Abstract
Very small embryonic-like (VSELs) and ovarian (OSCs) stem cells are located in adult mammalian ovary surface epithelium (OSE). OSCs can expand long-term and differentiate into oocyte-like structures in vitro and have resulted in birth of fertile pups. Lineage tracing studies have provided evidence to suggest OSCs differentiation into oocytes in vivo. But how these stem cells function under normal physiological conditions has not yet been well worked out. Besides studying STRA-8 and SCP-3 expression in enzymatically isolated OSE cells smears, mice were injected BrdU to track mitosis, meiosis and follicle assembly. H&E stained OSE cells during late diestrus and proestrus showed VSELs undergoing asymmetrical cell divisions to give rise to slightly bigger OSCs which in turn underwent symmetrical cell divisions followed by clonal expansion (rapid expansion with incomplete cytokinesis) during early estrus to form germ cell nests (GCN). OCT-4, SSEA-1, MVH and DAZL positive cells in GCN expressed Erα, Erβ and FSHR, were interconnected by ring canals (TEX-14), showed mitochondrial aggregation (Cytochrome C) and Balbiani Body (TRAL). Apoptosis in ‘nurse’ cells was marked by PARP and putative oocytes were clearly visualized. BrdU was detected in cells undergoing mitosis/meiosis and also in an oocyte of secondary follicle. FACS sorted, green fluorescent protein (GFP) positive VSELs upon transplantation resulted in GFP positive GCN suggesting crucial role for VSELs in adult ovaries. Results suggest that various events described during oogenesis and follicle assembly in fetal ovaries are recapitulated on regular basis in adult ovary and result in the formation of follicles.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Functionally active ovarian stem cells (OSCs) were reported in adult mouse ovary surface epithelium (OSE) in 2004 [1]. We have reported presence of two populations of stem cells in adult ovaries including very small embryonic-like stem cells (VSELs) and slightly bigger OSCs [2, 3]. VSELs are developmentally equivalent to primordial germ cells (PGCs) that survive in few numbers in multiple adult tissues including ovary [3, 4]. Failure to detect stem cells by single-cell RNAseq in human ovaries by Wagner et al. [5] was possibly technical [6, 7]. Stem cells are of small size and can be collected by centrifugation at 1000 g for 15 min whereas Wagner’s group subjected cells centrifuged at 300 g for 7 min to scRNAseq. Thus, their inability to detect stem cells in human ovaries was because stem cells were never subjected to scRNAseq [6, 7]. Presence of LIN-CD45-SCA-1 + VSELs was recently confirmed by other groups as well in adult mouse ovaries [8] and as small-sized stem cells in human ovaries [9, 10]. Several groups have reported functional potential of ovarian stem cells which readily differentiate into oocyte-like structures in vitro [11,12,13,14], undergo cortical reaction in presence of sperm [15] and have also resulted in birth of fertile mouse pups [16]. But whether similar events occur in vivo under physiological conditions has not yet been investigated extensively. Initial evidence was generated by lineage tracing studies tracking OCT-4 positive cells that underwent persistent meiosis to form new primordial follicles (PF), [17] and pre-meiotic STRA-8 + ve germ cells which developed into mature oocytes that fertilized to produce fertile offspring [16].
We have earlier reported that, VSELs undergo asymmetrical cell divisions (ACD) to self-renew and give rise to OSCs which divide by symmetrical cell divisions (SCD) and clonal expansion to form germ cell nests (GCN) when OSE cells isolated from adult sheep OSE were cultured in vitro in presence of FSH [2, 18], (Suppl Fig. 1). OCT-4, FSHR and PCNA expression has also been reported in germ cells, GCN and primordial follicles (PF) in sheep ovary cortical tissue sections [19]. But whether similar divisions and GCN formation occurs in vivo in adult mice ovaries under physiological conditions is not yet reported. Present study was undertaken to delineate stem cell dynamics and GCN differentiation in vivo under physiological conditions and possible regulation by circulatory hormones based on available knowledge how PF get assembled in fetal ovaries.
PGCs migrate into the gonadal ridge where they increase in number by accelerated mitotic divisions to form oogonial nests. Oogenesis in the fetal ovaries comprises a series of events including formation of GCN (cyst) by rapid proliferation and incomplete cytokinesis of the stem cells followed by meiosis, and GCN breakdown leading to PF assembly. Oogonial cells in fetal GCN are interconnected by ring canals [20], undergo meiosis and transition into oocytes. In the GCN, one germ cell matures into an oocyte whereas others act as ‘nurse cells’, transfer their contents including cytoplasmic organelles like mitochondria into the pre-destined oocyte through the ring canals and subsequently undergo apoptosis. Organelles transport from the nurse cells through the ring canals results in the formation of Balbiani body or mitochondrial cloud in the perinuclear cytoplasm of the developing oocyte [21] Eventually the nurse cells undergo apoptosis and the oocyte with the Balbiani body detaches from the GCN, gets surrounded by pre-granulosa cells resulting in the formation of a primordial follicle [22, 23]. Based on this understanding in fetal ovaries, carefully selected panel of markers were used to study stem/progenitor cells isolated from the adult mice ovaries.
Study Design and Methods
Adult Swiss and GFP [FVB.Cg-Tg(GFPU) 5NAGY/J] mice, maintained in the Institute Experimental Animal Facility were used for the present study, after approval from Institute Animal Ethics Committee. The mice were housed under controlled temperature (23 ± 1 °C) and humidity (55 ± 5%), with 14 h light/10 h dark cycle and with free access to food and water. Ovaries from 8 to 10 weeks old mice were used for the study.
Study Design
Dynamics of Ovary Surface Epithelium (OSE) Across Estrus Cycle
Histological Changes in OSE and Ovarian Cortex Across Estrus Cycle
Adult mice ovaries were collected during different stages of estrus cycle, fixed in neutral buffer formalin (NBF), paraffin embedded and 5 µm thick sections were cut and stained with Hematoxylin and Eosin (H & E). The representative areas were viewed under brightfield microscope (90i Nikon) and representative images were recorded.
Changes in OSE Cell Smears Across Estrus Cycle
OSE cells lodged in the ovary surface, were isolated from the mice ovaries and used to prepare cell smears during different stages of estrus cycle. Changes in the H&E stained smears and SCP-3 (meiotic marker, Synaptonemal Complex Protein 3) expression were studied across estrus cycle. Smears were also used for immuno-localization studies described ahead. Representative areas were photographed.
Enumeration of VSELs Across Estrus Cycle
VSELs were enumerated during different stages of estrus cycle by flow cytometry. Intact ovaries (from at least 3 mice per group) were collected during different stages of estrus cycle and subjected to flow cytometry to study and enumerate live (7AAD negative), 2-6 µm VSELs with a surface phenotype of LIN-CD45-SCA-1 + after doublets exclusion using gating strategy as described earlier [25, 26].
Delineating Ovarian Stem Cells Proliferation and Differentiation Including Meiosis
This was achieved by using carefully selected set of markers for immuno-localization and RT-PCR studies. Various events reported extensively in the fetal ovaries were studied in the stem cells and GCN in OSE cell smears prepared from the adult ovaries. Stem (OCT-4, SSEA-1) and germ (MVH, DAZL) cell markers along with Erα, Erβ and FSHR expression was studied to understand that the stem cells respond to circulatory hormones. STRA-8 responsible for pre-meiotic replication of germ cells and SCP-3 suggestive of meiosis were studied. TEX14 expression was suggestive of interconnecting ring canals [20] and Cytochrome C (Cyto C) marked the mitochondria and its expression reflected clustering of mitochondria around the pre-destined oocyte in a GCN [21]. Trailer Hitch (TRAL), a major component involved in transportation of RNA and organelles, is known to be associated with the Balbiani Body [21]. BrdU uptake in the OSE smears was studied and was suggestive of proliferation as well as meiosis. Co-expression of SCP-3 and BrdU provided strong evidence to support meiosis in the GCN. PARP enzyme expression was studied as it marks necrotic and apoptotic cells in the nest [23]. RNA extracted from the OSE cells was used to detect transcripts specific for pluripotent stem cells (Oct-4A, Sox2, Nanog), primordial germ cells (Stella), germ cells (Oct-4, Mvh), oocyte (ZP3) and receptors for estradiol and FSH receptors(Erα, Erβ, Fshr1, Fshr3) in addition to 18 s. Transcripts specific for pre-meiotic germ cells (Stra8), meiotic markers (Scp1, Dmc1, Spo11) and ring canals (Tex14) were studied in RNA extracted from intact ovary with adult testes RNA as a positive control.
To Examine the Fate of Transplanted VSELs In Vivo
The aim of this experiment was to delineate the role of VSELs in adult ovary. For this, FACS sorted 2–6 µm, SSEA-1 positive VSELs from GFP mice were transplanted in the ovaries of two Swiss mice. The mice were later sacrificed after two weeks, during early estrus and diestrus. The OSE cells were isolated from recipient mice, smears were prepared and studied for the expression of GFP and SSEA-1. SSEA-1 sorted VSELs from the mouse bone marrow were separately used to study MVH expression.
OSE Cells Culture and Live Cell Imaging
OSE cells were cultured to track various events during oogenesis by live cell imaging.
Methods
Dynamics of Ovary Surface Epithelium (OSE) Across Estrus Cycle
OSE Cells Smears Preparation
OSE cells were isolated using a method published by Gamwell et al. [24] with slight modifications by partial enzymatic digestion of intact ovaries during different stages of estrus cycle. The ovaries were placed singly in 500 µl of 0.25% of trypsin-EDTA at 37 °C for 30 min. The reaction was stopped by equal volume of DMEM-F12 media containing 20% fetal bovine serum (FBS). The tube was agitated gently back and forth ten times to release OSE cells. The cells were vortexed for one second to dislodge OSE cells. Denuded ovaries were removed and cells suspension was filtered through 40 μm nylon cell strainer (BD Falcon) and first spun at 250 g to collect bigger epithelial cells followed by 1000 g to collect stem cells, each time for 15 min. Later both the cell pellets were mixed and used to make cell smears, for RNA extraction and for immuno-localization studies. Use of two different speeds to collect cells for various experiments ensured collecting intact bigger somatic cells at 250 g and stem cells at 1000 g which otherwise inadvertently get discarded as discussed earlier [3, 6]. Cells smears were prepared by gently placing the cells in a small area pre-marked by a diamond pencil on poly-L-lysine (Sigma) coated slides, air dried, fixed with 4% paraformaldehyde (Sigma) for 15 min, washed three times with PBS (5 min per wash), air-dried and stored at 4 °C for future use. These smears were stained with H&E, viewed under microscope and representative images were taken under brightfield microscope (90i, Nikon).
Flow Cytometry Studies
Enumerating VSELs Across Estrus Cycle
Whole ovaries in different stages of estrus cycle were used to enumerate VSELs by flow cytometry using gating strategy described earlier [25, 26]. In brief, the ovaries were cleared of surrounding fat and collected in sterile Dulbecco phosphate buffer saline (DPBS), washed three times to remove blood cells, and then chopped and subjected to enzymatic digestion with collagenase type IV (Gibco, 1 mg/mL) dissolved in Dulbecco modified Eagle medium (DMEM/F12, Gibco) at 370C for 20 min. Intermittent gentle pipetting was done to break the cell clumps. Then DMEM medium containing 20% fetal bovine serum (FBS, Gibco) was added and the cell suspension was filtered through 40 µm cell strainer (Corning). Single cells suspension was spun at 250 g for 10 min to collect Pellet A and then the supernatant was further spun at 1000 g for 10 min to collect Pellet B. Both the pellets A and B were washed with 1X PBS and mixed before further processing for reasons described above. Approximately one million cells from total cells were subjected to flow cytometry studies to detect VSELs. VSELs were studied as viable (7AAD negative) cells, after doublets exclusion, in the size range of 2–6 µm with a surface phenotype of LIN-/CD45-/SCA-1 + . A total of one lakh events were counted per sample. Calibration size beads were used to gate 2–6 µm events. Antibodies used included FITC-conjugated rat anti mouse SCA-1 (#553335, BD Biosciences), PE rat anti mouse CD45 (#553081, BD Biosciences), APC Mouse Lineage antibody cocktail (#558074, BD Pharmingen). FMO controls were run to see true negative boundary for FITC. The samples were run on FACS Aria (BD Biosciences, San Jose, CA, USA). Results were analyzed by using BD FACS Diva software (BD Biosciences).
FACS Sorting of VSELs from Ovaries of FvB GFP MICE
Whole ovaries from 10 FvB GFP animals were chopped and processed as described above to obtain a single cells suspension. Cells were stained with PE tagged SSEA-1 antibody (BD Pharmingen, 560886, 5 µl/million cells). After 30 min, the cells were washed twice with DPBS containing 2% FBS. Antibody was omitted from unstained control tube. Unstained control tube was prepared from cells obtained from non-GFP mice. The tubes were run on FACS Aria and cells were collected in FBS coated tubes. Similarly, SSEA-1 positive cells were isolated from mouse bone marrow (refer to Supplementary section for further details).
Immuno-Localization Studies
For immuno-fluorescence studies, smears were first washed thrice with TRIS buffered saline (TBS) and then blocked for 1 h at room temperature with 3% BSA in a humidified chamber and incubated with primary antibody diluted in Antibody Signal Enhancer Solution consisting of 10 mM Glycine, 0.1% Tween20, 0.1% H2O2 (3% stock solution) at 4 °C in a moist chamber for 12–16 h as described earlier [28]. The primary antibody was omitted for the negative controls. Various primary antibodies used in the study are provided in Suppl Table 1. For co-expression studies, cells were incubated with both antibodies at 4 °C. Next day, the cell smears were brought to room temperature, washed with wash buffer [0.1% tween-20 in TRIS buffer saline (TBS)] three times followed by incubation with secondary antibody (Alexafluor-488/568/633; 1:500, diluted in 0.1% of Tween −20) for 2 h at room temp in dark. The cells were washed thrice with TBS-Tween 20 and counterstained with DAPI (Thermo Fisher) for 30 min. For co-expression of OCT-4 with ERα and ERβ, the cells were treated with 0.2% Triton-X 100 for 10 min followed by blocking and antibody incubation. Negative controls were run with all experiments with omission of primary antibody (Suppl Fig. 2). All images were captured by laser scanning confocal microscope (Fluoview 3000, Olympus).
BrdU Incorporation Studies
Mice in late diestrus were injected with 200 mg/Kg of BrdU (Sigma) intra-peritoneally on daily basis and later sacrificed during estrus/ late metestrus to study proliferating and meiotic nests and after 11 days to detect BrdU in growing follicles. BrdU uptake was studied in both OSE cells smears and ovarian sections. For studying expression of BrdU, cells were permeabilized with 0.2% triton X-100 for 30 min, washed thrice with PBS. Cells were treated with pre-warmed 1.5 N HCL at 37 °C for 20 min to denature DNA followed by two washes of PBS (15 min each). The cells smears were then treated with 3% BSA for 1 h at room temperature to block non-specific staining followed by overnight incubation with anti-BrdU antibody. Second day procedure was similar as described above. BrdU expression on paraffin sections is described in the Suppl section (Pg 3).
RT-PCR Studies
For RNA extraction, OSE cells were collected as described above using trypsin containing 0.12% of 4% sodium hypochlorite to avoid RNA degradation [27] at 37 °C for 30 min. Trypsin was pre-treated with sodium hypochlorite for half an hour before placing the ovaries. The tube was shaken gently back and forth 10 times or pipetted up and down 5 times very gently. Cell suspension obtained after digestion was spun at 1000 g for 15 min, washed with ice-cold PBS and placed in Trizol (Invitrogen, Carlsbad, CA, USA) at −80 °C. Intact ovaries were directly placed in Trizol to extract RNA. RNA was extracted using standard protocol and treated with DNase I (Fermentas) to remove any genomic DNA present. First-strand cDNA was synthesized using the iScript cDNA synthesis Kit (Bio-Rad, USA, Hercules, CA, USA) according to the manufacturer’ instructions. Briefly, 500 ng of total RNA was incubated with 5 × iScript reaction mix and reverse transcriptase. The reaction was carried out in G-STORM Thermocycler (Gene Technologies, UK) as per manufacturer’s instructions. 18S was used as housekeeping gene. PCR conditions were 1 cycle at 94 °C for 3 min followed by 35 cycles, with each cycle consisting of denaturation at 94 °C for 30 s, annealing at the specified temperature for 40 s, and extension at 72 °C for 40 s and a final extension cycle at 72 °C for 10 min. The PCR products were later examined on a 2% agarose gel. Primer details and annealing temperatures for various transcripts are provided in Suppl Table 2.
Transplantation of VSELs in Ovaries
Approximately 1.5 × 105 FACS sorted SSEA-1 and GFP positive VSELs were transplanted into the ovaries of normal mice. Recipient mice were anaesthetized with intraperitoneal injection of 1% sodium pentobarbitone. 10 μl of cells suspension, containing approximately 5 × 104 cells, was injected into ovaries using a 10 μl NanoFil syringe with a 33-gauge beveled needle. The animals were sacrificed two weeks later during estrus and diestrus.
OSE Cells Culture and Live Cell Imaging
OSE cells were isolated and cultured for 24–48 h. Images were captured using an inverted microscope (Eclipse TE2000-S; Nikon, Japan) with Hoffman optics. Live cell imaging was done for 24 h using inverted microscope with live cell imaging attachment (Fluoview 3000, Olympus). Cells were also labelled with 150 nM of Mitotracker Red FM (M22425, Invitrogen) and Hoechst 33342 (Thermo Scientific) for 30 min followed by one PBS wash and imaged under Olympus microscope.
Results
Dynamics of Ovary Surface Epithelium (OSE) Across Estrus Cycle
Histological Changes in Ovarian Cortex Across Estrus Cycle
Histological changes in OSE and the underlying ovarian cortex were studied across estrus cycle in Hematoxylin and Eosin (H&E) stained ovarian sections (Suppl Fig. 3). Number of PF just beneath the OSE appeared to be more during diestrus stage of estrus cycle in agreement with earlier reports [20]. GCN with cytoplasmic connectivity were observed in the cortex (Suppl Fig. 4, 5, Fig. 3b). Adjacent to the GCN, newly formed oocytes (green arrows) in the process of getting assembled as PF were clearly visualized (Suppl Figs. 3, 5, Fig. 3b).
Stem Cells in OSE Cell Smears
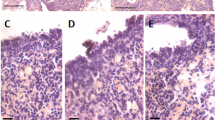
Mice ovaries were subjected to partial enzymatic digestion to collect OSE cells and prepare smears. H&E-stained smears showed distinctly spherical stem cells with dark stained nuclei and high nucleo-cytoplasmic ratio including the smaller VSELs and slightly bigger OSCs (Fig. 1A, Suppl Figs. 6–10) present singly or in doublets suggestive of ACD and SCD and also at places they existed in clusters with cytoplasmic continuity and were the GCN that represent clonal expansion of stem cells with incomplete cytoplasmic divisions (Fig. 1B-E). Pale blue, bigger cells were the epithelial cells and their pink cytoplasm was possibly lost while centrifuging the cells at 1000 g to pellet down the stem cells (Fig. 1A, D). Modified protocol where epithelial cells were collected by centrifuging at 1000 rpm (250 g) and then stem cells at 1000 g and preparing the smears by mixing both the pellets resulted in clear visualization of intact epithelial cells (Fig. 1E). Suppl Fig. 11 shows differential expression of various markers (NUMB, OCT-4, MVH) in stem cells undergoing ACD, SCD and clonal expansion.
Stem cells heterogeneity and dynamics in OSE. A Two distinct populations of stem cells were clearly evident in the H&E-stained cells smear including smaller sized VSELs (red broken circle), slightly bigger OSCs (blue broken circle) present singly or as doublets. The cells in the doublets were of unequal size (green broken circle, suggestive of asymmetric cell division) or of similar size (orange broken circle suggestive of symmetrical cell divisions). Pale blue nuclei represent epithelial cells whose cytoplasm was probably damaged by centrifuging at 3000 rpm. B-D At places, cell clusters with cytoplasmic connectivity were observed and were suggestive of clonal expansion due to rapid proliferation and incomplete cytokinesis. These clonally expanding stem cell clusters are termed as putative germ cell nest (GCN) and are reported to exist only in fetal mammalian ovaries. Here we show putative GCN/ germline cyst in adult ovary smears as well. E This smear was prepared by mixing cells collected by centrifuging at 1000 rpm and 1000 g. This allowed visualization of intact epithelial cells with abundant pink cytoplasm along with the stem cells. Epithelial cells had pale stained nucleus and abundant cytoplasm. Germ cells with dark stained nuclei showed variable amount of pink cytoplasm suggesting different stages of differentiation and development. Scale: 20 µm
Cyclic Changes in OSE Cell Smears Across Estrus Cycle
Cyclic changes in smears across estrus cycle are shown in Fig. 2. Stem cells were present singly during early proestrus and undergo ACD and SCD. GCN are formed during late proestrus and estrus stage when circulatory levels of FSH and estradiol are high and their breakdown occurs during late metestrus. Maximal numbers of GCN were visualized during estrus and metestrus. These results are based on observing smears from 4 mice per stage of the estrus cycle. The epithelial cells varied in size and shape in different stages of estrus cycle and red blood cells were noticed in the smears around the time of ovulation (Suppl Fig. 7). The relatively neglected and poorly studied OSE harbors stem cells which undergo characteristic ACD and SCD and GCN formation regularly during estrus cycle. Extreme right panel of immunofluorescence images shows SCP-3 (meiotic marker) expression in the GCN. SCP3 expression remained cytoplasmic in DE and PE stage. Meiosis occurred during E and ME when SCP3 showed nuclear expression associated with distinct chromatin changes. SCP-3 is reported in the cytoplasm prior to meiosis and translocates to the nuclei during meiosis (Li et al. 2016). Single channel images for SCP-3 expression are provided as Suppl Fig. 12.
Distinct changes on OSE cell smears during different stages of estrus cycle. Epithelial cells remain pale stained and with abundant cytoplasm (K). Stem cells are darkly stained and spherical in shape, presence interspersed amongst the epithelial cells. Note presence of single and dividing doublets during proestrus. GCN were observed maximally during estrus and metestrus stage. Stem cell activity was minimal during diestrus. Scale: 20 µm A-D 10X, E–H 20X, I-L 40X. Figs M-P show SCP-3 expression in cells collected during different stages of estrus cycle. SCP-3 was cytoplasmic during diestrus and proestrus stages of estrus cycle and became nuclear (suggestive of meiosis) during estrus and metestrus stage. Note the changing chromatin pattern during different stages of estrus cycle showing progression of cells through prophase of meiosis 1. Scale bar is 5 µm
Enumeration of VSELs Across Estrus Cycle by Flow Cytometry
VSELs were enumerated during different stages of estrus cycle by flow cytometry (Fig. 3A, B) as viable (7AAD negative) cells after doublets exclusion in size range of 2–6 µm for surface phenotype of LIN-CD45-SCA-1 + . VSELs enumerated during different stages (Fig. 3B) as % total events studied were 0.16 ± 0.03% in proestrus, 0.41 ± 0.05% in estrus, 0.96 ± 0.03% in metestrus and 0.36 ± 0.06% in diestrus.
Characterization of stem cells enriched from adult mouse ovary surface epithelium. A Enumeration of VSELs by flow cytometry. B Bar graph showing percent total VSELs during different stages of estrus cycle. Results are an average of 3 independent experiments and depicted as mean ± SE. **P ˂ 0.01, ***P ˂ 0.001. Maximal numbers of VSELs were observed by flow cytometry during ME. Results are an average of 3 independent experiments. C RT-PCR analysis of OSE cells for transcripts specific for pluripotency (Oct4A, Sox2, Nanog), primordial germ cells (Stella), germ cells (Oct-4, Mvh, Dazl), gonadotropin and steroid receptors (FSHR1, FSHR3, ERα, ERβ). D Detection of meiotic markers Stra8, Scp1, Dmc1, Spo11 and Tex14 in RNA extracted from intact ovary and testes (used as positive control). Absence of a band for ZP3 suggested no oocyte contamination and 18S was used as housekeeping gene
Detection of Stem Cell Specific Transcripts in OSE
RNA extracted from OSE cells upon RT-PCR showed transcripts specific for PGCs, VSELs, and OSCs/germ cells (Fig. 3C). Expression of Stella is suggestive of VSELs being developmentally linked to PGCs [4]. Besides Erα and ERβ, Fshr3 was detected but canonical Fshr1 was not amplified (Fig. 3C).
Immuno-Expression of Stem and Germ Cell Specific Markers
The stem/progenitor cells lodged in OSE express pluripotent as well as germ cell markers and have been well characterized by several groups [13, 19, 29, 30]. Expression of pluripotent (OCT-4, SSEA-1) and germ cells (DAZL, MVH) specific markers (Fig. 4) was studied in adult mice OSE cells smears. Cells existed singly or in small clusters and expressed nuclear or cytoplasmic OCT4 (A). The cells also expressed cell surface SSEA-1 (B). ACD was observed wherein two stem cells of different sizes and expressing SSEA-1 were observed as a doublet (C). As these stem cells initiate differentiation, they start expressing MVH and DAZL resembling more differentiated germ cells. MVH expressing cells were studied by evaluating SSEA-1 sorted cells in mouse bone marrow (Suppl Fig. 13). VSELs were observed with nuclear and cell surface MVH (arrow) along with slightly bigger cells with cell surface to cytoplasmic MVH expression (D). Nuclear (E) and cytoplasmic (F) expression of MVH was also observed in germ cells/GCN (Fig. 4). Pre-meiotic germ cell marker DAZL was visualized in single cells (G, H) and also in the GCN (I, J). Cells expressed nuclear (arrow) and cytoplasmic DAZL and only a few co-expressed cytoplasmic OCT-4. Some GCN expressed nuclear DAZL and these may be putative immature oogonial nests (I) whereas few expressed cytoplasmic DAZL and were relatively mature GCN supposedly undergoing meiosis (J). Similar gradual increase in size and cytoplasm of cells was observed when human ovarian stem cells enriched from the OSE were cultured in vitro [30].
Germ stem cells and putative nests in OSE cells smears collected from adult mouse ovary. A Note nuclear and cytoplasmic OCT-4 expression in the small VSEL and slightly bigger OSC respectively. B-C SSEA-1 expression on stem cells and a dividing doublet. Differentiation of stem cells was associated with gradual loss of surface SSEA1 and it shifted to the cytoplasm in the bigger cell. D MVH expression was studied in 2-6 μm, SSEA-1 FACS sorted cells from mouse bone marrow. Note both nuclear and cell surface expression of MVH is detected E-F Co-expression of MVH and OCT-4 showing gradual loss of OCT-4 in MVH positive doublet and in a putative germ cell nest. G-H Co-expression of DAZL and OCT-4 revealing its distinct subcellular localization. DAZL was exclusively localized in nuclei of pre-meiotic germ cells (arrowheads) whereas it was cytoplasmic in differentiated cells. Note a single OCT-4 + DAZL- cell (broken circle) in a DAZL + GCN suggestive of clonal expansion to form the GCN. I Germ cell nest with nuclear DAZL represents an oogonial nest. J GCN with cytoplasmic DAZL represents a relatively differentiated nest. Cells smears were prepared by isolating OSE cells from estrus and metestrus stage adult murine ovaries separately. Scale: A 5 µm, B-C 4 µm, D 10 µm, E–F 3 µm, G-J: 10 µm
Figure 5 shows GCN in ovarian sections with MVH expression. Similar clusters were also found to express SSEA-1 (Suppl Figs. 14–16). Z-stacks of MVH positive clusters are also included (Suppl Figs. 17,18). These clusters are not surrounded by granulosa cells and thus are pre-meiotic germ cells rather than PF.
Putative germ cell nests in ovarian sections in estrus stage A-B H&E-stained sections showing small clusters of cells with distinct cytoplasmic connectivity. GCN in A is further magnified and shown in B. C-F Cluster of cells resembling GCN were observed subjacent to the OSE and expressed MVH. Cell clusters show a common ring of MVH surrounding them. These are not single cells and this was further confirmed by Z-stack imaging (Suppl Fig. 14). Note these clusters are not surrounded by granulosa-like cells and thus could not be primordial follicles. Scale: A-B 20 µm, IF 3 µm
Stem Cells Express FSHR, Erα and Erβ
Earlier immuno-localization and in situ hybridization studies showed FSHR expression on ovarian stem cells whereas the epithelial cells remained distinctly negative [18]. Expression of receptors for gonadotropin (FSHR) and steroid hormones (ERα, Erβ) hormones were documented on the ovarian stem cells (Fig. 6) in agreement with the RT-PCR results (Fig. 3c).
A Ovarian stem cells express gonadotropin and estrogen receptors. Small cell clusters expressed ERβ, FSHR expression was observed in small, supposedly immature oogonial GCN whereas ERα was expressed in bigger and mature GCN, where the germ cells were of bigger size and possibly undergoing meiosis as evident from DAPI stained distinct nuclear chromatin pattern. Scale bar A: 5 µm, B: 4 µm & C: 3 µm
Tracking Oogenesis in Germ Cell Nests (GCN) in Adult Mouse Ovary
Oogenesis occurs in fetal ovaries and has been well documented in published literature (discussed above in Introduction). Similar events were studied in adult ovary. Transcripts specific for pre-meiotic germ cells (Stra-8), early meiosis (Spo11, Dmc1, Scp1) and ring canals (Tex14) were studied by RT-PCR. The transcripts were found at relatively lower abundance in RNA extracted from the intact ovaries compared to adult testes where these were readily detectable (Fig. 3D).
OCT-4, SSEA-1, MVH and DAZL positive GCN were observed in adult ovary (Fig. 7). Figure 7 and Suppl Figs. 19–20 are a compilation of immuno-localization results to support various events involved in the differentiation of pre-meiotic germ cells in GCN into oocytes with a Balbiani Body. Pre-meiotic germ cells marker (nuclear STRA-8) was observed in dividing germ cells (A) and shifted to the cytoplasm in the GCN (C). Synaptonemal complex protein SCP-3 is required for synapsis of homologous chromosomes during early meiosis. SCP-3 was expressed in nuclei counterstained with DAPI in the germ cells indicating early stages of meiosis (B, D). Presence of ring canals was confirmed by studying expression of TEX14 (Testis Expressed 14) which is an intercellular bridge forming factor. SSEA-1 positive cells in GCN remained interconnected through TEX14 positive ring canals (E, E’). Mitochondrial transport and perinuclear mitochondrial aggregation were studied by the expression of Cytochrome C (CYTO C), a peripheral protein of the mitochondrial inner membrane. Aggregation of CYTO C was observed in the perinuclear region suggestive of Balbiani Body formation (F, G, F’). In one of the DAZL expressing cells in the GCN, all the mitochondria were present in the perinuclear region of a single cell (G). The GCN were apparently in different stages of development. Balbiani Body was further studied by the expression of TRAILER HITCH (TRAL), which is a major component and member of ribonucleoprotein complex. TRAL was clearly detected and enriched in a small area in the cells comprising the GCN and co-expressing DAZL, (H-I). These oocytes with TRAL positive Balbiani Body later get separated and are surrounded by the pre-granulosa cells to assemble as PF.
Immuno-expression of markers suggestive of oogenesis in adult ovary. A STRA8 immuno-expression is suggestive of pre-meiotic replication B SCP3 expression suggests later stages of meiosis in germ cell nest C Cytoplasmic STRA8 suggesting that the nest is differentiated D Downregulation of SSEA1 in SCP3 positive nest suggesting that nest has entered meiosis. E Overlay of TEX14 (marker for ring canal) with SSEA1. E’ is the Z stack projection of E. F Co-expression of DAZL and Cytochrome C (CYTO C) suggesting mitochondrial transport in GCN. F’ is Z stack projection of F G Mitochondrial cloud (Balbiani body) expressing CYTO C in a DAZL positive nest. downregulation of DAZL expression in the GCN suggests it is in later stages of maturation. H-I. DAZL positive Balbiani Body co-expresses Trailer Hitch (TRAL). Scale: A 3 µm, B 2 µm, C 4 µm, D E’ & F’ 3 µm
BrdU Uptake by Stem Cells and GCN Suggestive of Ongoing Mitosis and Meiosis
BrdU incorporation could demonstrate mitosis, mitochondrial replication, DNA repair and meiosis in germ cells/oogonia [31] but DNA synthesis in mitotic and meiotic cells has distinct features [32]. Based on the results described above (Fig. 7), cytoplasmic DAZL was suggestive of the cells had already entered meiosis.
Figure 8, A-D represent cells in various stages of mitosis or DNA repair whereas the staining pattern in E-J were suggestive of meiosis. Chromatin pattern of DAPI stained nuclei was carefully observed along with BrdU staining pattern and DAZL expression. Cell clusters undergoing mitosis expressed nuclear MVH and chromatin was uniformly stained with DAPI and BrdU. Meiosis was basically marked by speckled staining pattern of BrdU. The GCN were bigger (E–G) and doublets were also observed with cytoplasmic DAZL and speckled BrdU (H–J). Note the differential MVH expression in the GCN and few cells expressing speckled BrdU (E). Few nests were observed with speckled BrdU expression in all the MVH positive cells (F, G) suggestive of a later stage of development. A cell doublet was observed with cytoplasmic DAZL and speckled BrdU suggesting it has broken down from a nest (H). Doublets with speckled BrdU and minimal DAZL expression were also observed (I–J). Co-expression of BrdU was studied along with SCP3 which marked cells undergoing meiosis. Figure 9 shows co-expression of SCP-3 with BrdU (A–B) during metestrus providing additional evidence to support prophase of meiosis 1 in adult ovary. Nuclear expression of BrdU was evident. The staining pattern is very dynamic in nature and had we made the smears 3–4 h earlier, we would have observed greater nuclear expression. Ovarian tissue collected after 11 days of BrdU injection, showed BrdU in the oocyte of a secondary follicle (D) whereas the negative control with omission of primary antibody showed no staining (C). These results are intriguing and suggest that stem cells took up BrdU, underwent mitosis/ meiosis, retained BrdU and matured into a secondary follicle within 11 days. Earlier, GFP positive oocytes have been reported to be formed within 1–2 weeks of transplantation [34].
BrdU staining in MVH and DAZL positive germ cells. 5A. A-D BrdU expression in MVH positive germ cells reveals mitotic/DNA repair/ mitochondrial replication. E-G MVH positive cells showed BrdU uptake in perinuclear area dispersed throughout the nucleus as speckled pattern suggestive of meiosis. Scale: A-B & E-J 3 µm, C-D 2 µm
Co-expression of SCP3 with BrdU (A-B) and BrdU in the oocyte of a secondary follicle in ovary section (D). Co-expression of BrdU and SCP3 was studied in late metestrus. Scale: A-B: 10 µm. D Co-expression of BrdU with MVH in an oocyte of secondary follicle whereas C negative control with omission of primary antibody showed no staining. MVH was cytoplasmic whereas BrdU showed both nuclear and cytoplasmic expression. Non-specific red color in D was essentially observed in between cells and granulosa cells remained negative
Apoptosis in the Developing Germ Cell Nests
After the dumping of cytoplasm and cytoplasmic organelles into the developing oocyte, the nurse cells regress and die and massive cell loss occurs in the fetal ovaries [23, 33]. Co-expression of PARP (apoptosis marker) and SSEA1 was studied in OSE cells smears (Fig. 10). Few GCN were visualized with PARP expressing cells whereas in some, a large cell was visualized negative for PARP which could be the possible future egg (Fig. 10A).
Co-expression of SSEA-1 with PARP to mark apoptosis within the nurse cells. A Dying cells indicated by the expression of nuclear PARP. B Note one of the cells in few of the GCN survives apoptosis does not express PARP and is destined to form the future oocyte. C-D PARP positive nests indicating the massive apoptosis happening in adult ovary. Cell smears for these experiments were prepared from mouse ovaries collected during metestrus. Scale: 3 µm
Fate of Transplanted VSELs In Vivo
To confirm the role of VSELs in oogenesis, FACS sorted SSEA-1 positive VSELs in the size range of 2–6 μm from GFP mice were transplanted in 2–3 months adult ovary (Suppl Fig. 21). GFP positive GCN in OSE cells smears were observed after 14 days of transplantation. Previous studies have shown that OSCs upon transplantation results in oocyte formation and birth of fertile pups [34]. Transplanted VSELs co-expressed SSEA-1 and GFP (Fig. 11) whereas the resident VSELs expressed SSEA-1 and remained negative for GFP (Suppl Fig. 22). GFP and SSEA-1 positive cell doublets were clearly observed. Besides SSEA-1 positive GCN expressing GFP (from transplanted VSELs) and negative for GFP (from tissue-resident VSELs) were observed. Suppl Figs. 23–24 shows different stages of GCN maturation. z-stack of a GFP + GCN with a developing oocyte (Suppl Fig. 23) whereas in Suppl Fig. 24 a putative oocyte is clearly observed. Note gradual loss of SSEA-1 as the GCN develop further (Suppl Fig. 24). Results confirm a crucial role of VSELs during neo-oogenesis in adult ovaries.
Differentiation potential of 2–6 µm, SSEA-1 and GFP positive, FACS sorted VSELs upon direct transplantation in Swiss mice ovaries. OSE cells smears of recipient mice were studied after 2 weeks of transplantation. A-B SSEA-1 positive cell doublets were observed which were GFP positive (A) and also GFP negative (B) implying both transplanted and tissue-resident stem cells divide. C-H GFP positive GCN were clearly observed which expressed SSEA-1 and (E) SSEA-1 expression was gradually lost as differentiation proceeded further and putative oocyte becomes evident
OSE Cells Culture and Live Cell Imaging
OSE cells were isolated and cultured overnight and later subjected to live cell imaging. Suppl Fig. 25 shows cells under an inverted microscope, epithelial-mesenchymal transition was clearly evident leading to the formation of a feeder layer. Stem cells were clearly visualized as small, spherical cells which remained non-adherent in vitro. These cells were subjected to live cell imaging, under an inverted microscope, and as shown in Suppl Fig. 26, the cells were observed in different stages of development. GCN of varying sizes were observed. Bigger oocyte was observed (Suppl Fig. 26D) along with a cluster of putative ‘nest cells’ undergoing cell death. GFP + ve GCN were observed in OSE cell smears prepared from OCT-4-eGFP mutant mice (Pou5f1-EGFP mice, Jackson Laboratory, USA). These mice are generally used to study GFP positive primordial germ cells and are not supposed to express GFP in adult tissues. But since VSELs are developmentally equivalent to primordial germ cells [4], GFP positive VSELs are expected to exist in adult ovaries and eGFP positive GCN were visualized (Suppl Fig. 27). MitoTracker (dye that stains mitochondria in live cells) expression was also observed in eGFP + OCT-4 + cluster of cells with nuclei counterstained with Hoechst (Suppl Fig. 28). Suppl Video 1 shows oocyte developing from a GCN. Oocyte/future egg grows in a nest and later detaches from it whereas egg in upper left corner seems to release zona pellucida and polar body. It is clearly evident from the video that the egg is newly formed from GCN and is not pre-existing.
Discussion
Key finding of the present study is the successful demonstration of various events whereby ovarian stem cells lodged in the OSE undergo differentiation, neo-oogenesis and follicle assembly in adult mice ovaries under physiological conditions without utilizing any genetic tool to track the events (Fig. 12). Ovarian VSELs undergo ACD to self-renew and give rise to slightly bigger OSCs which in turn undergo SCD and clonal expansion to form GCNs (by rapid proliferation and incomplete cytokinesis) and all these events were clearly evident in OSE cell smears and were stage-specific during estrus cycle. Maximal numbers of GCN in different stages of development were visualized in Estrus and Metestrus stage of the estrus cycle. We had earlier contradicted [35] the findings of Lei and Spradling [36] who denied the presence of stem cells and their differentiation into oocytes in adult mouse ovary, and had dissected out all the events in fetal mouse ovaries [33]. Present study was focused on the formation of GCN under physiological conditions in vivo and also gathered evidence to support various events suggestive of neo-oogenesis in the adult ovary. Also, we successfully show formation of a secondary follicle with BrdU positive nuclei after 11 days of BrdU injection which suggests that the oocytes formed by differentiation of stem cells are indeed functional. Potential of VSELs to form GCN is clearly demonstrated since GFP positive VSELs formed GCN upon transplantation. Contrary to the current understanding of the presence of a single wave of GCN formation during early development, we report regular formation of GCN from the ovarian stem cells (VSELs and OSCs) that undergo meiosis and eventually break down once every estrus cycle to form oocytes whereas the nurse cells undergo apoptosis under the influence of circulatory gonadotropin and ovarian hormones in adult mice ovaries resulting in regular neo-oogenesis and PF assembly (Fig. 12).
Our Postulate based on the results of the present study. We propose a model for PF assembly from the stem cells (VSELs and OSCs) in adult mouse ovary under physiological conditions. Quiescent VSELs undergo asymmetrical cell divisions to self-renew and give rise to OSCs which in turn undergo symmetrical cell divisions during diestrus and early proestrus. Gradually rising levels of FSH and elevated estradiol levels during PE cause OCT-4 positive OSCs to clonally expand to form GCN comprising of oogonial cells which remain interconnected through the TEX14 positive ring canals. Estradiol levels later decline concomitant with the upregulation of progesterone and LH and FSH surge by late PE during the ‘active dark period’. OSE smears prepared during diestrus, showed maximum ACD and SCD whereas smears taken during afternoon of proestrus showed oogonial nest. Elevated levels of FSH and LH during late PE are responsible for the expansion of pre-meiotic STRA-8 positive germ cells in the GCN. Meiosis occurs in the GCN during E and ME with expression of meiotic transcripts (Scp1, Spo11, Dmc1) and characteristic changes in the chromatin observed by BrdU staining. These GCN develop further, one of the cells gets identified as the oocyte and transfer of cytoplasmic organelles including mitochondria occurs from the sister cells into it through the ring canals resulting in the formation of a Balbiani Body. With falling estradiol levels in late metestrus, these nests break down to form oocytes which get surrounded by pre-granulosa cells to form new primordial follicle. Maximal numbers of PF in DE correspond to similar increase reported in ovarian sections. A newly formed oocyte can be distinguished by the presence of BB that later gets dispersed in the perinuclear region
Martin et al. [8] confirmed the presence of VSELs in adult mouse ovary as viable cells with surface phenotype of LIN-CD45-SCA-1 + but upon sorting, these cells were found negative for MVH. Authors argued that various studies reported so far in vitro focus on MVH positive OSCs and thus questioned the role of VSELs. Results of the present study showing ability of VSELs to form GCN should address this concern. Moreover, it is important to realize that OSCs can be expanded for months in vitro but they have a finite life span in vivo. Once a cell gets committed, it differentiates and enters a path of no return. VSELs are the most primitive and relatively quiescent stem cells that function in a subtle manner in the adult ovary to give rise to OSCs by undergoing regular ACD and OSCs further undergo SCD and GCN formation to further differentiate into oocytes. Thus, both VSELs and OSCs have crucial and specific roles to play in ovarian stem cells biology.
Results of the present study showed nuclear and surface/cytoplasmic MVH in SSEA-1 sorted cells (from bone marrow) and in BrdU positive germ cells/GCN in OSE smears. Using MVH to sort OSCs had earlier resulted in a controversy but MVH is expressed on cell surface as well as is cytoplasmic in the oocytes [37]. It will be ideal to study MVH expression in SSEA-1 sorted cells to get the full spectrum since nuclear MVH cannot be detected without permeabilization and this explains inability of Martin et al. [8] to detect MVH positive VSELs by flow cytometry. Excessive triton treatment could have led to non-specific nuclear MVH expression in the present study results, but this is not likely since BrdU positive cells express distinct nuclear and cytoplasmic MVH. Nuclear MVH positive small stem cells have been reported by other groups as well [38, 39] and PGCs also express nuclear MVH [40]. Similarly, DAZL is also expressed on PGCs, VSELs as well as on the OSCs.
Zou et al. (2009) cultured female germline stem cells (FGSCs/OSCs), transplanted in ovaries where they formed normal follicles and resulted in the birth of pups. Abban and Johnson (2009) wrote an editorial on this study and wondered the origin of FGSCs. Are FGSCs equivalent to PGCs or oogonia that survive in adult ovaries as such without forming cysts/primordial follicles assembly (arrested in prophase of meiosis 1). The authors argued that FGSCs could not be PGCs as they do not form teratoma whereas PGCs in culture (EGCs) readily form tumor. They suggested that FGSCs arise between the border of PGC and oogonia development and the initiation of germline cysts. We show FGSCs/OSCs arise from the VSELs (developmentally linked to PGCs) and various fetal events including GCN formation are indeed recapitulated in adult ovaries.
Increased FSH and E2 levels are responsible for the proliferation of germ cells whereas meiosis is understood to be regulated by increased LH and P in circulation [41]. We have earlier provided in vitro evidence showing FSH role in stimulating ovarian stem cells and GCN formation [18]. Mitotic to meiotic switch is an important event during gametogenesis and STRA8 has a crucial role [42]. Nuclear, longer isoform of STRA8 containing bHLH domain is crucial for meiotic initiation [42]. STRA8 has been reported across estrus cycle in mouse ovaries [20]. We presume that similar cues might be responsible for initial proliferation/clonal expansion of stem cells under influence of FSH and E2 whereas meiotic initiation in GCN and STRA8 expression might by associated with increased LH and P (Fig. 6). Results provide scope for further research to understand the variation across different stages of estrus cycle.
To conclude, ovarian stem cells express receptors for gonadotropin and ovarian hormones, show cyclic variation across estrus cycle and undergo normal proliferation, clonal expansion, differentiation resulting in neo-oogenesis followed by follicle assembly in adult life. We describe serendipitous findings and everything made sense once we started looking for estrus cycle specific changes. Injecting BrdU in any other stage other than metestrus/diestrus may not show its uptake in newly formed oocytes. How this process of neo-oogenesis and primordial follicle assembly gets affected with age and upon exposure to endocrine disruption is currently being investigated. Hopefully the results of the present study will encourage more research in the field and bring about a paradigm shift.
Availability of Data and Materials
All data is available in the manuscript and in supplement.
Change history
19 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12015-021-10284-x
References
Johnson, J., Canning, J., Kaneko, T., Pru, J. K., & Tilly, J. L. (2004). Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature, 428(6979), 145–150.
Bhartiya, D., & Patel, H. (2018). Ovarian stem cells-resolving controversies. Journal of Assisted Reproduction and Genetics, 35(3), 393–398.
Bhartiya, D., Shaikh, A., Anand, S., Patel, H., Kapoor, S., Sriraman, K., Parte, S., & Unni, S. (2016). Endogenous, very small embryonic-like stem cells: Critical review, therapeutic potential and a look ahead. Human Reproduction Update, 23(1), 41–76.
Ratajczak, M. Z., Ratajczak, J., & Kucia, M. (2019). Very small embryonic-like stem cells (VSELs): An update and future directions. Circulation Research, 124(2), 208–210.
Wagner, M., Yoshihara, M., Douagi, I., Damdimopoulos, A., Panula, S., Petropoulos, S., Lu, H., Pettersson, K., Palm, K., Katayama, S., Hovatta, O., Kere, J., Lanner, F., & Damdimopoulou, P. (2020). Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nature Communications, 11(1), 1147.
Bhartiya, D., & Sharma, D. (2020). Ovary does harbor stem cells-size of the cells matter! Journal of Ovarian Research, 13(1), 39.
Bhartiya, D., Kaushik, A., Singh, P., & Sharma, D. (2021). Will Single-Cell RNAseq decipher stem cells biology in normal and cancerous tissues? Human Reproduction Update, 27(2), 421.
Martin, J. J., Woods, D. C., & Tilly, J. L. (2019). Implications and current limitations of oogenesis from female germline or oogonial stem cells in adult mammalian ovaries. Cells, 8(2), 93.
Silvestris, E., Cafforio, P., D’Oronzo, S., Felici, C., Silvestris, F., & Loverro, G. (2018). In vitro differentiation of human oocyte-like cells from oogonial stem cells: Single-cell isolation and molecular characterization. Human Reproduction, 33(3), 464–473.
Clarkson, Y. L., McLaughlin, M., Waterfall, M., Dunlop, C. E., Skehel, P. A., Anderson, R. A., & Telfer, E. E. (2018). Initial characterization of adult human ovarian cell populations isolated by DDX4 expression and aldehyde dehydrogenase activity. Scientific Reports, 8(1), 6953.
Virant-Klun, I. (2015). Postnatal oogenesis in humans: A review of recent findings. Stem Cells and Cloning: Advances and Applications, 8, 49–60.
Woods, D. C., & Tilly, J. L. (2013). Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nature Protocols, 8(5), 966–988.
Parte, S., Bhartiya, D., Telang, J., Daithankar, V., Salvi, V., Zaveri, K., & Hinduja, I. (2011). Detection, characterization, and spontaneous differentiation in vitro of very small embryonic-like putative stem cells in adult mammalian ovary. Stem Cells and Development, 20(8), 1451–1464.
Virant-Klun, I., Zech, N., Rozman, P., Vogler, A., Cvjeticanin, B., Klemenc, P., Malicev, E., & Meden-Vrtovec, H. (2008). Putative stem cells with an embryonic character isolated from the ovarian surface epithelium of women with no naturally present follicles and oocytes. Differentiation, 76(8), 843–856.
Virant-Klun, I. (2018). Functional testing of primitive oocyte-like cells developed in ovarian surface epithelium cell culture from small VSEL-like stem cells: Can they be fertilized one day? Stem Cell Reviews and Reports, 14(5), 715–721.
Wang, N., Satirapod, C., Ohguchi, Y., Park, E. S., Woods, D. C., & Tilly, J. L. (2017). Genetic studies in mice directly link oocytes produced during adulthood to ovarian function and natural fertility. Scientific Reports, 7(1), 10011.
Guo, K., Li, C. H., Wang, X. Y., He, D. J., & Zheng, P. (2016). Germ stem cells are active in postnatal mouse ovary under physiological conditions. Molecular Human Reproduction, 22(5), 316–328.
Patel, H., Bhartiya, D., Parte, S., Gunjal, P., Yedurkar, S., & Bhatt, M. (2013). Follicle stimulating hormone modulates ovarian stem cells through alternately spliced receptor variant FSH-R3. Journal of Ovarian Research, 6, 52.
Patel, H., Bhartiya, D., & Parte, S. (2018). Further characterization of adult sheep ovarian stem cells and their involvement in neo-oogenesis and follicle assembly. Journal of Ovarian Research, 11(1), 3.
Lei, L., & Spradling, A. C. (2016). Mouse oocytes differentiate through organelle enrichment from sister cyst germ cells. Science, 352(6281), 95–99.
Pepling, M. E., Wilhelm, J. E., O’Hara, A. L., Gephardt, G. W., & Spradling, A. C. (2007). Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body. Proceedings of the National Academy of Sciences of the USA, 104(1), 187–192.
Pepling, M. E. (2012). Follicular assembly: Mechanisms of action. Reproduction, 143(2), 139–149.
Pepling, M. E., & Spradling, A. C. (2001). Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Developmental Biology, 234(2), 339–351.
Gamwell, L. F., Collins, O., & Vanderhyden, B. C. (2012). The mouse ovarian surface epithelium contains a population of LY6A (SCA-1) expressing progenitor cells that are regulated by ovulation-associated factors. Biology of Reproduction, 87(4), 80.
Kaushik, A., & Bhartiya, D. (2020). Additional evidence to establish existence of two stem cell populations including VSELs and SSCs in adult mouse testes. Stem Cell Reviews and Reports, 16(5), 992–1004.
Zuba-Surma, E. K., Kucia, M., Wu, W., Klich, I., Lillard, J. W., Jr., Ratajczak, J., & Ratajczak, M. Z. (2008). Very small embryonic like stem cells are present in adult murine organs: Image Stream based morphological analysis and distribution studies. Cytometry Part A, 73A, 1116–1127.
Vrtačnik, P., Kos, Š, Bustin, S. A., Marc, J., & Ostanek, B. (2014). Influence of trypsinization and alternative procedures for cell preparation before RNA extraction on RNA integrity. Analytical Biochemistry, 463, 38–44.
Rosas-Arellano, A., Villalobos-González, J. B., Palma-Tirado, L., Beltrán, F. A., Cárabez-Trejo, A., Missirlis, F., & Castro, M. A. (2016). A simple solution for antibody signal enhancement in immunofluorescence and triple immunogold assays. Histochemistry and Cell Biology, 146(4), 421–430.
Sriraman, K., Bhartiya, D., Anand, S., & Bhutda, S. (2015). Mouse ovarian very small embryonic-like stem cells resist chemotherapy and retain ability to initiate oocyte-specific differentiation. Reproductive Sciences, 22(7), 884–903.
Parte, S., Bhartiya, D., Patel, H., Daithankar, V., Chauhan, A., Zaveri, K., & Hinduja, I. (2014). Dynamics associated with spontaneous differentiation of ovarian stem cells in vitro. Journal of Ovarian Research, 7, 25.
Notarianni, E. (2011). Reinterpretation of evidence advanced for neo-oogenesis in mammals, in terms of a finite oocyte reserve. Journal of Ovarian Research, 4(1), 1.
Muñoz-Velasco, I., Ortíz, R., Echeverría, O. M., Escobar, M. L., & Vázquez-Nin, G. H. (2013). Characterization of the pre-meiotic S phase through incorporation of BrdU during spermatogenesis in the rat. The Journal of Histochemistry and Cytochemistry, 61(9), 680–689.
Lei, L., & Spradling, A. C. (2013). Mouse primordial germ cells produce cysts that partially fragment prior to meiosis. Development, 140(10), 2075–2081.
White, Y. A. R., Woods, D. C., Takai, Y., Ishihara, O., Seki, H., & Tilly, J. (2012). Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nature of Medicine, 18, 413–421.
Bhartiya, D., Sriraman, K., Parte, S., & Patel, H. (2013). Ovarian stem cells: Absence of evidence is not evidence of absence. Journal of Ovarian Research, 6(1), 65.
Lei, L., & Spradling, A. C. (2013). Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. Proceedings of the National Academy of Sciences, 110(21), 8585–8590.
Woods, D. C., White, Y. A., & Tilly, J. L. (2013). Purification of oogonial stem cells from adult mouse and human ovaries: An assessment of the literature and a view toward the future. Reproductive Sciences, 20(1), 7–15.
Wu, M., Xiong, J., Ma, L., Lu, Z., Qin, X., Luo, A., Zhang, J., Xie, H., Shen, W., & Wang, S. (2018). Enrichment of female germline stem cells from mouse ovaries using the differential adhesion method. Cell and Physiology Biochemistry, 46, 2114–2126.
Kenda Suster, N., & Virant-Klun, I. (2019). Presence and role of stem cells in ovarian cancer. World Journal of Stem Cells., 11(7), 383–397.
Toyooka, Y., Tsunekawa, N., Takahashi, Y., Matsui, Y., Satoh, M., & Noce, T. (2000). Expression and intracellular localization of mouse VASA-homologue protein during germ cell development. Mechanisms of Development, 93(1–2), 139–149.
He, B., Mi, Y., & Zhang, C. (2013). Gonadotropins regulate ovarian germ cell mitosis/meiosis decision in the embryonic chicken. Molecular and Cellular Endocrinology, 370(1–2), 32–41.
Kojima, M. L., de Rooij, D. G., & Page, D. C. (2019). Amplification of a broad transcriptional program by a common factor triggers the meiotic cell cycle in mice. eLife, 8, e43738.
Acknowledgements
We acknowledge the help provided by Dr SM Metkari at the Animal Experimental Facility, NIRRH for transplanting VSELs in mice ovaries. Help from Gayatri and Sushma for flow cytometry and Shobha, Reshma and Swati for confocal microscopy is acknowledged.
Funding
Financial support was provided by Indian Council of Medical Research, Government of India, New Delhi, India. DS acknowledges SRF fellowship from ICMR (#10832).
Author information
Authors and Affiliations
Contributions
DS performed the experiments, generated results, data interpretation and manuscript preparation. DB designed the study, arranged funds, data interpretation and manuscript preparation. Both authors agree to the final version of submitted manuscript.
Corresponding author
Ethics declarations
Ethical Approval
All experiments carried out in the present study were approved by NIRRH Institutional Animal Ethics Committee (78/GO/ReBi/SL/99/CPCSEA dated-11/03/1999).
Consent to Publish
Institute accession number NIRRH No. REV/1074/05-2021.
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The wrong Supplementary file for Supplementary file 1 was originally published with this article; it has now been replaced with the correct file.
Supplementary Information
Below is the link to the electronic supplementary material.
(AVI 486 KB)
Rights and permissions
About this article
Cite this article
Sharma, D., Bhartiya, D. Stem Cells in Adult Mice Ovaries Form Germ Cell Nests, Undergo Meiosis, Neo-oogenesis and Follicle Assembly on Regular Basis During Estrus Cycle. Stem Cell Rev and Rep 17, 1695–1711 (2021). https://doi.org/10.1007/s12015-021-10237-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-021-10237-4