dialdehyde–gelatin hydrogel. International Journal of Biological Macromolecules, 166, 999–1008. https://doi.org/10.1016/j.ijbiomac.2020.10.256
Ranjbar-Mohammadi, M., & Bahrami, S. H. (2015). Development of nanofibrous scaffolds containing gum tragacanth/poly (ε-caprolactone) for application as skin scaffolds. Materials Science and Engineering: C, 48, 71–79. https://doi.org/10.1016/j.msec.2014.10.020
Ranjbar-Mohammadi, M., & Bahrami, S. H. (2016). Electrospun curcumin loaded poly (ε-caprolactone)/gum tragacanth nanofibers for biomedical application. International Journal of Biological Macromolecules, 84, 448–456. https://doi.org/10.1016/j.ijbiomac.2015.12.024
Ranjbar, J., Koosha, M., Chi, H., Ghasemi, A., Zare, F., Abdollahifar, M. A., . . . Li, T. (2021). Novel chitosan/gelatin/oxidized cellulose sponges as absorbable hemostatic agents. Cellulose, 28, 3663-3675. https://doi.org/10.1007/s10570-021-03699-9
Richards, D., Jia, J., Yost, M., Markwald, R., & Mei, Y. (2017). 3D bioprinting for vascularized tissue fabrication. Annals of Biomedical Engineering, 45, 132–147. https://doi.org/10.1007/s10439-016-1653-z
Robertson, M. J., Dries-Devlin, J. L., Kren, S. M., Burchfield, J. S., & Taylor, D. A. (2014). Optimizing recellularization of whole decellularized heart extracellular matrix. PLoS ONE, 9, e90406. https://doi.org/10.1371/journal.pone.0090406
Shang, Y., Tamai, M., Ishii, R., Nagaoka, N., Yoshida, Y., Ogasawara, M., . . . Tagawa, Y.-i. (2014). Hybrid sponge comprised of galactosylated chitosan and hyaluronic acid mediates the co-culture of hepatocytes and endothelial cells. Journal of Bioscience and Bioengineering, 117, 99-106https://doi.org/10.1016/j.jbiosc.2013.06.015
Sharifzadeh, G., & Hosseinkhani, H. (2017). Biomolecule-responsive hydrogels in medicine. Advanced Healthcare Materials, 6, 1700801. https://doi.org/10.1002/adhm.201700801
Sharma, A., Rawal, P., Tripathi, D. M., Alodiya, D., Sarin, S. K., Kaur, S., & Ghosh, S. (2021). Upgrading Hepatic Differentiation and Functions on 3D Printed Silk–Decellularized Liver Hybrid Scaffolds. Acs Biomaterials Science & Engineering, 7, 3861–3873. https://doi.org/10.1021/acsbiomaterials.1c00671
Sharma, S., & Tiwari, S. (2020). A review on biomacromolecular hydrogel classification and its applications. International Journal of Biological Macromolecules, 162, 737–747. https://doi.org/10.1016/j.ijbiomac.2020.06.110
She, Z., Liu, W., & Feng, Q. (2009). Self-assembly model, hepatocytes attachment and inflammatory response for silk fibroin/chitosan scaffolds. Biomedical Materials, 4, 045014. https://doi.org/10.1088/1748-6041/4/4/045014
Shi, X., Fang, Q., Ding, M., Wu, J., Ye, F., Lv, Z., & Jin, J. (2016). Microspheres of carboxymethyl chitosan, sodium alginate and collagen for a novel hemostatic in vitro study. Journal of Biomaterials Applications, 30, 1092–1102. https://doi.org/10.1177/0885328215618354
Sk, M. M., Das, P., Panwar, A., & Tan, L. P. (2021). Synthesis and characterization of site selective photo-crosslinkable glycidyl methacrylate functionalized gelatin-based 3D hydrogel scaffold for liver tissue engineering. Materials Science and Engineering: C, 123, 111694. https://doi.org/10.1016/j.msec.2020.111694
Souza, G. R., Molina, J. R., Raphael, R. M., Ozawa, M. G., Stark, D. J., Levin, C. S., . . . Georgescu, M.-M. (2010). Three-dimensional tissue culture based on magnetic cell levitation. Nature Nanotechnology, 5, 291-296. https://doi.org/10.1038/nnano.2010.23
Starokozhko, V., & Groothuis, G. M. (2018). Challenges on the road to a multicellular bioartificial liver. Journal of Tissue Engineering and Regenerative Medicine, 12, e227–e236. https://doi.org/10.1002/term.2385
Sun, Y., Yang, C., Zhu, X., Wang, J. J., Liu, X. Y., Yang, X. P., . . . Jiang, W. (2019). 3D printing collagen/chitosan scaffold ameliorated axon regeneration and neurological recovery after spinal cord injury. Journal of Biomedical Materials Research Part A, 107, 1898-1908. https://doi.org/10.1002/jbm.a.36675
Suo, H., Zhang, J., Xu, M., & Wang, L. (2021). Low-temperature 3D printing of collagen and chitosan composite for tissue engineering. Materials Science and Engineering: C, 123, 111963. https://doi.org/10.1016/j.msec.2021.111963
Taddei, P., Chiono, V., Anghileri, A., Vozzi, G., Freddi, G., & Ciardelli, G. (2013). Silk Fibroin/G elatin Blend Films Crosslinked with Enzymes for Biomedical Applications. Macromolecular Bioscience, 13, 1492–1510. https://doi.org/10.1002/mabi.201300156
Taheri, P., Jahanmardi, R., Koosha, M., & Abdi, S. (2020). Physical, mechanical and wound healing properties of chitosan/gelatin blend films containing tannic acid and/or bacterial nanocellulose. International Journal of Biological Macromolecules, 154, 421–432. https://doi.org/10.1016/j.ijbiomac.2020.03.114
Taylor, D. A., Sampaio, L. C., Ferdous, Z., Gobin, A. S., & Taite, L. J. (2018). Decellularized matrices in regenerative medicine. Acta Biomaterialia, 74, 74–89. https://doi.org/10.1016/j.actbio.2018.04.044
Uygun, B. E., Soto-Gutierrez, A., Yagi, H., Izamis, M.-L., Guzzardi, M. A., Shulman, C., . . . Berthiaume, F. (2010). Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nature Medicine, 16, 814-820. https://doi.org/10.1038/nm.2170
Velasco-Rodriguez, B., Diaz-Vidal, T., Rosales-Rivera, L. C., García-González, C. A., Alvarez-Lorenzo, C., Al-Modlej, A., . . . Soltero Martínez, J. F. A. (2021). Hybrid Methacrylated Gelatin and Hyaluronic Acid Hydrogel Scaffolds. Preparation and Systematic Characterization for Prospective Tissue Engineering Applications. International Journal of Molecular Sciences, 22, 6758. https://doi.org/10.3390/ijms22136758
Vishwakarma, S. K., Bardia, A., Lakkireddy, C., Raju, N., Paspala, S. A. B., Habeeb, M. A., & Khan, A. A. (2019). Intraperitoneal transplantation of bioengineered humanized liver grafts supports failing liver in acute condition. Materials Science and Engineering: C, 98, 861–873. https://doi.org/10.1016/j.msec.2019.01.045
Wang, X., Ding, B., & Li, B. (2013). Biomimetic electrospun nanofibrous structures for tissue engineering. Materials Today, 16, 229–241. https://doi.org/10.1016/j.mattod.2013.06.005
Wei, G., Wang, J., Lv, Q., Liu, M., Xu, H., Zhang, H., . . . Wang, X. (2018). Three‐dimensional coculture of primary hepatocytes and stellate cells in silk scaffold improves hepatic morphology and functionality in vitro. Journal of Biomedical Materials Research Part A, 106, 2171-2180. https://doi.org/10.1186/s40824-021-00206-w
Wolf, M. T., Dearth, C. L., Sonnenberg, S. B., Loboa, E. G., & Badylak, S. F. (2015). Naturally derived and synthetic scaffolds for skeletal muscle reconstruction. Advanced Drug Delivery Reviews, 84, 208–221. https://doi.org/10.1016/j.addr.2014.08.011
Xie, X., Li, D., Chen, Y., Shen, Y., Yu, F., Wang, W., . . . Mo, X. (2021). Conjugate Electrospun 3D Gelatin Nanofiber Sponge for Rapid Hemostasis. Advanced Healthcare Materials, 2100918. https://doi.org/10.1002/adhm.202100918
Yajima, Y., Lee, C. N., Yamada, M., Utoh, R., & Seki, M. (2018). Development of a perfusable 3D liver cell cultivation system via bundling-up assembly of cell-laden microfibers. Journal of Bioscience and Bioengineering, 126, 111–118. https://doi.org/10.1016/j.jbiosc.2018.01.022
Yang, X., Liu, W., Shi, Y., Xi, G., Wang, M., Liang, B., . . . Shi, C. (2019). Peptide-immobilized starch/PEG sponge with rapid shape recovery and dual-function for both uncontrolled and noncompressible hemorrhage. Acta Biomaterialia, 99, 220-235https://doi.org/10.1016/j.actbio.2019.08.039
Yap, K. K., Dingle, A. M., Palmer, J. A., Dhillon, R. S., Lokmic, Z., Penington, A. J., . . . Mitchell, G. M. (2013). Enhanced liver progenitor cell survival and differentiation in vivo by spheroid implantation in a vascularized tissue engineering chamber. Biomaterials, 34, 3992-4001https://doi.org/10.1016/j.biomaterials.2013.02.011
Yin, J., & Xu, L. (2020). Batch preparation of electrospun polycaprolactone/chitosan/aloe vera blended nanofiber membranes for novel wound dressing. International Journal of Biological Macromolecules, 160, 352–363. https://doi.org/10.1016/j.ijbiomac.2020.05.211
Zhang, H., Siegel, C. T., Li, J., Lai, J., Shuai, L., Lai, X., . . . Bai, L. (2018). Functional liver tissue engineering by an adult mouse liver‐derived neuro‐glia antigen 2‐expressing stem/progenitor population. Journal of Tissue Engineering and Regenerative Medicine, 12, e190-e202. https://doi.org/10.1002/term.2311
Zhang, S., Li, J., Chen, S., Zhang, X., Ma, J., & He, J. (2020). Oxidized cellulose-based hemostatic materials. Carbohydrate Polymers, 230, 115585. https://doi.org/10.1016/j.carbpol.2019.115585
Zhang, Y.-Q., Shen, Y., Liao, M.-M., Mao, X., Mi, G.-J., You, C., . . . Lin, N. (2019). Galactosylated chitosan triptolide nanoparticles for overcoming hepatocellular carcinoma: Enhanced therapeutic efficacy, low toxicity, and validated network regulatory mechanisms. Nanomedicine: Nanotechnology, Biology and Medicine, 15, 86-97. https://doi.org/10.1016/j.nano.2018.09.002
Zhang, Y. S., & Khademhosseini, A. (2017). Advances in engineering hydrogels. Science, 356(6337). https://doi.org/10.1126/science.aaf3627
Zhao, C., Li, Y., Peng, G., Lei, X., Zhang, G., & Gao, Y. (2020). Decellularized liver matrix-modified chitosan fibrous scaffold as a substrate for C3A hepatocyte culture. Journal of Biomaterials Science, Polymer Edition, 31, 1041–1056. https://doi.org/10.1080/09205063.2020.1738690
Zhao, X., Guo, B., Wu, H., Liang, Y., & Ma, P. X. (2018). Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nature Communications, 9, 1–17. https://doi.org/10.1038/s41467-018-04998-9
Zhong, S., Zhang, Y., & Lim, C. (2010). Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 2, 510–525. https://doi.org/10.1002/wnan.100
Zhu, J., & Marchant, R. E. (2011). Design properties of hydrogel tissue-engineering scaffolds. Expert Review of Medical Devices, 8, 607–626. https://doi.org/10.1586/erd.11.27
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vazirzadeh, M., Azarpira, N., Davoodi, P. et al. Natural Scaffolds Used for Liver Regeneration: A Narrative Update. Stem Cell Rev and Rep 18, 2262–2278 (2022). https://doi.org/10.1007/s12015-022-10362-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10362-8