Abstract
Mesenchymal stem/ stromal cell (MSC) exhaustion has been suggested to be a hallmark of aging. Osteoarthritis has a complex etiology that comprises several factors. Dysplasia has been shown to be an individual risk factor for osteoarthritis. Subchondral bone changes are often the first detectable alterations in osteoarthritis. In this study, we aimed to determine whether skeletal MSCs are differentially affected in patients with primary versus dysplastic osteoarthritis. Patients undergoing hip arthroplasty due to primary osteoarthritis (n = 11) and osteoarthritis with hip dysplasia (n = 10) were included in the study. Femoral head subchondral bone was used for isolation of MSCs. The cells were compared using detailed ex-vivo and in-vitro analyses, which included immunophenotyping, colony-forming-unit fibroblast assay, growth kinetics, senescence, multilineage potential, immunophenotyping, and MSC marker-gene expression profiling. Isolated cells from primary osteoarthritis patients showed decreased viability in comparison with those from dysplasia patients, with similar mesenchymal fractions (i.e., CD45/ CD19/ CD14/ CD34-negative cells). In-vitro expanded MSCs from primary osteoarthritis patients showed reduced osteogenic and chondrogenic potential in comparison with dysplasia patients. There were no differences in clonogenicity, growth kinetics, senescence, adipogenic potential, and immunophenotype between these groups. Gene expression profiling showed well-known marker of bone marrow MSCs, the leptin receptor, to be significantly lower for primary osteoarthritis patients. Our study shows that the pathology of primary osteoarthritis is accompanied by bone MSC exhaustion, while biomechanical dysfunction associated with hip dysplasia can induce secondary osteoarthritis without this MSC impairment. Our study suggests that subchondral bone MSC exhaustion is implicated in the pathology of primary osteoarthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several structures of adult synovial joints have been shown to harbor rare populations of cells that have tissue-regeneration properties. These unique cells are often referred to as mesenchymal stem/ stromal cells (MSCs), and they represent common progenitors for multiple connective tissues, such as bone, cartilage and adipose tissue. Joint-resident MSCs occupy several bone and joint cavity niches, which include subchondral bone [1], periosteum [2], synovium [3], adipose tissue [4], superficial cartilage [5], and others. The most well recognized are MSCs in mouse bone marrow [6]. Using transgenic mice, we can trace MSCs from early development of the musculoskeletal system throughout adult life, and determine their roles in health and disease [7]. This allowed identification of several rare subpopulations of MSCs that can contribute to cartilage and fracture repair in adult life. Gremlin1 expression identifies distinct connective-tissue MSCs in the metaphysis of long bones that are needed for bone development, bone remodeling, and fracture repair [8]. Leptin receptor identifies MSCs that are the major source of bone and adipocytes in adult bone marrow, and that also regenerate bone after irradiation and fractures [9]. Endeavors to identify human skeletal MSCs were recently successful, with the identification of self-renewing and multipotent human skeletal stem cells that can generate progenitors of bone, cartilage and stroma, but not of fat [10].
Our increasing understanding of native MSCs in the joint microenvironment provides new hope to find strategies to exploit the regenerative features of these cells in degenerative disorders like osteoarthritis. However, to exploit endogenous MSCs, we need to first identify their roles in joint degeneration. There is some evidence that bone marrow-derived MSCs from patients with osteoarthritis have reduced chondrogenic and adipogenic activities in vitro [11]. In addition, the proportion of CD271 positive MSCs is higher in femoral head bone-marrow lesions in hip osteoarthritis patients [1]. Accumulation of CD271-positive cells was also noted in bone adjacent to cartilage defects, and in areas of osteochondral angiogenesis. Recently, two different MSCs phenotypes have been shown in cartilage of osteoarthritis patients: one with preferential chondrogenic potential, and the other with osteogenic potential [5].
Osteoarthritis is a disease of the whole joint, to which changes in cartilage, bone and bone marrow, synovium, menisci, ligaments, and neural tissue contribute [12]. Although pathological processes might selectively target a single joint tissue, ultimately cartilage and subchondral bone will be affected because of their biological and physical cross-talk [13]. Subchondral bone changes with increased metabolism and sclerosis are often the first detectable alterations in the osteoarthritis process [13].
Although osteoarthritis presupposes interactions of systemic and/ or local factors, congenital and developmental malformations, such as hip dysplasia, have been shown to constitute an individual risk factor for premature degeneration and osteoarthritis [14, 15]. The more severe the dysplasia the earlier the likely development of osteoarthritis. Biomechanics is of utmost importance for joint homeostasis. Abnormal joint biomechanical stress is likely to make a joint environment hostile, even if the native MSC repair strategies function optimally [16].
Based on these findings, we hypothesized that skeletal MSCs in primary osteoarthritis patients would have alterations that show their exhaustion and decreased regenerative potential. On the other hand, MSCs derived from dysplasia patients would show superior features, which would suggest that osteoarthritis in these patients is a consequence of abnormal joint biomechanical stress.
To test this hypothesis, we compared skeletal MSCs from patients with primary hip osteoarthritis and patients with osteoarthritis as a consequence of hip dysplasia. We show that MSCs in primary osteoarthritis patients have inferior biological properties compared to those of dysplasia patients, including lower viability, osteogenesis and chondrogenesis. MSCs from primary osteoarthritis patients also showed lower expression of the leptin receptor, a well-known marker of bone-marrow MSCs that has been shown to contribute to fracture repair in adult organisms.
Methods
Donor Inclusion and Bone Sampling
Patients undergoing routine total hip arthroplasty at the Valdoltra Orthopaedic Hospital (Ankaran, Slovenia) were included in this study. Osteoarthritis and dysplasia were diagnosed by clinical examinations and plain X-rays (Fig. 1a). The exclusion criteria included history of inflammatory arthritis, metastatic cancer, and disorders that affect bone. Approval for this study was obtained from the National Medical Ethics Committee of the Republic of Slovenia (reference numbers: 0120–523/2016–2, KME 45/10/16). Written informed consent to participate in this study was obtained from all of donors. Detailed patient information is provided in Table 1. There were no differences in age, sex, body mass index, C-reactive protein levels or blood monocytes between the patient groups.
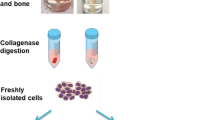
Overview of the donors included in the study, and the tissue harvesting and study protocol. (a) Representative X-ray of the donors undergoing hip replacement surgery included in the study. (b) Scheme of sampling site (indicated by dashed cylinder shape) for subchondral trabecular bone. (c) Scheme of isolation of the bone MSCs, and their ex-vivo and in-vitro analyses. OA, osteoarthritis
A cylindrical section of trabecular bone with bone marrow was sampled from all of the patients from the femoral neck side of the femoral head, as shown on Fig. 1b, using a TRAP8G system (H.S. Hospital Services). The bone biopsy samples were ~15 mm in length (Fig. 1b, c, top). The bone biopsies were immediately placed in growth medium of low glucose Dulbecco’s modified Eagle’s medium (DMEM; Biowest; Cat# L0064–500) supplemented with 1% glutamine (Biowest; Cat# X0550–100), 2% penicillin and streptomycin (Biowest; Cat# L0022–020) and 10% fetal bovine serum (Gibco; Cat# 10270–106).
Cell Isolation
Primary MSCs were isolated from bone biopsies following previously published protocols [17], with some modifications. Briefly, the cylindrical bone sections were washed three times in sterile phosphate buffered saline (PBS) to remove visible blood cells. The sections were cut with a scalpel into smaller fragments, which were then digested in 1 mg/mL collagenase D (Roche; Cat# 11088882001) for 3 h at 37 °C. The digested cells were filtered through a 70-μm nylon filter strainer (Corning). An additional 10 mL fresh media was added to the bone remnants, which were then vortexed for 10 s. This was repeated two more times. The pooled suspensions (~30 mL) were centrifuged at 300×g for 10 min. After the final centrifugation, the cell pellets from bone tissue were resuspended in 1.0 mL MSC medium (Expansion Media Kit XF, human; Miltenyi Biotec; Cat# 130–104-182) supplemented with 2% penicillin and streptomycin and 1% glutamine. Aliquots of freshly isolated cells (100 μL) were used for immunophenotyping, and the rest of the samples (900 μL) were seeded as described below. The cells were incubated at 37 °C in a humidified atmosphere of 5% CO2 and O2. The study design and the analyses are summarised in Fig. 1C.
Immunophenotyping
Flow cytometry was performed on a small aliquot of freshly isolated cells, and on the culture-expanded MSCs between passages 3 and 5 (p3-p5). The samples were resuspended in cell dissociation buffer (2 mM EDTA [Sigma; Cat# 60–00-4] in PBS with 0.5% fetal bovine serum). Before staining, the freshly isolated cells were treated with BD FACS Lysing solution (BD Biosciences; Cat# 349202) for 10 min, and then incubated with monoclonal antibodies against CD45 (Thermo Fischer Scientific, Cat# 48–9459-42; 2D1), CD19 (Thermo Fischer Scientific; Cat# 48–0198-42; SJ25C1) and CD14 (Thermo Fischer Scientific; Cat# 48–0149-41; 61D3), all of which were conjugated with eFluor 450 and CD34 VioBlue (Miltenyi Biotec; Cat# 170–080-023; AC136). The culture-expanded MSCs were immunophenotyped using anti-CD105 FITC (Milteny Biotec; Cat# 130–112-169; REA794), anti-CD90 FITC (Milteny Biotec; Cat# 130–117-684; DG3) and anti-CD73 APC antibodies (Miltenyi Biotec; Cat# 130–095-183; AD2). The fixable viability dye eFluor 780 (Thermo Fisher Scientific; Cat# 65–0865) was used to determine cell viability. The cells were resuspended in PBS and analyzed using a flow cytometer (Attune NxT; Thermo Fisher Scientific).
Colony-Forming-Unit Fibroblast Assay
The colony-forming-unit fibroblast (CFU-F) assay was performed using freshly isolated MSCs plated as nine replicates in six-well plates. Once colonies were observed (i.e., from 10 to 14 days), six wells were trypsinized and the viable MSCs were counted. The remaining three wells were stained with methyl violet (Merck; Cat# 115940) to count the colonies. The CFU-F assay data at p0 were calculated as percentages of methyl-violet-positive colonies per cells counted. The CFU-F assays were repeated at p1. MSCs were seeded at 2000 cells/well in six-well plates in three replicates. The numbers of colonies were counted after 12–14 days. The data for the CFU-F assays at p1 were calculated as percentages of methyl-violet-positive colonies per seeded MSCs.
Cell-Growth Kinetics
For the growth rates and doubling times, the MSCs were seeded at p1 to p3 as four replicates in 12-well plates at 5000–10,000 cells/cm2. For the cumulative population doubling, the MSCs were seeded at p1 and counted every 10 days. The mean cell counts were used to calculate the population doubling for each sample according to Equation: 3.32*(logN2-logN1),
where N1 is the number of seeded cells, and N2 is the number of counted cells, at each passage.
Senescence Analysis
For the senescence analysis, the cells were seeded in three or four replicates in 12-well plates at 5000 cells/cm2. After 2–3 days, when the cultures reached approximately 30% confluence, the senescence was evaluated using senescence β-galactosidase staining kits (Cell Signaling Technology; Cat# 9860), following the manufacturer instructions. The wells were imaged using a microscope (Primovert) mounted with a digital camera (AxioCam ICc5; Zeiss). The numbers of senescent cells and the total numbers of cells were counted independently by two investigators. Senescence is expressed as the senescence-associated β-galactosidase-positive cells as percentages of the total numbers of cells.
Multilineage Differentiation
Adipogenic and Osteogenic Differentiation
Osteogenic and adipogenic differentiation was assessed histologically and at the gene expression level. For each analysis, the cells were seeded in four replicates in 24-well plates at 25,000 cells/cm2. Two of each four replicates were used for histological assessment (one as treated, the other as control), and the remaining two replicates for RNA isolation and gene expression analysis (one as treated, the other as control). After reaching confluence, the growth medium for the treated wells was replaced with either adipogenic or osteogenic medium. Adipogenic medium consisted of growth medium supplemented with 500 nM dexamethasone (Sigma; Cat# 50–02-2), 10 μM indomethacine (Sigma; Cat# 53–86-1), 50 μM iso-butylmethyl xanthine (Sigma; Cat# 28822–58-4), and 10 μg/mL insulin (Sigma; Cat# 11061–58-0). Osteogenic medium consisted of growth medium supplemented with 5 mM β-glycerophosphate (Sigma; Cat# 154804–51-0), 100 nM dexamethasone, and 50 mg/mL ascorbic acid-2-phosphate (Sigma; Cat# 56170–10-3). Control wells received growth media without supplements. The protocol was continued for 21 days with media changes every 2 days to 3 days. Adipogenic differentiation was assessed using Oil Red O (Sigma; Cat# 1320-06-5) histochemistry. After staining, the wells were imaged (Evos XL, LifeTechnologies), and the number of Oil-Red-O-positive adipocytes was counted using the ImageJ software [18]. The adipogenic efficiency was expressed as the number of Oil-Red-O-positive adipocytes per number of cells originally seeded. Osteogenesis was assessed using Alizarin Red S (Sigma; Cat# 130–22-3) histochemistry. The wells were imaged (Evos XL, LifeTechnologies). The bound Alizarin Red S was subsequently extracted using acetic acid, and quantified as described previously [19].
Chondrogenic Differentiation
Chondrogenic pellets were formed as two replicates (one treated, one control) by resuspending 150,000 cells in chondrogenic medium, which consisted of high glucose DMEM (Biowest; Cat# L0106–500), 100 nM dexamethasone, 1% insulin–transferrin–selenium (Sigma; Cat# I221), 50 mg/mL ascorbic acid-2-phosphate, and 1% penicillin/ streptomycin. The cell suspensions were centrifuged for 5 min at 380×g in 15-mL conical tubes. The cell pellets were then incubated at 37 °C, in 5% CO2 for 24 h, after which the chondrogenic medium was supplemented with 10 ng/mL TGF-β1 (Thermo Fisher Scientific; Cat#PHG9204). The control pellets received medium without TGF-β1. The protocol was continued for 21 days, with medium changes every 2 days to 3 days. After 21 days, the pellets were washed with PBS and fixed in 10% neutral buffered formalin (Sigma; Cat# HT50–1-320) for 20 min. The pellets were then transferred to 15% sucrose (Sigma; Cat# 57–50-1) solution for 1 h, and after that to a 30% sucrose solution overnight. The pellets were then embedded in Tissue Freezing Medium (Leica; Cat# 14020108926), snap frozen in liquid nitrogen, and cut into 8-μm-thick sections using a cryostat (Leica). Cryosections were used for toluidine blue (Sigma; Cat# 6586-04-5) staining and for immunofluorescence for collagen type II (Col2). For the Col2 staining, a goat anti-Col2 antibody conjugated with Alexa Fluor 488 (SouthernBiotech; Cat# 1320–30) was used (dilution, 1:50). The slides were mounted with Prolong Gold Antifade Reagent with DAPI (Life Technologies; Cat# P36941), and imaged (Evos FL; Life Technologies). The chondrogenic potential was evaluated as the positive toluidine blue or Col2 staining, and the toluidine blue stained sections were also evaluated using the Bern score [20].
RNA Isolation and Gene Expression Profiling
Total RNA was isolated from cells subjected to adipogenesis and osteogenesis, as well as during MSC culture expansion, using peqGOLD Total RNA kits (VWR; Cat# 732–2868). cDNA was synthesized using High-Capacity cDNA Reverse Transcription kits (Thermo Fisher Scientific; Cat# 4368814). Gene expression analysis was performed according to the MIQE guidelines [21]. The primer sequences used in the quantitative PCR (qPCR) are given in Supplementary Table S1. The qPCR was performed using 5× HOT FIREPol EvaGreen qPCR Supermix (Solis BioDyne; Cat# 08–24-00020) and gene-specific primers (Macrogen, Sigma-Aldrich) that were optimized to produce the specific PCR products. To select the most stable reference genes between the treated cells and the controls for adipogenesis and osteogenesis, seven commonly used reference genes were tested in a subset of 32 samples (i.e., β-actin, ribosomal protein 13a, β2-microglobulin, glyceraldehyde-3-phosphate dehydrogenase [GAPDH], ribosomal protein large P0, glucoronidase-β, ubiquitin C), with the best combination of three genes selected with NormFinder [22]. All of the PCR amplifications were performed in triplicate in a 15-μL reaction volume using a LightCycler 480 Instrument II (Roche). The gene expression data were obtained using the standard curve and the second derivative maximum method (LightCycler 480 software, version 1.5.0). With the demonstration that the gene expression data for a subset of 10 samples of MSCs under osteogenesis do not differ when normalized to the three best reference genes as selected by NormFinder (Supplementary Fig. S1) in comparison to GAPDH, all of the data were normalized to GAPDH.
Statistical Analysis
The normalities of the data distributions were tested with Shapiro-Wilk tests. To compare data between the primary osteoarthritis patients and patients with dysplasia, either Student’s t-tests or Mann–Whitney tests were used. Where the groups compared had unequal variances and unequal sample sizes, Welch’s t-tests were used. Two-way ANOVA with Bonferroni corrections for multiple testing was used for the gene expression and in-vitro immunophenotyping analysis. All statistical analyses were performed using GraphPad Prism, version 6.07 for Windows (GraphPad Software, La Jolla, CA, USA; www.graphpad.com. P values <0.05 were considered as statistically significant. The Figures were created using Mind the Graph.
Results
Skeletal MSCs from primary osteoarthritis patients show impaired viability Ex Vivo
The viability of the freshly isolated cells from primary osteoarthritis patients was significantly lower in comparison with the cells derived from patients with dysplasia (p = 0.0311; Fig. 2A). The proportions of the freshly isolated cells that were negative for the combination of the CD45/ CD19/ CD14/ CD34 markers were similar between the two cell groups (Fig. 2B).
Ex-vivo analyses of the freshly isolated cells. (a) Viability of the collagenase-digested cells before plating, as the proportions of single live cells. Significantly improved viability was seen for cells from the patients with dysplasia (Mann-Whitney test). (b) Mesenchymal lineage cells defined as the CD45/ CD34/ CD14/ CD19-negative fraction in the freshly isolated cells was obtained using flow cytometry. No differences in mesenchymal lineage was observed between the two groups of patients. OA, osteoarthritis
Skeletal MSCs from primary osteoarthritis and dysplasia patients show similar culture-expansion properties In Vitro
Primary cultures of skeletal MSCs were successfully established for 17 out of 21 donors. In four cases (one with primary osteoarthritis, three with dysplasia), the cultures had to be discarded due to bacterial or fungal contamination. There were no differences in CFU-F data between the tested groups at p0 (Fig. 3a, left) or p1 (Fig. 3a, middle). Culture expanded MSCs showed similar growth rates (Fig. 3b, left), doubling times (Fig. 3b, middle), and number of cumulative population doublings (Fig. 3c, right). The MSCs from both of these groups also showed fibroblast-like morphology and similar proportions of senescent β-galactosidase–positive cells (Fig. 3c).
Culture expansion of MSCs. (a) Colony-forming-unit fibroblast (CFU-F) assays at p0 (left) and p1 (middle), which show no differences between the two groups of patients. Representative images of bone-derived MSCs at p0 showing colonies stained with methyl violet for quantification in the CFU-F assays. (b) Culture expanded bone-derived MSCs showed no differences in growth rate (left), doubling time (middle), and mean cumulative population doublings (right) between the two groups of patients. (c) Representative images of senescence-associated β-galactosidase staining for MSCs from both groups. Scale bars: 200 μm. OA, osteoarthritis
Skeletal MSCs from primary osteoarthritis patients show inferior osteogenesis and chondrogenesis In Vitro
To demonstrate the multipotency of the culture-expanded MSCs, in-vitro adipogenic, osteogenic, and chondrogenic differentiation assays were performed (Fig. 4). MSCs from primary osteoarthritis patients showed similar adipogenic potential to those from patients with dysplasia (Fig. 4a). In contrast, the MSCs from primary osteoarthritis patients showed inferior osteogenesis (Fig. 4b) and chondrogenesis (Fig. 4c), compared to those from dysplasia patients. MSCs from primary osteoarthritis patients showed significantly lower levels of Alizarin Red S (Fig. 4b, left, right), while the expression of the osteogenesis-specific genes was similar between the two groups (Fig. 4b, middle). Potentially, after 21 days of osteogenic treatment, the expression of the selected osteogenic genes did not adequately reflect the rate of mineralization measured with Alizarin Red S. There was significantly lower Bern score in the group of patients with primary osteoarthritis (Fig. 4c, upper row, left). The diameters of the chondrogenic pellets tended to be lower for the primary osteoarthritis patients in comparison to those with dysplastic osteoarthritis (Fig. 4c, upper row, middle); however, this difference did not reach statistical significance (p = 0.056). Moreover, the percentage of toluidine blue and Col2-positive chondrogenic pellets was lower in patients with primary osteoarthritis (Fig. 4c, lower row, left).
Multilineage differentiation of MSCs. (a) Chondrogenesis assessments using toluidine blue (bottom left) and collagen type II (Col2) staining (bottom right) showed significant differences between MSCs from both patient groups. Subchondral-bone-derived MSCs from patients with primary osteoarthritis (OA) showed significantly lower Bern score (upper left, Welch’s t-tests). The percentage of toluidine blue and Col2-positive pellets were also lower in this group of patients (upper middle). No differences in chondrogenic pellet diameter were seen between patients with primary osteoarthritis (OA) or osteoarthritis with hip dysplasia, i.e., dysplastic OA (upper right, Welch’s t-tests). (b) Osteogenesis assessments using quantification of Alizarin Red S staining (left, Welch’s t-tests) was significantly lower in MSCs from patients with primary osteoarthritis (OA). Analysis of osteogenic gene expression (centre, two-way ANOVA) showed no differences between MSCs from the two patient groups. Representative images for Alizarin Red S staining analysis for MSCs from the two patient groups (right). (c) Adipogenesis assessments using quantification of Oil Red O staining (left, Welch’s t-tests) and analysis of adipogenic gene expression (centre, two-way ANOVA) were similar between the MSCs from the patient groups. Representative images for Oil Red O staining analysis are shown (right). Scale bar in all panels: 200 μm. OA, osteoarthritis. ALP, Alkaline phosphatase, RUNX2, Runt-related transcription factor 2, COL1A1, Collagen type 1 alpha 1, OC/ BGLAP, Osteocalcin/ Bone gamma-carboxyglutamate protein, PPARG, peroxisome proliferator-activated receptor gamma, ADIPOQ, Adiponectin, C1Q and collagen domain containing, FAPB4, Fatty acid binding protein 4
Skeletal MSCs from primary osteoarthritis and dysplasia patients show similar In-Vitro immunophenotypes
To further determine whether these culture-expanded cells had features of MSCs in vitro, their immunophenotypes were determined, according to the International Society of Cellular Therapy (ISCT) [23] (Fig. 5). Both groups showed similar immunophenotypes for the skeletal MSCs, with high expression of the positive markers CD73, CD90, and CD105, and low expression of the negative markers CD45, CD14, and CD19 (Fig. 5), as indicated by the ISCT guidelines [23]. The criteria set by the ISCT require that positive markers are expressed by >95% of all of the cells. This was only achieved for CD73 and CD90 for the primary osteoarthritis patients (means: CD73, 95.8%; CD90, 95.7%), while the MSCs from osteoarthritis and dysplasia patients showed lower expression of these markers (means: CD73, 91.4%; CD90, 84.6%) (Fig. 5a, b). CD105 was low in both groups (means: primary osteoarthritis, 83.5%, osteoarthritis and dysplasia, 84.6%). The ISCT criteria also suggest that <2% of the cells should express the negative markers; this was fulfilled by the MSCs from both groups (primary osteoarthritis, 0.104%; osteoarthritis and dysplasia, 1.122%; Fig. 5b).
In-vitro immunophenotype of MSCs. Immunophenotyping for CD73, CD90, and CD105 (a) and negative markers CD45, CD14, and CD19 (b) of in-vitro expanded cells between p1 and p5. No significant differences were observed for positive markers (two-way ANOVA) and negative markers (Welch’s t-tests). OA, osteoarthritis
Skeletal MSCs from Primary Osteoarthritis Patients Show Lower Gene Expression of the Leptin Receptor MSC Marker
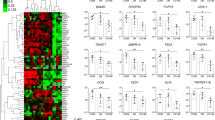
To determine whether there is a preferential MSC subpopulation that expands in vitro in these particular patient groups, the gene expression profiles of 10 previously identified MSC subpopulation markers were compared between these groups (Fig. 6a). The data for the multitest comparison showed that LEPR expression was significantly lower for primary osteoarthritis patients (mean, 0.019) compared to dysplasia patients (mean, 4.420) (Fig. 6).
Discussion
Despite numerous efforts to reveal the pathophysiological mechanisms of primary osteoarthritis, no concrete targets that can be used to slow down joint degeneration have been identified to date. As osteoarthritis is a “wear-and-tear” disorder that ultimately results in degeneration of the entire joint, regenerative approaches that target exhausted or impaired MSCs might provide new promise for future treatments of this major cause of disability in adults.
Synovial joints in adult organisms have been shown to harbor rare populations of MSCs within several tissue structures [7]. Bone marrow is currently the most well-recognized site of these progenitors [8, 9, 16, 17]. In general, tissue repair and regeneration require a viable pool of MSCs. Stem cell exhaustion and the changes in the regenerative potential of MSCs have been previously associated with osteoarthritis [1, 5, 11, 24]. Subchondral bone has often been identified as the initiator of early osteoarthritis changes in joints [12, 13]. Impaired joint biomechanics, in particular joint dysplasia, has also been shown to be an independent risk factor for osteoarthritis [14, 15].
Based on these findings, we hypothesized that primary osteoarthritis might be the consequence of the exhaustion of skeletal MSCs derived from subchondral bone. To this end, we performed detailed ex-vivo and in-vitro analyses of MSCs derived from subchondral trabecular bone; i.e., skeletal MSCs from two groups of distinct etiology of hip osteoarthritis patients, as primary osteoarthritis patients versus hip dysplasia osteoarthritis patients.
Our data indicated inferior biological properties of freshly isolated and culture-expanded skeletal MSCs from primary osteoarthritis patients. These included significantly lower ex-vivo viability and poorer osteogenic and chondrogenic potential. Gene expression profiling for previously identified MSC subpopulation markers revealed that altered features of skeletal MSCs in primary osteoarthritis patients might be associated with lower expression of the leptin receptor, a well-known marker of bone replenishing MSCs in adult bone marrow [9].
Previously MSCs from patients with osteoarthritis have been compared to normal donors without any signs of osteoarthritis [11]. Murphy et al. showed that bone-marrow-derived MSCs in osteoarthritis patients have reduced proliferative capacity as well as chondrogenic and adipogenic potential, in comparison with healthy controls [11]. These were bone- marrow MSCs derived from iliac crest, tibia, or femur. Given that bone-marrow MSCs become virtually identical in vitro to trabecular-bone MSCs isolated with collagenase [17], our data can be compared to this study. Similarly, we observed lower chondrogenesis associated with primary osteoarthritis; however, Murphy et al. used glycosaminoglycan quantification, while we used Bern scores, although the collagen type II analyses were similar.
With regard to proliferation of the culture-expanded MSCs, the cells from both groups of patients reached much lower mean population doublings in comparison with what has been reported in the literature for MSCs derived from healthy individuals [25]. Therefore, our data build on a previously identified difference in MSCs derived from healthy donors and from patients with osteoarthritis, further suggesting stratification of osteoarthritis patients based on the properties of their subchondral-bone-derived MSCs.
Comparing MSCs derived from bone marrow lesions with those derived from healthy regions of subchondral bone in patients with late-stage hip osteoarthritis, functional and gene expression perturbations were reported for MSCs from lesions [1]. These included higher numbers of CD45-negative and CD271-positive MSCs; however, these cells had lower proliferation and mineralization capacities in vitro. In the present study, MSCs from primary osteoarthritis patients also showed lower osteogenesis. Similar to our study, no differences were seen for clonogenicity of the MSCs between these two groups. We also screened for the CD271 marker, which was shown here to be associated with the bone-marrow lesions; however, we did not see any differences between these for our groups of patients. The regions of subchondral bone where bone-marrow lesions occur have been associated with greater trabecular bone area and cartilage damage [1]. This evidence raises the possibility that the increase in bone density and loss of cartilage that are characteristic of osteoarthritis might result from changes in MSCs, which are believed to be responsible for the homeostatic maintenance of the joint tissues. These MSCs that have altered features might initiate or escalate joint damage. Age-dependent decreases in proliferation and osteoblast differentiation and increases in senescence-associated β-galactosidase–positive cells and apoptosis have also been shown in bone-marrow-derived MSCs obtained from patients undergoing primary hip arthroplasty [26].
Looking at the immunophenotypes of the MSCs, we observed large variations in the expression of the common positive markers, in particularly for CD105. The ISCT recommends that the minimal expression of these markers to be >95% [23]. However, according to the US Food & Drug Administration, many studies have reported much lower values, such as 87% ±7% for CD90, 86% ±7% for CD73,and 88% ±8% for CD105 [27]. Kohno et al. reported values of CD105 as low as 62.7% in synovial MSCs from osteoarthritis patients, without apparent loss of multilineage capabilities [28], which was similar to the present study.
Skeletal stem cells have been clearly identified in mouse bone marrow [6, 8, 9], and very recently in humans as well [10]. Chan et al. suggested that mouse skeletal stem cells would be negative for CD45, Ter119, Tie2, Thy, 6C3, and CD105, and positive for integrin subunit αV (ITGAV/CD51) and CD200 [6]. Very recently, human skeletal stem cells were identified as negative for CD45, CD235, TIE2, CD31, and CD146, and positive for PDPN, CD73, and CD164 [10]. The contribution of this cell population to osteoarthritis pathology is yet to be established. In the current study, we screened for 10 markers of previously identified subpopulations of MSCs that have shown roles in replenishing bone and cartilage in adulthood. These included Gremlin1 [8], leptin receptor [9], ITGAV [6], platelet-derived growth factor receptor (PDGFR)α, PDGFRβ, chondroitin sulfate proteoglycan 4 (CSPG4/NG2) [29, 30], and nerve growth factor receptor (NGFR/CD271) [1], and some populations identified in nonskeletal tissues, such as PEG3 (paternally expressed 3, PW1) [31], neural cell adhesion molecule 1 (NCAM1/CD56) [32], and sirtuin 1 (SIRT1), a factor required for chondrogenic differentiation of MSCs [33, 34]. Multiple comparisons showed that only LEPR was differentially expressed between the cell groups tested here. Interestingly, there was significantly lower expression of leptin receptor in MSCs from the primary osteoarthritis patients compared to those from the patients with dysplastic osteoarthritis. Subpopulations of leptin-receptor-expressing MSCs have been shown to give rise to most bone and adipocytes formed in adult bone marrow, including bone regenerated after irradiation or fracture [9].
The major drawback of this study is that we were not able to perform all of the analyses for the complete study cohort, as shown in Table 1. Isolation and culture expansion of primary cells is a tedious procedure and it is difficult to expand sufficient quantities of the cells at similar passages to perform multiple analyses. However, this is a genuine situation and the size of the present study cohort is comparable to previous studies [1, 5, 11, 28]. Additionally, the groups compared in the present study were balanced in the factors that have been suggested to influence MSCs, such as, in particular, the age of the donor. Another drawback is the lack of healthy control donors in our study. These would need to be age and sex matched with no degenerative joint disorders. Ethical issues associated with obtaining subchondral bone from femoral head of healthy donors that would be needed for unbiased comparison are reasonable. Additionally, a previous study showed that bone-marrow-derived MSCs from iliac crest, tibia, or femur of patients submitted to knee or hip arthroplasty due to osteoarthritis have reduced proliferation and adipogenic and chondrogenic potential, in comparison with normal donors without any signs of osteoarthritis [11].
In the current study we have only focused on bone-derived MSCs, based on previous suggestions of their role in osteoarthritis and the considerable amount of knowledge for the identification of skeletal MSCs. Bearing in mind that osteoarthritis is a disorder of the whole joint, MSCs derived from other joint structures, such as synovium, cartilage, and tendons, might have roles in osteoarthritis etiology as well [16].
The significance and novelty of our findings is that skeletal MSCs are impaired to some extent in primary hip osteoarthritis in comparison to dysplastic osteoarthritis. They show lower ex-vivo viability and poorer osteogenic and chondrogenic potential. These features might be associated with lower expression of the leptin receptor MSC marker. Leptin-receptor-expressing MSCs have been identified as major subpopulations of MSCs that form bone, cartilage, and adipocytes in adult life. In primary osteoarthritis patients, leptin-receptor-expressing MSCs might be depleted, which would suggest exhaustion of endogenous regenerative potential as their contribution to osteoarthritis etiology.
Conclusions
To summarize, our study provides evidence that subchondral skeletal MSC exhaustion might be important in primary osteoarthritis pathology, whilst biomechanical dysfunction, such as dysplasia, can induce secondary osteoarthritis without MSC dysfunction. MSC insufficiency would therefore represent a novel target to tackle this degenerative disorder in primary osteoarthritis.
References
Campbell, T. M., Churchman, S. M., Gomez, A., McGonagle, D., Conaghan, P. G., Ponchel, F., & Jones, E. (2016). Mesenchymal stem cell alterations in bone marrow lesions in patients with hip osteoarthritis. Arthritis & Rheumatology, 68, 1648–1659. https://doi.org/10.1002/art.39622.
De Bari, C., & Dell ‘Accio, F., Karystinou, A., et al. (2008). A biomarker-based mathematical model to predict bone-forming potency of human synovial and periosteal mesenchymal stem cells. Arthritis and Rheumatism. https://doi.org/10.1002/art.23143.
De Bari, C., & Dell ‘Accio, F., Tylzanowski, P., Luyten, F. P. (2001). Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis and Rheumatism. https://doi.org/10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P.
Xu, L., Liu, Y., Sun, Y., Wang, B., Xiong, Y., Lin, W., & Li, G. (2017). Tissue source determines the differentiation potentials of mesenchymal stem cells: A comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Research & Therapy, 8, 1–11. https://doi.org/10.1186/s13287-017-0716-x.
Jayasuriya, C. T., Hu, N., Li, J., Lemme, N., Terek, R., Ehrlich, M. G., & Chen, Q. (2018). Molecular characterization of mesenchymal stem cells in human osteoarthritis cartilage reveals contribution to the OA phenotype. Scientific Reports, 8, 1–14. https://doi.org/10.1038/s41598-018-25395-8.
Chan, C. K. F., Seo, E. Y., Chen, J. Y., et al. (2015). Identification and specification of the mouse skeletal stem cell. Cell. https://doi.org/10.1016/j.cell.2014.12.002.
Čamernik, K., Barlič, A., Drobnič, M., Marc, J., Jeras, M., & Zupan, J. (2018). Mesenchymal stem cells in the musculoskeletal system: From animal models to human tissue regeneration? Stem Cell Reviews and Reports. https://doi.org/10.1007/s12015-018-9800-6.
Worthley, D. L., Churchill, M., & Compton, et al. (2015). Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. https://doi.org/10.1016/j.cell.2014.11.042.
Zhou, B. O., Yue, R., Murphy, M. M., Peyer, J. G., & Morrison, S. J. (2014). Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. https://doi.org/10.1016/j.stem.2014.06.008.
Chan, C. K. F., Gulati, G. S., Sinha, R., et al. (2018). Identification of the human skeletal stem cell. Cell. https://doi.org/10.1016/j.cell.2018.07.029.
Murphy, J. M., Dixon, K., Beck, S., Fabian, D., Feldman, A., & Barry, F. (2002). Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis and Rheumatism. https://doi.org/10.1002/art.10118.
Lories, R. J., & Luyten, F. P. (2011). The bone–cartilage unit in osteoarthritis. Nature Reviews Rheumatology. https://doi.org/10.1038/nrrheum.2010.197.
Goldring, S. R., & Goldring, M. B. (2016). Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage–bone crosstalk. Nature Reviews Rheumatology. https://doi.org/10.1038/nrrheum.2016.148.
Jacobsen, S., & Sonne-Holm, S. (2005). Hip dysplasia: A significant risk factor for the development of hip osteoarthritis. A cross-sectional survey. Rheumatology. https://doi.org/10.1093/rheumatology/keh436.
Jacobsen, S. (2006). Adult hip dysplasia and osteoarthritis. Studies in radiology and clinical epidemiology. Acta Orthopaedica. Supplementum, 77(324), 1–37.
McGonagle, D., Baboolal, T. G., & Jones, E. (2017). Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nature Reviews Rheumatology, 13, 719–730. https://doi.org/10.1038/nrrheum.2017.182.
Sakaguchi, Y., Sekiya, I., Yagishita, K., Ichinose, S., Shinomiya, K., & Muneta, T. (2009). Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Stem Cells. https://doi.org/10.1182/blood-2003-12-4452.
Schneider, C. A., Rasband, W. S., & Eliceiri, K. W. (2012). NIH image to ImageJ: 25 years of image analysis. Nature Methods, 9(7), 671–675.
Gregory, C. A., Grady Gunn, W., Peister, A., & Prockop, D. J. (2004). An alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Analytical Biochemistry. https://doi.org/10.1016/j.ab.2004.02.002.
Grogan, S. P., Barbero, A., Winkelmann, Rieser, F., Fitzsimmons, J. S., O’Driscoll, S., Martin, I., Mainil-Varlet, P. (2005). Visual histological grading system for the evaluation of in vitro-generated neocartilage. Tissue Engineering 2006; 12(8):2141–9.
Bustin, S. A., Benes, V., Garson, J. A., Hellemans, J., Huggett, J., Kubista, M., & Wittwer, C. T. (2009). The MIQE Guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clinical Chemistry. https://doi.org/10.1373/clinchem.2008.112797.
Andersen, C. L., Jensen, J. L., & Ørntoft, T. F. (2004). Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Research. https://doi.org/10.1158/0008-5472.CAN-04-0496.
Dominici, M., Le Blanc, K., Mueller, I., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. https://doi.org/10.1080/14653240600855905.
Partridge, L., Deelen, J., & Slagboom, P. E. (2018). Facing up to the global challenges of ageing. Nature. https://doi.org/10.1038/s41586-018-0457-8.
Tarte, K., Gaillard, J., Lataillade, J.-J., et al. (2009). Clinical-grade production of human mesenchymal stromal cells: Occurrence of aneuploidy without transformation. Blood. https://doi.org/10.1182/blood-2009-05-219907.
Zhou, S., Greenberger, J. S., Epperly, M. W., Goff, J. P., Adler, C., Leboff, M. S., & Glowacki, J. (2008). Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. https://doi.org/10.1111/j.1474-9726.2008.00377.x.
Mendicino, M., Bailey, A. M., Wonnacott, K., Puri, R. K., & Bauer, S. R. (2014). MSC-based product characterization for clinical trials: An FDA perspective. Cell Stem Cell. https://doi.org/10.1016/j.stem.2014.01.013.
Kohno, Y., Mizuno, M., Ozeki, N., Katano, H., Komori, K., Fujii, S., Otabe, K., Horie, M., Koga, H., Tsuji, K., Matsumoto, M., Kaneko, H., Takazawa, Y., Muneta, T., & Sekiya, I. (2017). Yields and chondrogenic potential of primary synovial mesenchymal stem cells are comparable between rheumatoid arthritis and osteoarthritis patients. Stem Cell Research and Therapy, 8, 1–11. https://doi.org/10.1186/s13287-017-0572-8.
Crisan, M., Yap, S., Casteilla, L., et al. (2008). A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. https://doi.org/10.1016/j.stem.2008.07.003.
Futami, I., Ishijima, M., Kaneko, H., et al. (2012). Isolation and characterization of multipotential mesenchymal cells from the mouse synovium. PLoS One. https://doi.org/10.1371/journal.pone.0045517.
Cottle, B. J., Lewis, F. C., Shone, V., & Ellison-Hughes, G. M. (2017). Skeletal muscle-derived interstitial progenitor cells (PICs) display stem cell properties, being clonogenic, self-renewing, and multi-potent in vitro and in vivo. Stem Cell Research and Therapy, 8, 1–16. https://doi.org/10.1186/s13287-017-0612-4.
Arrighi, N., Moratal, C., Clément, N., et al. (2015). Characterization of adipocytes derived from fibro/adipogenic progenitors resident in human skeletal muscle. Cell Death & Disease. https://doi.org/10.1038/cddis.2015.79.
Zainabadi, K. (2018). The variable role of SIRT1 in the maintenance and differentiation of mesenchymal stem cells. Regenerative Medicine. https://doi.org/10.2217/rme-2017-0128.
Vrtačnik, P., Zupan, J., Mlakar, V., Kranjc, T., Marc, J., Kern, B., & Ostanek, B. (2018). Epigenetic enzymes influenced by oxidative stress and hypoxia mimetic in osteoblasts are differentially expressed in patients with osteoporosis and osteoarthritis. Scientific Reports, 8, 1–12. https://doi.org/10.1038/s41598-018-34255-4.
Acknowledgements
The authors thank Gorana Furlanič and Helena Poniž for their valuable assistance at Valdoltra Orthopaedic Hospital, Laura Poženel, Tina Levstek, Sandra Mramor, Iva Blaževič, Kyriaki Hadjianastasi, Lina Andersson, Matjaž Jeras and Majda Sirnik for their much appreciated technical and general help. The authors acknowledge Chris Berrie for scientific English editing of the manuscript.
Funding
This study was funded by the Slovenian Research Agency, P3–298 Research Programme and J3–7245 research project and partially funded by the ARTE Project EU INTERREG ITALIA SLOVENIA 2014 2020 and J3–1749 research project funded by the Slovenian Research Agency.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Klemen Čamernik, Anže Mihelič, Rene Mihalič, Rihard Trebše, Darja Marolt Presen and Janja Zupan. The first draft of the manuscript was written by Klemen Čamernik and Janja Zupan, and all of the authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interests
R. Trebše declares payment for development of educational presentations for Zimmer, Medacta, De Puy, Link and Hareus, and R. Mihalič declares consultancy for Medacta International. The rest of the authors declare that they have no conflicts of interest regarding this study.
Ethics Approval and Consent to Participate
Approval for this study was obtained from the National Medical Ethics Committee of the Republic of Slovenia (reference numbers: 0120–523/2016–2, KME 45/10/16). Written informed consent was obtained from all of the donors included in this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Selection of the best reference gene for qPCR for adipogenesis and osteogenesis. NormFinder [22] was used to select the most stable reference gene between the treated cells and the controls for adipogenesis and osteogenesis, in a subset of 32 samples. The best combination of the three genes was GAPDH, RPL13A and RPLP0. Shown is the expression of the osteogenic genes (COL1A1, OC, parathyroid hormone 1 receptor [PTHR1], RUNX2) in another subset of 10 pairs of MSC samples treated with osteogenic media. There was no difference in the normalization of these genes to all three reference genes (GAPDH, RPL13A and RPLP0) and to GAPDH only (two-way ANOVA with Bonferroni correction: p = 0.3003 for COL1A1; p > 0.9999 for OC, PTHR1 and RUNX2). (PNG 242 kb)
ESM 2
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Čamernik, K., Mihelič, A., Mihalič, R. et al. Increased Exhaustion of the Subchondral Bone-Derived Mesenchymal Stem/ Stromal Cells in Primary Versus Dysplastic Osteoarthritis. Stem Cell Rev and Rep 16, 742–754 (2020). https://doi.org/10.1007/s12015-020-09964-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-09964-x