Abstract
Mesenchymal stem cells (MSC) have been extensively studied over the past years for the treatment of different diseases. Most of the ongoing clinical trials currently involve the use of MSC derived from adult tissues. This source may have some limitations, particularly with therapies that may require extensive and repetitive cell dosage. However, nowadays, there is a staggering growth in literature on a new source of MSC. There is now increasing evidence about the mesenchymal differentiation from pluripotent stem cell (PSC). Here, we summarize the current knowledge of pluripotent-derived mesenchymal stem cells (PD-MSC). We present a historical perspective on the subject, and then discuss some critical questions that remain unanswered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the last decade we have been witnessing a new frontier in therapeutic research opportunities, and the clinical translation of their results is inminent. Surgery and pharmacology were the predominant medical models applied in the past, but now the therapeutic spectrum is shared with more sophisticated and complex treatment, including bioprosthetics, recombinant growth factors and complex molecules, and cellular products. In this ever evolving context, mesenchymal stem cells (MSC) are being evaluated thoroughly in clinical trials. Over the past ten years there has been numerous clinical trials using MSC employed in numerous diseases (www.clinicaltrials.gov). However, the conditions in which these clinical trials were carried out were not uniform, including MSC originating from different sources, claiming their usefulness for a numerous diseases and different mechanism proposals of their therapeutic action spanning from immunomodulation to tissue regeneration.
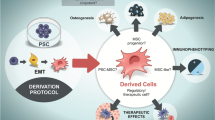
It is now well-established that MSC can be derived from pluripotent stem cells (PSC). Therefore, this review intends to summarize the expanding field of pluripotent derived mesenchymal stem cells. Since the literature is now flooded with different names for these cells, we propose to unify the terminology and name them as PD-MSC. We will go over the published research over the past years, including the many different protocols for PD-MSC differentiation as well as the initial animal experiments aiming for clinical application. More importantly, we will discuss two fundamental issues regarding PD-MSC: Are these cells true MSC and, fundamentaly, what is their potential for clinical application?
The Stone Age: Mesenchymal Versus Pluripotent Stem Cells
For many years the stem cell field was divided into the study of adult stem cells (mostly, but not only, MSC) and the study of PSC, although we acknowledge that this is a rather simplistic classification, as there are many other stem cells that do not fit into it.
PSC can be divided roughly into Embryonic Stem Cells (ESC) and induced Pluripotent Stem Cells (iPSC). ESC were originally derived from the inner cell mass (ICM) of mouse blastocysts [16], and later on from human ICM [56]. PSC are now mostly generated as iPSC by cell reprogramming based on the original work from Yamanaka’s group [53, 54]. The main feature of these cells is their ability to indefinitely self-renew and their ability to differentiate into any adult tissue cell, a property known as pluripotency. Pluripotency is readily demonstrable in vitro and in vivo. Firstly, the cells are grown in a spheroid structure known as embryoid body, a three-dimensional cell package where the self-secreted morphogens stimulate the differentiation of PSC into any kind of cells. In vivo demonstration comes from the teratoma assay, in which PSC are injected in nude mice, inducing a few weeks later a tumor formed by different adult tissues. Ultimately, complementation assays provide a definitive confirmation of pluripotency [33].
In the sixties, Friedenstein and coworkers discussed the existence of cells present in bone marrow that were unrelated to the hematopoietic lineage [19]. By implanting a bone marrow extract into an organ, they concluded that a mesenchymal stem-like cell should have been present in that extract as they later observed bone formation. In the nineties, Pittenger et al. published the description of a bone marrow cell population with a mesenchymal phenotype [45]. This description set off a variety of studies concerning the cellular regenerative and immunomodulation properties.
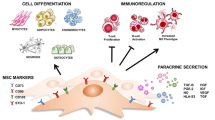
Current evidence shows that MSC are able to differentiate into chondroblast, osteoblasts or adipocytes, and hence they are known as multipotent [45]. Although initially MSC were considered capable of repairing damaged tissue by replacing the local cell phenotype, it is now believed that this is not the only mechanism taking place. Today we know that MSCs secrete a variety of molecules that includes cytokines, growth factors, antioxidants and pro-angiogenic factors that can contribute to tissue repair in a paracrine way [8, 57]. These factors would increase the regeneration of damaged tissue by decreasing the response to stress and apoptosis of damaged cells and regulating the local and systemic inflammatory and immune response. MSCs have demonstrated strong immunomodulatory properties. Multiple studies consistently show that these cells are capable of inhibiting stimulated T lymphocyte proliferation, suppress proliferation and differentiation of B lymphocytes, inhibit differentiation of dendritic cells to monocytes, prevent their maturation and activation, and induce the formation of regulatory T lymphocytes [23, 46].
The Modern Age: Mesenchymal from Pluripotent Stem Cells
Pluripotent stem cells can differentiate into any adult cell, and therefore the description of mesenchymal stem cells arising from PSC was not surprising at all. What is surprising, as we will see, is the easiness with which this specific differentiation occurs. Hence, nowadays PSC and MSC are well established different areas of study, but MSC can be reprogrammed into PSC as PSC can be differentiated to MSC.
The Context of the Embryo Development
For a better comprehension of PD-MSC it is necessary to understand the first stages of embryo development. The formation of the initial embryonic structures involves major structural changes in the pluripotent stem cells. Epiblast cells invaginate in the middle line and migrate towards the lateral processes, giving way to the formation of the mesoderm and endoderm. This is called the primary epithelial-to-mesenchymal transition (EMT). This is a critical step for embryos, although for some cells this is not the only EMT they suffer: some structures undergo a couple of EMT and mesenchymal-to-epithelial transition (MET) before they differentiate into the adult cells [32].
During EMT, epithelial cells lose their membrane junctions and apical-basal polarity, reorganize their cytoskeleton, undergo a change in the signaling pathways that define cell shape and reprogram gene expression. The epithelial cell suffer a number of distinct molecular changes such as activation of transcription factors, expression or repression of specific cell-surface proteins, reorganization and expression of cytoskeletal proteins, production of ECM-degrading enzymes, and changes in the expression of specific microRNAs.
The process in which PSC undergo the EMT has been consistent with the more abundant literature describing the same process in tumor cells. Several transcription factors and growth factors that orchestrate EMT during epiblast-to-mesoderm transition in vivo in the primitive streak during gastrulation are also involved in the initial stages of mesodermal differentiation of PSC. Multiple reports emphasize the importance of the EMT in the formation of the mesoderm-derived adult cells. Moreover, Evseenko and co-workers identified and characterized a unique population of human embryonic mesodermal progenitor (hEMP) cells, which arose from hESCs through the process of EMT [17]. These events can be easily tracked by the combined loss of the epithelial adhesion marker CD326 (EpCAM) and up-regulation of CD56 (NCAM). The analysis of hEMP cells shows the expected markers of cells undergoing EMT.
However, the information is scarce regarding EMT in the development of PD-MSC. We have analyzed the expression of some critical transcription factors that govern the EMT process such as Zeb1, Zeb2 and Snail, and found that they increase during the differentiation of PD-MSC [40]. Many cell surface markers are also down-regulated or up-regulated as expected during the EMT. Furthermore, there are publications about microRNAs which are critical regulators of EMT and that have been found to be active in PSC, including the mir-200 family. Hence, we believe that most of the events that occur in EMT are expected to be critical for the derivation of PD-MSC.
There is no information regarding which are the critical growth factors that signal the formation of PD-MSC. Although there are a few papers that, based on the morphogens known to induce mesoderm formation [36] use these growth factors for the generation of PD-MSC, these papers only make use of this previous knowledge in order to get a population enriched in mesenchymal cells. Which one of them are critical for the differentiation of PSC into PD-MSC remains to be determined.
Derivation and Characterization of Mesenchymal Stem Cells from Pluripotent Stem Cells
There has been a growing literature in the past ten years on how to develop MSC from PSC. It should not be surprising that a pluripotent cell can generate a mesenchymal stem cell, since PSC are, by definition, able to rise all kind of adult cells, including those with some features of stem cells. What is somehow intriguing is the easiness of developing pluripotent derived mesenchymal stem/stromal cells, as opposed to the obtention of other adult cells where complex protocols with several stages may be needed. We have recently developed a simple protocol based on the use of platelet lysate as cell media supplement, observing that there is a straightforward process where PSC enters in an epithelial-to-mesenchymal transition (EMT) and eventually, and uniformly, becomes cells with all features of MSC.
It has come to our attention that it makes no difference which supplement is present in the culture medium (i.e., platelet lysate, fetal bovine serum or defined components), a PSC cultured in the proper manner will eventually generate PD-MSC ([40] and unpublished results). Interstingly enough, provided that there are sufficient nutrients and survival signals in the medium, there is no evidence of cell death, suggesting that most PSC that were induced to differentiate will eventually do so. Although this is experimentally challenging to demonstrate, it is tempting to suggest that every PSC will become a MSC by default, an event that occurs spontaneously while PSC are cultured under less stringent pluripotent conditions. Then, it is interesting that a stem cell with pluripotent and epithelial properties can directly be differentiated into a stem/progenitor cell with mesenchymal features, as if they were the two faces of the same coin.
After human PSC were originally described scientist focused on improving culture methods to keep pluripotency. Even today there are papers describing how to achieve a “higher” state of pluripotency represented by pluripotent stem cells called naïve or ground state pluripotent stem cell [12, 30]. Originally, PSC were co-cultured with inactivated mouse embryonic fibroblasts (iMEF). These cells were supposed to nourish PSC by secreting growth factors to the medium. Xu et al. in 2001 described different substrates to culture PSC and comparing them to iMEF which represent the original method [63]. They showed that Matrigel®; and laminin, and to some extent fibronectin were good enough to maintain pluripotency when cultured with iMEF conditioned medium, but not on gelatin or plastic, which eventually will induce differentiation of the cells. However, they noticed that PSC grown under these cell culture conditions were surrounded by a dense mesh of differentiated cells. These cells were observed migrating from the border of the colonies. By morphology and in retrospective, this is probably the first description of cells resembling MSC outgrowing PSC. The same group later described another protocol to derive fibroblast-like cells with the purpose of using them to support PSC culture [64]. Although the authors performed a limited characterization of the fibroblast-like cells, they found that they expressed CD44 and CD90, two known markers of MSC. These differentiated cells also lost the ability to produce teratomas, and hence, pluripotency. In 2007, however, Ullmann et al. provided more insights into these cells that arose around the pluripotent colonies of hESC by demonstrating a mesenchymal origin of these outgrowths [59]. These authors grew the cells in Matrigel®; and conditionated medium from iMEF. These cells lost the pluripotent identity and gained mesenchymal markers such as vimentin. Furthermore, these cells lost the gap junction proteins E-cadherin and connexin-43, a hallmark of the EMT in this cell population. However, the authors did not attempt to establish any connection between these cells and adult MSC.
In 2005 Barberi et al. published a paper in which, for the first time, the derivation of MSC from PSC was claimed [2]. The method in which this was obtained consisted in co-culturing PSC with OP9 cells, a mesoderm embryonic cell line. After a long period of time (40 days), they sorted the CD73(+) cell population and found that these cells expressed several markers usually found in MSC, including CD166, CD54, CD29, CD105, CD44, and STRO-1. They also performed a genome-wide expression analysis and found a significant overlap between the genes expressed between adult bone marrow-derived MSC and those derived from PSC, particularly in genes associated to the mesenchymal state. Therefore, this paper established for the first time that it was possible to derive mesenchymal stem cells from a pluripotent stem cell.
A year later Olivier et al. published a paper where they described a cumbersome method for deriving PD-MSC [43]. They called it the Raclure method after the french word raclures, which means scrapping. Essentially, the method consists in scrapping the differentiated cells from a standard PSC culture on iMEF. The scrapped cells were grown for 4 weeks or more and formed what they called a thick epithelial layer, although they do not provide any evidence that these cells were, indeed, epithelial. These cells were then passed and after two more weeks in culture they confirmed their mesenchymal origin. The list of subsequent publications on methods to derive PD-MSC is long. However, all these methods can be grouped according to a general criteria: either PSC are left to differentiate spontaneously or differentiation to MSC is directed specifically. There is a first group in which the main idea behind the method is to let them differentiate by drepriving the medium of pluripotent signals. For example, Trivedi et al. grew PSC with iMEF conditionated-media but spacing the medium changes from 3 to 5 days [58]. This step induced the appearance of cells with a MSC morphology around the PSC colonies. Although this efficiently differentiated cells into a MSC-like phenotype, they still had to manually dissect and extract the undifferentiated colonies, a step that had to be repeated several times. Another publication also mechanically separated the mesenchymal cells appearing from embryoid bodies attached after 10 days of differentiation [31]. Thus, coming from undifferentiated PSC in colonies or differentiated PSC in the form of embryoid bodies did not seem to make any difference. Therefore, these initial approaches to PD-MSC derivation were generated by the spontaneous appearance of mesenchymal cells coming from undifferentiated or pre-differentiated cells. One common denominator to the first papers on PD-MSC was the lack of specific stimulus was given to the PSC in order to generate mesenchymal stem/stromal cells. In fact, these cells can be seen as by-stander of the spontaneous differentiation of PSC, which may happen when the culture conditions are not sufficient to maintain the pluripotent state. Given adverse conditions to sustain pluripotency, PSC enters in EMT and acquires a mesenchymal phenotype. These previous methods to derive PD-MSC were effective, but inefficient: they still required some specific manipulation and the use of FBS to induce differentiation.
A second group of papers were published with more specific methods. Stavropoulos et al. showed that it was possible to obtain PD-MSC after culturing hESC in ITS (insulin-transferrin-selenite) [50]. After this period they sorted CD73(+) cells, which presented all features of MSC. Karlsson et al. also showed that it was possible to differentiate PSC into PD-MSC by several passages with trypsin and using a medium supplemented with FBS and bFGF [35]. During the same year Boyd et al. showed a similar result by growing PSC in endothelial medium for a few weeks [7].
A third group with protocols which use specific inhibitors and/or growth factors were finally published. All previous protocols were performed with medium supplemented with fetal bovine serum, which provides multiple growth factors with non-specific signals to the cells. Instead of inducing a non-specific differentiation signal, these protocols include specific signals by incubating the cells with known morphogens that drives the PSC to mesoderm formation. For example, Mahmood et al. used the TGF- β inhibitor SB-431542 during the differentiation of pluripotent stem cells in embryoid body structures [41]. Another paper by Sanchez et al. used a similar strategy by using the same TGF- β inhibitor, but this time in two dimensional growing cells [48]. The fact that these authors used an inhibitor of the TGF- β family is interesting since the inhibition of TGF- β is implicated in the maintenance of the undifferentiated state of the pluripotent stem cells [14, 55]. Moreover, in a complex protocol Kimbrel et al. developed PD-MSC by growing the cells first in embryoid bodies and then in 2D conditions, incubating them with bFGF, Vascular Endothelial Growth Factor (VEGF), Bone Morphogenetic Protein 4 (BMP4), and thrombopoietin [36]. Wu et al. used the ROCK inhibitor Y-27632 and the neural stem cell supplements B27 and N2, obtaining a defined medium for the differentiation of PSC into MSC [62]. Again, these protocols were shown to be effective in differentiating PSC into PD-MSC, either by finding expression of the usual mesenchymal surface markers, multipotentiality or by immmunomodulation. Even though these protocols can be perceived as being more simple and clinically compatible, they are usually much more expensive.
We have recently published our experience with a new protocol to derive PD-MSC [40]. We found that these cells can be easily grown from hESC or iPSC when they are cultured for at least 3 weeks in a medium supplemented with human platelet lysate. We analyzed the temporal pattern of the differentiation process, either by gene expression analysis or by flow cytometry and established that 21 days is approximately the time needed to fully differentiate. We compared the expression profile of PD-MSC with umbilical cord-derived MSC and fibroblasts, and found minor differences between them. We also performed the same protocol using platelet lysate instead of FBS, or defined supplements such as a combination of growth factors and small molecules (bFGF/BMP4/Lithium chloride or the TGF-beta inhibitor SB431542) and found that they also were able to differentiate PSC into PD-MSC with no major differences (unpublished results). A similar finding was done by Diederichs and Tuan [11]. They analyzed PD-MSC derived by four different protocols and found no major differences in terms of MSC differentiation. Therefore, these findings stressed that there are many ways to obtain PD-MSC, and although we performed some comparisons between different supplements, there is no formal comparison between all these published protocols. To what extent the obtained PD-MSC by these different protocols are equivalent, or if all of them correspond to a similar mesenchymal cell type, is unknown, but they seem to share all the classical features of a mesenchymal phenotype.
In summary, there is a long list of differentiation protocols that would eventually produce PD-MSC from PSC. These protocols can be grouped in three general concepts. First, there are protocols that are mainly based on the isolation of differentiated cells that arise around PSC colonies, usually after a change in the usual culture techniques that keep the cells in a pluripotent state. These protocols mechanically collect the cells that grow with a distinct MSC morphology. A second group of protocols include those that involve a more active induction of the mesenchymal differentiation. In these cases, the protocols usually introduce defined cell culture media and eventually separate the differentiated cells by surface markers. Finally, more stringent protocols have been published where PSC are ushered to differentiate by means of specific growth factors and pathway inhibitors. These protocols are based in specific signals that are known to induce mesoderm formation. Despite the general differences within the protocols, certain similarities are worth being mentioned. First, it usually takes several weeks to get fully differentiated PD-MSC. Second, all these protocols result in a homogeneous mesenchymal cell population, without contamination with other cellular phenotypes. Third, differentiation is complete, and no remnants of undifferentiated cells are found. Finally, all these protocols are described as robust and consistent in their results.
A critical question rooted within PD-MSC is about their definition and characterization. The question if a PD-MSC is a true MSC has been challenged, but there are still some controversies about these facts. A few years ago an expert commitee recommended a list of characteristics a true MSC should have [13]. Despite this effort, these criteria can be considered to be an approximation to (and a reduction of) causing an oversimplification to a complex subject, and hence many other criteria can be properly used to assert that a cell is a MSC. The application of these criteria to PD-MSC was immediate, and it can be observed that PD-MSC are positive for all these criteria with minor differences. However, we think that it is also important to demonstrate that the PD-MSC population is originated from undifferentiated cells that underwent EMT with all the features mesenchymal cells usually present. This finding supports the notion that the cells are indeed a mesenchymal derivation. Finally, the demonstration that there is no persistent feature of pluripotency is also important.
The tissue source of MSC is widely used to help define MSC, but even tissue-specific populations seem to contain subsets of MSC. So, how equivalent are PD-MSC to MSC from other sources? This question is hard to answer and it will depend on the criteria of each reader to say how similar a PD-MSC is to a MSC. It cannot be denied, however, that in general PD-MSC present many of the structural and functional features of a MSC. We attempted to clarify this issue by comparing PD-MSC with umbilical cord-derived MSC (a cell population developmentally closer to pluripotent cells than adult MSC) [40], as well as other authors have used mesenchymal cell lines derived from embryo tissues [43], and no major differences were found between mesenchymal cell populations in regard to their surface markers composition.
Another approach to answer this question has been the use of genome-wide expression analysis [2, 3, 5, 39, 43]. As expected, these papers describe a significant overlap in gene expression between PD-MSC and bone marrow-derived MSC. These genes include many that are well-recognized markers of mesenchymal cells. Moreover, the differences can be obviously expected considering that the source may imprint the cells in a niche-related way. One interesting finding is that mesenchymal cells inherit some important features from their origin. For example, cells derived from PSC have a shorter doubling time and longer telomeres than MSC derived from the bone marrow, a feature that resembles the characteristics from the pluripotent stage [44]. These findings give PD-MSC the property of a fast expansion after differentiation, making them attractive to use for experiments and preclinical trials. Recently Billings et al. compared PD-MSC with BM-MSC using genome-wide and proteomic analysis [5]. This extensive analysis of both MSC strongly support that PD-MSC are indeed a mesenchymal cell.
Surface markers have been a standard tool for the identification of the MSC population. There is a vast array of markers that have been found on the surface of these cells, and there is no unique marker or pattern that distinguishes MSC from other cells. The classical pattern of CD90(+)/CD73(+)/CD105(+) is also present in PD-MSC, although we found that CD90 is also highly expressed in undifferentiated cells [40]. Most MSC markers described in literature have also been found to be expressed in PD-MSC. An exception could be the mesenchymal marker Stro-1; we and others found that PD-MSC are negative for this marker [62]. Interestingly, fibroblasts also expressed all these markers, a finding that supports the few differences that can be found when MSC are compared to fibroblasts [26]. Moreover, there is also a clear change in the pattern of marker expression compared to PSC. As previously explained, the development of PD-MSC involves an EMT. Therefore, there is a switch in the surface marker expression where PSC lose their pluripotent markers (SSEA-4, Tra-1-60, CD-326, E-cadherin, etc.) and gain mesenchymal ones.
Mesenchymal stem cells also present the multipotent ability by which they can differentiate into adipocyte, chondrocytes and osteoblasts. Again, multipotency has also been widely used to demonstrate the MSC phenotype of the PD-MSC cells. Most publications readily demonstrate this ability in these cells, although it has been reported that these cells are less multipotent when compared to the adult bone marrow-derived MSC [11, 34].
Finally, another characteristic of MSC is their immunomodulatory ability. Many papers have also reproduced this ability in PD-MSC. Moreover, some papers suggested that this feature is even more potent in PD-MSC [61], though this finding failled to be confirmed in another in vitro study [21]. In any case, PD-MSC reproduce MSC strong inhibition of activated lymphocyte proliferation [36, 40, 48, 58, 61]. Finally, studies regarding the mechanisms by which PD-MSC immunomodulate are scarse [10, 22, 24], but it is believed that they are probably similar to those found in MSC from both adult and neonatal tissues.
The Future Age: Experimental Therapies with PD-MSC
MSC are a promising source of cells for therapeutic purposes. Currently there are many clinical trials evaluating the effects of MSC in a variety of diseases, including osteoarthritis, wound healing, degenerative disease, and autoimmune disorders (U.S. National Institutes of Health; www.clinicaltrials.gov (2016)). One of the proposed advantages of MSC for cell therapy is that these cells are able to evade immune detection. Although the exact mechanism of this property is still not clear, now we know that MSC do not express co-stimulatory molecules such as CD80 or CD40, they may express HLA-G and a non-canonical MHC class I molecule [37] along with a serine protease inhibitor of the immune response [15]. All these mechanisms may contribute to their immunopriviliged status.
Although MSC may be readily isolated from several different adult tissues and are being incorporated as an alternative cell source in regenerative therapy, from a pharmaceutical point of view there are still some issues left to solve in order to make MSC readily available. On the one hand, MSC loose their multipotency and immunomodulatory properties when cultured for long periods of time [4, 6]. This poses several concerns regarding the possibility of scaling up MSC culture to meet clinical demands. On the other hand, the isolation of adult MSC from different sources or different donors contribute to cell heterogeneity, complicating cGMP validation. In this regard, PD-MSC might be a clever solution for the medical industry. Since PSC can self-renew, the source for MSC derivation can be validated and reproducibly differentiated to produce large quantities of young, fresh MSC at low passages. Recently, a publication supports this fact since it has been shown that epigenetic changes are compatible with a reversion of cellular aging [20]. The research with PD-MSC developed so far falls well behind to the adult or neonatal MSC, but recently an Australian-based company has announced that they will conduct a phase I clinical study with PD-MSC in graft-versus-host disease (Cynata Therapeutics).
A wave of animal studies using these cells have emerged in the past few years (Table 1). For example, in 2014 Kimbrel et al. showed that PD-MSCs have therapeutic efficacy in two different autoimmune disorder models, including a marked increase in survival of lupus-prone mice and a reduction of symptoms in an autoimmune model of uveitis [36]. Contemporaneously, Wang and co-workers postulated that PD-MSC have a significantly better performance than bone marrow MSC in treating an experimental autoimmune encephalomyelitis (EAE) model of Multiple Sclerosis [61]. In fact, bone marrow MSC were ineffective in this model. Many other animal studies are surfacing, replicating the findings of MSC from other sources. These works are showing that PD-MSC are effective and safe as immune modulators in animal models of inflammation and autoimmunity [18, 25, 27, 52, 68].
One of the current discussions in MSC therapy field is about the clinical use of autologous and allogeneic cells. Autologous MSC applications may have some potential limitations. In the first place, given that clinical trials generally use large numbers of cells per dose (above 200 million MSC) it may be troublesome to obtain a sufficient amount of cells, especially when multiple doses are needed. Secondly, MSC aging will be a major issue in this regard, since cells lose their therapeutic properties with the increasing number of passages [4, 6, 49]. The age of the patient may also affect the MSC cell therapeutic potential [51, 69]. MSCs have been shown to have low immunogenicity based on the lack of expression of markers such as CD45 and CD34 and HLA-DR surface molecules. Thus, it seems that both autologous and allogeneic cells could safely be applied, although clinical studies are still ongoing. Because of these immunosuppressive properties of MSCs, allogeneic MSCs are currently infused intravenously for the treatment of steroid-resistant graft-versus-host disease, acute respiratory distress syndrome and Crohns disease in clinical trials [60].
Clinical PD-MSC production make sense only when an allogeneic therapy is considered. Otherwise, PD-MSC would result in a time consuming and expensive protocol that will not be available for the general public. This involves isolating and expanding patientś cells for reprogramming, iPSC line validation and their posterior differentiation towards MSC. In contrast, PD-MSC may be obtained from a well validated iPSC line, and consistently be derived from these cells in order to create a stock of MSC ready to use in an allogeneic fashion. This will make MSC affordable and homogeneous from batch to batch, leading to improve the public access to these therapies.
Finally, exosomes collected from PD-MSC were shown to enhance cutaneous wound healing in rats by promoting collagen synthesis and angiogenesis [66]. There are a few publications with PD-MSC that showed in in vivo animal models a similar clinical efficacy than adult MSC [9, 29, 47]. The field of MSC-derived exosomes is rapidly growing [65], and it can be expected in the near future that PD-MSC derived exosomes will also be clinically investigated.
Unanswered Questions with PD-MSC
The field of PD-MSC is expanding every year. There are now many publications showing that these cells truly resemble MSC, and that they behave as adult MSC. However, several questions still remain unanswered about PD-MSC. Research in the future will probably address these topics.
-
What is the main driving force for differentiation? If many different signals induce the differentiation of PSC into MSC, with no major apparent differences in the final fate, which is the major signal or signals that lead to the straightforward differentiation, if there is any? The understanding about the signals that induced mesoderm differentiation in PSC are well described. However, is there any specific signaling for PD-MSC differentiation? Alternatively, could differentiation towards PD-MSC be a default process under still unknown circumstances? There are many ways to induce the differentiation of PD-MSC, but, are all these cells the same? Heterogeneity is well described in MSC isolated from adult tissue. However, there is no information if this heterogeneity exists in PD-MSC, or if they are a uniform cell population.
-
Do PD-MSC retain donnor cell epigenetic memory? It is well known that iPSC cells have an epigenetic bias towards the donor cell lineage. So, an obvious question arise: do MSC derived from iPSC have the same epigenetic background as the original differentiated cell that gave rise to that iPSC? Moreover, is PD-MSC secretome different depending on the origin of the parental iPSC line? To tackle this issue correctly a large scale study should be conducted, comparing MSC derived from iPSC that were reprogrammed from different cell lineages.
-
Are they safe? There is a vast literature showing that MSC from other sources are safe. Now, can we consider that PD-MSC are also safe? What do we need to be confident that these cells are stable, and that they will not produce a tumor? Considering their origin from PSC, this concern needs further research in the future.
Concluding Remarks and Future Directions
The ability of PSC to differentiate into MSC has been explored in the past ten years. There are multiple protocols that are able to produce cells with all the features that characterize a MSC. We believe that there are some reasons why these cells may become key players in the field of regenerative medicine and MSC research in the near future. First, they may be easier to produce and have a higher proliferation rate, with less senescence. Second, once iPSC cells are obtained from a patient, there is potentially an unlimited source of MSC to work with. iPSC can be considered as immortal, and hence they can be seen eventually as an off-the-shelf bone marrow-like tissue to produce MSC. Therefore, if an allogeneic aproach is to be chosen, large numbers of MSC can be achieved consistently from a same, well characterized, consistent source. Additionally, PD-MSC could be potentially derived from the biobanks that are currently storing iPS cells under GMP conditions, which may be HLA-matched source. Finally, we believe that exosomes from PD-MSC may well become a successful scheme for a therapeutic product, where iPSC generates an unlimited amount of MSC which eventually produce large quantities of exosomes in an easy and cGMP compatible way.
Some hurdles, however, should finally be mentioned. The research done so far with PD-MSC shows a promising future, but there are still critical unanswered questions. How are these cells formed, and which are the key signals necessary for an effective differentiation? Are all cells generated and all protocols producing the same type of PD-MSC? Is there any variability regarding the genetic background of the iPSC cell? Does any previous disease, such as diabetes, affect the source and outcome of PD-MSC production? Last, but not least, researchers should be sure that no PSC remain after the differentiation to MSC, since one pluripotent cell is enought to give rise to a teratoma. Although derivation of MSC from PSC is well described, it is presently taking its initial steps in terms of demonstrating their research and clinical utility. Therefore, we foresee an active and exciting incoming years in this field.
Abbreviations
- BM-MSC:
-
Bone marrow-derived mesenchymal stem cells
- EMT:
-
Epithelial-to-mesenchymal transition
- iPSC:
-
induced pluripotent stem cells
- MSC:
-
Mesenchymal stem cells
- PD-MSC:
-
Pluripotent-derived mesenchymal stem cells
- PSC:
-
Plurpotent stem cells
References
Arpornmaeklong, P., Brown, S.E., Wang, Z., & Krebsbach, P.H. (2009). Phenotypic characterization, osteoblastic differentiation, and bone regeneration capacity of human embryonic stem cell-derived mesenchymal stem cells. Stem cells and Development, 18(7), 955–968.
Barberi, T., Willis, L.M., Socci, N.D., & Studer, L. (2005). Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Medicine, 2(6), e161.
Barbet, R., Peiffer, I., Hatzfeld, A., Charbord, P., & Hatzfeld, J.A. (2011). Comparison of gene expression in human embryonic stem cells, hesc-derived mesenchymal stem cells and human mesenchymal stem cells. Stem Cells International, 2011(368), 192. doi:10.4061/2011/368192.
Baxter, M.A., Wynn, R.F., Jowitt, S.N., Wraith, J.E., Fairbairn, L.J., & Bellantuono, I. (2004). Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells, 22(5), 675–82. doi:10.1634/stemcells.22-5-675 10.1634/stemcells.22-5-675.
Billing, A.M., Ben Hamidane, H., Dib, S.S., Cotton, R.J., Bhagwat, A.M., Kumar, P., Hayat, S., Yousri, N.A., Goswami, N., Suhre, K., Rafii, A., & Graumann, J. (2016). Comprehensive transcriptomic and proteomic characterization of human mesenchymal stem cells reveals source specific cellular markers. Scientific reports, 6(21), 507–15.
Bonab, M.M., Alimoghaddam, K., Talebian, F., Ghaffari, S.H., Ghavamzadeh, A., & Nikbin, B. (2006). Aging of mesenchymal stem cell in vitro. BMC Cell Biology, 7, 14. doi:10.1186/1471-2121-7-14.
Boyd, N.L., Robbins, K.R., Dhara, S.K., West, F.D., & Stice, S.L. (2009). Human embryonic stem cell-derived mesoderm-like epithelium transitions to mesenchymal progenitor cells. Tissue Engineering Part A, 15 (8), 1897–1907.
Cantinieaux, D., Quertainmont, R., Blacher, S., Rossi, L., Wanet, T., Noël, A, Brook, G., Schoenen, J., & Franzen, R. (2013). Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: An original strategy to avoid cell transplantation. PLoS ONE, 8 (8), e69,515.
Chen, T.S., Arslan, F., Yin, Y., Tan, S.S., Lai, R.C., Choo, A.B.H., Padmanabhan, J., Lee, C.N., de Kleijn, D.P.V., & Lim, S.K. (2011). Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. Journal of Translational Medicine, 9(1), 47.
Cheng, P.P., Liu, X.C., Ma, P.F., Gao, C., Li, J.L., Lin, Y.Y., Shao, W., Han, S., Zhao, B., Wang, L.M., Fu, J.Z., Meng, L.X., Li, Q., Lian, Q.Z., Xia, J.J., & Qi, Z.Q. (2015). iPSC-MSCs combined with low-dose rapamycin induced islet allograft tolerance through suppressing Th1 and enhancing regulatory T-Cell differentiation. Stem cells and Development, 24(15), 1793–1804.
Diederichs, S., & Tuan, R.S. (2014). Functional comparison of human-induced pluripotent stem cell-derived mesenchymal cells and bone marrow-derived mesenchymal stromal cells from the same donor. Stem Cells and Development, 23(14), 1594–1610.
Dodsworth, B.T., Flynn, R., & Cowley, S.A. (2015). The current state of naive human pluripotency. Stem Cells, 33(11), 3181–3186.
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., Deans, R., Keating, A., Prockop, D., & Horwitz, E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy, 8(4), 315–317.
Du, J., Wu, Y., Ai, Z., Shi, X., Chen, L., & Guo, Z. (2014). Mechanism of sb431542 in inhibiting mouse embryonic stem cell differentiation. Cell Signal, 26(10), 2107–16. doi:10.1016/j.cellsig.2014.06.002.
El Haddad, N., Heathcote, D., Moore, R., Yang, S., Azzi, J., Mfarrej, B., Atkinson, M., Sayegh, M.H., Lee, J.S., Ashton-Rickardt, P.G., & Abdi, R. (2011). Mesenchymal stem cells express serine protease inhibitor to evade the host immune response. Blood, 117(4), 1176–83. doi:10.1182/blood-2010-06-287979.
Evans, M.J., & Kaufman, M.H. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature, 292(5819), 154–156.
Evseenko, D., Zhu, Y., Schenke-Layland, K., Kuo, J., Latour, B., Ge, S., Scholes, J., Dravid, G., Li, X., Maclellan, W.R., & Crooks, G.M. (2010). Mapping the first stages of mesoderm commitment during differentiation of human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America, 107(31), 13,742–13,747.
Ferrer, L., Kimbrel, E.A., Lam, A., Falk, E.B., Zewe, C., Juopperi, T., Lanza, R., & Hoffman, A. (2016). Treatment of perianal fistulas with human embryonic stem cell-derived mesenchymal stem cells: A canine model of human fistulizing Crohn’s disease. Regenerative Medicine, 11(1), 33–43.
Friedenstein, A.J., Piatetzky-Shapiro, I.I., & Petrakova, K.V. (1966). Osteogenesis in transplants of bone marrow cells. Journal of Embryology and Experimental Morphology, 16(3), 381– 390.
Frobel, J., Hemeda, H., Lenz, M., Abagnale, G., Joussen, S., Denecke, B., Sarić, T, Zenke, M., & Wagner, W. (2014). Epigenetic rejuvenation of mesenchymal stromal cells derived from induced pluripotent stem cells. Stem Cell Reports, 3(3), 414–422.
Fu, X., Chen, Y., Xie, F.N., Dong, P., Wb, Liu, Cao, Y., Zhang, W.J., & Xiao, R. (2015). Comparison of immunological characteristics of mesenchymal stem cells derived from human embryonic stem cells and bone marrow. Tissue Engineering Part A, 21(3-4), 616–626.
Giuliani, M., Oudrhiri, N., Noman, Z.M., Vernochet, A., Chouaib, S., Azzarone, B., Durrbach, A., & Bennaceur-Griscelli, A. (2011). Human mesenchymal stem cells derived from induced pluripotent stem cells down-regulate NK-cell cytolytic machinery. Blood, 118(12), 3254–3262.
Glennie, S., Soeiro, I., Dyson, P.J., Lam, E.W.F., & Dazzi, F. (2005). Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood, 105(7), 2821–2827.
Gonzalo-Gil, E., Pérez-Lorenzo, M J, Galindo, M., Díaz de la Guardia, R, López-Millán, B, Bueno, C., Menendez, P., Pablos, J.L., & Criado, G. (2015). Human embryonic stem cell-derived mesenchymal stromal cells ameliorate collagen-induced arthritis by inducing host-derived indoleamine 2,3 dioxygenase. Arthritis Research & Therapy, 18(1), 77.
Hajizadeh-Saffar, E., Tahamtani, Y., Aghdami, N., Azadmanesh, K., Habibi-Anbouhi, M., Heremans, Y., De Leu, N., Heimberg, H., Ravassard, P., Shokrgozar, M.A., & Baharvand, H. (2015). Inducible VEGF expression by human embryonic stem cell-derived mesenchymal stromal cells reduces the minimal islet mass required to reverse diabetes. Scientific Reports, 5, 9322.
Haniffa, M.A., Collin, M.P., Buckley, C.D., & Dazzi, F. (2009). Mesenchymal stem cells: the fibroblasts’ new clothes? Haematologica, 94(2), 258–63. doi:10.3324/haematol.13699.
Hao, Q., Zhu, Y.G., Monsel, A., Gennai, S., Lee, T., Xu, F., & Lee, J.W. (2015). Study of bone marrow and embryonic stem cell-derived human mesenchymal stem cells for treatment of escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells Translational Medicine, 4(7), 832–840.
Himeno, T., Kamiya, H., Naruse, K., Cheng, Z., Ito, S., Kondo, M., Okawa, T., Fujiya, A., Kato, J., Suzuki, H., Kito, T., Hamada, Y., Oiso, Y., Isobe, K., & Nakamura, J. (2013). Mesenchymal stem cell-like cells derived from mouse induced pluripotent stem cells ameliorate diabetic polyneuropathy in mice. BioMed Research International, 2013, 259,187.
Hu, G., Li, Q., Niu, X., Hu, B., Liu, J., Zhou, S., Guo, S., Lang, Hl, Zhang, C., Wang, Y., & Deng, Zf (2015). Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Research & Therapy, 6, 10. doi:10.1186/scrt546.
Huang, K., Maruyama, T., & Fan, G. (2014). The naive state of human pluripotent stem cells: a synthesis of stem cell and preimplantation embryo transcriptome analyses. Cell Stem Cell, 15(4), 410–415.
Hwang, N.S., Varghese, S., Lee, H.J., Zhang, Z., Ye, Z., Bae, J., Cheng, L., & Elisseeff, J. (2008). In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proceedings of the National Academy of Sciences, 105(52), 20,641–20,646.
Kalluri, R., & Weinberg, R.A. (2009). The basics of epithelial-mesenchymal transition. The Journal of Clinical Investigation, 119(6), 1420–1428.
Kang, L., Wang, J., Zhang, Y., Kou, Z., & Gao, S. (2009). iPS cells can support full-term development of tetraploid blastocyst-complemented embryos. Cell Stem Cell.
Kang, R., Zhou, Y., Tan, S., Zhou, G., Aagaard, L., Xie, L., Bünger, C, Bolund, L., & Luo, Y. (2015). Mesenchymal stem cells derived from human induced pluripotent stem cells retain adequate osteogenicity and chondrogenicity but less adipogenicity. Stem Cell Research & Therapy, 6, 144. doi:10.1186/s13287-015-0137-7.
Karlsson, C., Emanuelsson, K., Wessberg, F., Kajic, K., Axell, M.Z., Eriksson, P.S., Lindahl, A., Hyllner, J., & Strehl, R. (2009). Human embryonic stem cell-derived mesenchymal progenitors–potential in regenerative medicine. Stem Cell Research, 3(1), 39–50. doi:10.1016/j.scr.2009.05.002.
Kimbrel, E.A., Kouris, N.A., Yavanian, G.J., Chu, J., Qin, Y., Chan, A., Singh, R.P., McCurdy, D., Gordon, L., Levinson, R.D., & Lanza, R. (2014). Mesenchymal stem cell population derived from human pluripotent stem cells displays potent immunomodulatory and therapeutic properties. Stem Cells and Development, 23(14), 1611–1624.
Le Blanc, K., Tammik, C., Rosendahl, K., Zetterberg, E., & Ringdén, O (2003). HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Experimental Hematology, 31 (10), 890–896.
Li, O., Tormin, A., Sundberg, B., Hyllner, J., Le Blanc, K., & Scheding, S. (2013). Human embryonic stem cell-derived mesenchymal stroma cells (hES-MSCs) engraft in vivo and support hematopoiesis without suppressing immune function: implications for off-the shelf ES-MSC therapies. PLoS ONE, 8(1), e55,319.
Lian, Q., Lye, E., Suan Yeo, K., Khia Way Tan, E., Salto-Tellez, M., Liu, T.M., Palanisamy, N., El Oakley, R.M., Lee, E.H., Lim, B., & Lim, S.K. (2007). Derivation of clinically compliant mscs from cd105+, cd24- differentiated human escs. Stem Cells, 25(2), 425–36. doi:10.1634/stemcells.2006-0420.
Luzzani, C., Neiman, G., Garate, X., Questa, M., Solari, C., Fernandez Espinosa, D., Garcia, M., Errecalde, A.L., Guberman, A., Scassa, M.E., Sevlever, G.E., Romorini, L., & Miriuka, S.G. (2015). A therapy-grade protocol for differentiation of pluripotent stem cells into mesenchymal stem cells using platelet lysate as supplement. Stem Cell Research & Therapy, 6, 6. doi:10.1186/scrt540.
Mahmood, A., Harkness, L., Schroder, H.D., Abdallah, B.M., & Kassem, M. (2010). Enhanced differentiation of human embryonic stem cells to mesenchymal progenitors by inhibition of tgf-beta/activin/nodal signaling using sb-431542. Journal of Bone and Mineral Research, 25(6), 1216–33. doi:10.1002/jbmr.34.
Miao, Q., Shim, W., Tee, N., Lim, S.Y., Chung, Y.Y., Ja, K.P.M.M., Ooi, T.H., Tan, G., Kong, G., Wei, H., Lim, C.H., Sin, Y.K., & Wong, P. (2014). iPSC-derived human mesenchymal stem cells improve myocardial strain of infarcted myocardium. Journal of Cellular and Molecular Medicine, 18(8), 1644–1654.
Olivier, E.N., Rybicki, A.C., & Bouhassira, E.E. (2006). Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem Cells, 24(8), 1914–22. doi:10.1634/stemcells.2005-0648 10.1634/stemcells.2005-0648.
de Peppo, G.M., & Marolt, D. (2013). Modulating the biochemical and biophysical culture environment to enhance osteogenic differentiation and maturation of human pluripotent stem cell-derived mesenchymal progenitors. Stem Cell Research & Therapy, 4(5), 106. doi:10.1186/scrt317.
Pittenger, M.F., Mackay, A.M., Beck, S.C., Jaiswal, R.K., Douglas, R., Mosca, J.D., Moorman, M.A., Simonetti, D.W., Craig, S., & Marshak, D.R. (1999). Multilineage potential of adult human mesenchymal stem cells. Science, 284(5411), 143–147.
Potian, J.A., Aviv, H., Ponzio, N.M., Harrison, J.S., & Rameshwar, P. (2003). Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. Journal of immunology (Baltimore Md: 1950), 171(7), 3426–3434.
Qi, X., Zhang, J., Yuan, H., Xu, Z., Li, Q., Niu, X., Hu, B., Wang, Y., & Li, X. (2016). Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. International Journal of Biological Sciences, 12(7), 836–49. doi:10.7150/ijbs.14809.
Sanchez, L., Gutierrez-Aranda, I., Ligero, G., Rubio, R., Munoz-Lopez, M., Garcia-Perez, J.L., Ramos, V., Real, P.J., Bueno, C., Rodriguez, R., Delgado, M., & Menendez, P. (2011). Enrichment of human esc-derived multipotent mesenchymal stem cells with immunosuppressive and anti-inflammatory properties capable to protect against experimental inflammatory bowel disease. Stem Cells, 29(2), 251–62. doi:10.1002/stem.569.
Sethe, S., Scutt, A., & Stolzing, A. (2006). Aging of mesenchymal stem cells. Ageing Res Rev, 5(1), 91–116. doi:10.1016/j.arr.2005.10.001.
Stavropoulos, M.E., Mengarelli, I., & Barberi, T. (2009). Differentiation of multipotent mesenchymal precursors and skeletal myoblasts from human embryonic stem cells. Current Protocols in Stem Cell Biology Chapter 1:Unit 1F.8.
Stolzing, A., Jones, E., McGonagle, D., & Scutt, A. (2008). Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mechanisms of Ageing and Development, 129(3), 163–173. doi:10.1016/j.mad.2007.12.002.
Sun, Y.Q., Zhang, Y., Li, X., Deng, M.X., Gao, W.X., Yao, Y., Chiu, S.M., Liang, X., Gao, F., Chan, C.W., Tse, H.F., Shi, J., Fu, Q.L., & Lian, Q. (2015). Insensitivity of human iPS cells-derived mesenchymal stem cells to interferon- γ-induced HLA expression potentiates repair efficiency of hind limb ischemia in immune humanized NOD Scid Gamma Mice. Stem Cells, 33(12), 3452–3467.
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663–676.
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., & Yamanaka, S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131(5), 861–872.
Tan, F., Qian, C., Tang, K., Abd-Allah, S.M., & Jing, N. (2015). Inhibition of transforming growth factor β (tgf- β) signaling can substitute for oct4 protein in reprogramming and maintain pluripotency. The Journal of Biological Chemistry, 290(7), 4500–11. doi:10.1074/jbc.M114.609016.
Thomson, J.A., Itskovitz-Eldor, J., Shapiro, S.S., Waknitz, M.A., Swiergiel, J.J., Marshall, V.S., & Jones, J.M. (1998). Embryonic stem cell lines derived from human blastocysts. Science, 282(5391), 1145–1147.
Timmers, L., Lim, S.K., Arslan, F., Armstrong, J.S., Hoefer, I.E., Doevendans, P.A., Piek, J.J., El Oakley, R.M., Choo, A., Lee, C.N., Pasterkamp, G., & de Kleijn, D.P.V. (2007). Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res, 1(2), 129–137. doi:10.1016/j.scr.2008.02.002.
Trivedi, P., & Hematti, P. (2008). Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Experimental hematology, 36(3), 350–359.
Ullmann, U., In’t Veld, P., Gilles, C., Sermon, K., De Rycke, M., Van de Velde, H., Van Steirteghem, A., & Liebaers, I. (2007). Epithelial-mesenchymal transition process in human embryonic stem cells cultured in feeder-free conditions. Molecular human reproduction, 13(1), 21–32.
Wang, S., Qu, X., & Zhao, R.C. (2012). Clinical applications of mesenchymal stem cells. Journal of hematology & oncology, 5, 19.
Wang, X., Kimbrel, E.A., Ijichi, K., Paul, D., Lazorchak, A.S., Chu, J., Kouris, N.A., Yavanian, G.J., Lu, S.J., Pachter, J.S., Crocker, S.J., Lanza, R., & Xu, R.H. (2014). Human ESC-Derived MSCs Outperform Bone Marrow MSCs in the Treatment of an EAE Model of Multiple Sclerosis. Stem cell reports, 3(1), 115–130.
Wu, R., Gu, B., Zhao, X., Tan, Z., Chen, L., Zhu, J., & Zhang, M. (2013). Derivation of multipotent nestin(+)/CD271 (-)/STRO-1 (-) mesenchymal-like precursors from human embryonic stem cells in chemically defined conditions. Human cell, 26(1), 19–27.
Xu, C., Inokuma, M.S., Denham, J., Golds, K., Kundu, P., Gold, J.D., & Carpenter, M.K. (2001). Feeder-free growth of undifferentiated human embryonic stem cells. Nature Biotechnology, 19(10), 971–974.
Xu, C., Jiang, J., Sottile, V., McWhir, J., Lebkowski, J., & Carpenter, M.K. (2004). Immortalized fibroblast-like cells derived from human embryonic stem cells support undifferentiated cell growth. Stem Cells, 22 (6), 972–980.
Yeo, R.W.Y., Lai, R.C., Zhang, B., Tan, S.S., Yin, Y., Teh, B.J., & Lim, S.K. (2013). Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Advanced drug delivery reviews, 65(3), 336–341.
Zhang, J., Guan, J., Niu, X., Hu, G., Guo, S., Li, Q., Xie, Z., Zhang, C., & Wang, Y. (2015a). Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. Journal of Translational Medicine, 13, 49.
Zhang, Y., Liao, S., Yang, M., Liang, X., Poon, M.W., Wong, C.Y., Wang, J., Zhou, Z., Cheong, S.K., Lee, C.N., Tse, H.F., & Lian, Q. (2012). Improved cell survival and paracrine capacity of human embryonic stem cell-derived mesenchymal stem cells promote therapeutic potential for pulmonary arterial hypertension. Cell Transplantation, 21(10), 2225–2239.
Zhang, Y., Liang, X., Liao, S., Wang, W., Wang, J., Li, X., Ding, Y., Liang, Y., Gao, F., Yang, M., Fu, Q., Xu, A., Chai, Y.H., He, J., Tse, H.F., & Lian, Q. (2015b). Potent Paracrine Effects of human induced Pluripotent Stem Cell-derived Mesenchymal Stem Cells Attenuate Doxorubicin-induced Cardiomyopathy. Scientific reports 5:11, 235.
Zhou, S., Greenberger, J.S., Epperly, M.W., Goff, J.P., Adler, C., Leboff, M.S., & Glowacki, J. (2008). Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell, 7(3), 335–343. doi:10.1111/j.1474-9726.2008.00377.x.
Acknowledgments
This work is possible thanks to supporting grants from CONICET (PIP-112-20150100723) and FONCYT (PID-2014-0052, PICT-2015-1469, PICT-2015-0868, y PICT-2015-3850). We thank Fundación FLENI and Fundación Pérez Companc for their support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Conflict of Interests
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Luzzani, C.D., Miriuka, S.G. Pluripotent Stem Cells as a Robust Source of Mesenchymal Stem Cells. Stem Cell Rev and Rep 13, 68–78 (2017). https://doi.org/10.1007/s12015-016-9695-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-016-9695-z