Abstract
Stem cells are characterized by their self-renewal and multi-lineage differentiation potential. Stem cell differentiation is a prerequisite for the application of stem cells in regenerative medicine and clinical therapy. In addition to chemical stimulation, mechanical cues play a significant role in regulating stem cell differentiation. The integrity of mechanical sensors is necessary for the ability of cells to respond to mechanical signals. The nucleus, the largest and stiffest cellular organelle, interacts with the cytoskeleton as a key mediator of cell mechanics. Nuclear mechanics are involved in the complicated interactions of lamins, chromatin and nucleoskeleton-related proteins. Thus, stem cell differentiation is intimately associated with nuclear mechanics due to its indispensable role in mechanotransduction and mechanical response. This paper reviews several main contributions of nuclear mechanics, highlights the hallmarks of the nuclear mechanics of stem cells, and provides insight into the relationship between nuclear mechanics and stem cell differentiation, which may guide clinical applications in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stem cells, characterized by their self-renewal and multi-lineage potential, hold great promise in cytotherapy and tissue engineering [1–3]. Controlled differentiation is a prerequisite for stem cell application in clinical treatments. In addition to chemical factors, mechanical stimulation has drawn extensive attention as a key modulator in the regulation of stem cells. For instance, stem cells differentiate into distinct lineages depending on the elasticity and stiffness of their microenvironment [4]. Regardless of whether they are cultured in vitro or in vivo, stem cells inevitably encounter complex mechanical microenvironments. The integrity of the mechanical sensory system is thus indispensable for sensing and transmitting mechanical cues from the environment of a cell to its nucleus. These sensors include integrin proteins, the actin cytoskeleton, the nucleus and others, with the nucleus being one of most important contributors [5].
The nucleus is approximately 5–10 times stiffer than the cytoskeleton [6]. The mechanical stability of the nucleus is required for cellular functions because many fundamental biological behaviors occur in the nucleus. In addition, the interaction of the nuclear mechanical structure with the cytoskeleton defines the structural and mechanical integrity of cells [6, 7]. Alterations in nuclear mechanics may cause inefficient adaptation and transduction of mechanical signals, which could result in unusual reactions [7].
Interestingly, stem cell differentiation is accompanied by real-time changes in nuclear mechanical properties due to the diverse expression of nucleoskeleton components [8–11]. Nuclear mechanics are also indispensable for the multi-lineage potential of stem cells. For example, MSCs derived from the iPSCs of a patient who suffered from a truncated form of Lamin A/C called progerin had limited viability both in vitro and in vivo after being treated for stress and hypoxia [2]. This paper reviews the primary contributors to nuclear mechanics, summarizes the nuclear mechanics of stem cells, and provides insight into the relevance of nuclear mechanics and stem cell differentiation, which may guide clinical applications of stem cells in the future.
Contributors to Nuclear Mechanics
Based on structure and function, the nucleus is divided into the nuclear envelope and the nuclear interior (Fig. 1) [12–14]. The outer nuclear membrane (ONM), the inner nuclear membrane (INM) and the nuclear lamina compose the nuclear envelope, which separates the nucleus from the cytoplasm. The ONM and the INM consist of phospholipid bilayers that contain a multitude of randomly dispersed nuclear pore complexes. These nuclear pore complexes enable bidirectional, passive movement of small molecules (less than 40 kDa) and benefit the transport of larger (>40 kDa) ones with the mediation of nuclear import and export proteins [12]. The nuclear lamina underlies the INM and is a layer of dense protein networks mainly composed of lamins and lamin-associated proteins [14]. Various lamin-binding proteins facilitate the connection of the lamina, the INM and chromatin, sustaining the stabilization of nuclear mechanics [15]. The nuclear interior primarily contains chromatin and structural proteins [14]. Formed by DNA and histones, chromatin fibers occupy distinct and nonrandom chromosome territories within an inter-phase nuclear interior. Other nuclear structures, such as the nucleoli, Cajal bodies and promyelocytic leukemia (PML) proteins, are localized in different regions of the nucleus [13]. In addition, other structural proteins, such as lamin A/C and nuclear actins, are found in abundance in the nuclear interior [12].
Schematic diagram of the nuclear structure and components. The nucleus is divided into the nuclear interior and the nuclear envelope. The nuclear envelope separates the nucleus from the cytoplasm and is composed of the outer nuclear membrane (ONM), the inner nuclear membrane (INM) and the nuclear lamina. The ONM and INM consist of phospholipid bilayers with a multitude of nuclear pore complexes randomly distributed on the nuclear envelope. Three components of the cytoskeleton, microtubules, actin and intermediate filaments, physically connect the nuclear envelope through nuclear binding proteins, such as Nesprin 1/2, Plectin and Sun 1/2. In addition, these proteins sustain nuclear mechanical stabilization by connecting the lamina with the INM and chromatin. Underlying the INM is the nuclear lamina, which is a layer of dense protein networks composed of lamin proteins and lamin-associated proteins. The nuclear interior contains chromatin and a number of other nuclear structures, such as nucleoli, Cajal bodies and promyelocytic leukemia (PML) protein bodies. Composed of DNA and histones, chromatin fibers occupy nonrandom chromosomal territories within the inter-phase nuclear interior. Moreover, abundant structural proteins, such as lamin A/C and nuclear actins, are found in the nuclear interior
The nucleus is generally the largest and stiffest organelle in a eukaryotic cell and, as such, it defines the cell’s geometrical shape [13]. It is also the principal organelle that responds to mechanical signals, which ultimately cause a series of reactions ranging from gene expression to the reorganization of cellular structures [7, 14, 16, 17]. In general, lamin proteins, chromatin and other nucleoskeleton-related proteins are considered the main contributors to nuclear mechanics (Table 1) [13–15, 23].
Lamin Proteins
Lamins are type V intermediate filament proteins localized under the INM. Lamins combine with nucleoskeleton-related proteins to form a stable and dense layer called the nuclear lamina. Lamins are divided into A-type and B-type lamins, which are encoded by different genes [26]. Lamin A and lamin C, the A-type lamins, are the products of alternatively spliced isoforms of the LMNA gene; B-type lamins can be subdivided into lamin B1 and lamin B2, encoded by LMNB1 and LMNB2, respectively [26]. Lamins exist in a dynamic equilibrium between the nuclear lamina at the periphery of the nucleus and the nuclear interior [22]. Lamin B is ubiquitously expressed in all types of mammalian cells. Lamin B appears to be dispensable for nuclear stiffness, but defects in this protein result in an increase in nuclear blebbing [27]. Lamin A/C is expressed in almost all cell types, except ESCs and HSCs [18]. Intriguingly, the nuclei of ESCs are softer than those of differentiated cells, whereas HSCs and MSCs have an intermediate level of stiffness and deform irreversibly [9]. Furthermore, mouse embryonic fibroblasts lacking lamin A/C have a significantly reduced nuclear stiffness and an increased percentage of misshapen nuclei [20]. Together, these data explicitly indicate the vital role of lamin proteins, particularly lamin A/C, in nuclear mechanics.
The nuclei of endothelial cells are elongated and have a lower height following stimulation with a fluid shear stress of 2 Pa [21]. Furthermore, the deformation of nuclei induced by fluid shear stress appears to be irreversible; nuclei isolated from endothelial cells after an exposure to fluid shear stress retain their elongated shape. The elastic modulus of elongated nuclei was 0.627 ± 0.15 kPa, significantly higher than that of control cells, which was 0.427 ± 0.12 kPa [21]. In another study in HeLa cells, lamin A/C was up-regulated and translocated from the nuclear interior to the nuclear periphery in response to shear stress, resulting in stiffened nuclei [16]. However, cells transfected with lamin A△50, a mutated phenotype of lamin A, formed stiffer nuclei with thicker nuclear lamina, thus reducing their response to shear stress [16]. This effect was likely due to a reduced mechanical response of the mutated lamin A compared with normal lamin A. In addition, strain decreased the viability of mouse embryonic fibroblasts with lamin A/C defects [16]. These results further emphasize the significant role of lamin A in nuclear mechanics and demonstrate that cells can actively adjust mechanical stimulation to remodel their nuclear mechanical structure by redistributing lamins in the nucleus.
Lamin A/C also plays an essential role in the mechano-transduction of cells in response to mechanical signals. Lamins, chromatin, actin and linkers of the nucleus and the cytoskeleton (LINC) complexes interact with each other, mutually organizing a stable structure to link the nucleus and cytoplasm [13]. Defects in A-type lamins in fibroblasts result in the loss of emerin and nesprin-3 from the nuclear envelope, leading to an abnormal connection between the nucleus and cytoskeleton [13]. Delayed wound healing has also been found, possibly due to the deformed orientation of the nucleus and microtubule-organizing center and the loss of nuclear oscillatory rotation [24, 28, 29]. LMNA−/− mouse embryonic fibroblasts exhibit increased nuclear fragility and sensitivity to mechanical strain [28]. However, HGPS patients, who have increased expression of wild-type and mutant lamin A, have stiffer nuclei [30]. In fact, nearly all load-bearing tissues, such as the bones and cardiovascular tissues, in HGPS patients have a severe prematurely aged phenotype [30]. However, these symptoms are rarely found in soft tissues such as the brain and other internal organs. These data further support the importance of lamin A/C in mechanotransduction. Moreover, mouse embryonic fibroblasts lacking lamin A/C display decreased nuclear stiffness due to the disrupted links between the nucleus and the cytoskeleton. This leads to altered nuclear dynamics, cell polarization and cell migration [20]. In summary, lamins, particularly lamin A/C, are indispensable and predominant contributors to nuclear mechanics. Lamins are critical proteins that support the nucleus and the cell; cells lacking lamins cannot mechanotransduce effectively or respond to mechanical signals.
Chromatin
Chromatin, a major component of the inner nucleus, is a complex of DNA and histones that can be found as euchromatin or heterochromatin. Generally, euchromatin is located in the nuclear interior and is abundant in active genes. Conversely, heterochromatin is a densely packed chromatin that binds with lamins and histones at the periphery of nucleus and contains a large number of genes that have been silenced due to their altered chromatin configuration [13, 14, 24].
ESCs are the best candidates for investigating the role of chromatin on nuclear mechanics due to their lack of lamin A/C in the nucleus. Hence, the nuclear mechanics of ESCs are dependent on chromatin mechanics. The nuclei of ESCs display a remarkable compliance that implies a high accessibility to chromatin [9]. Although chromatin was easily deformed during initial periods of mechanical stress, it displayed an increased resistance to deformation in later periods [9]. Furthermore, chromatin was sensitive to the density of divalent cations such as Ca2+ and Mg2+. Salt-condensed chromatin showed an increased stiffness and decreased pliability, which probably resulted from a change in the conformation of the chromatin and the intensive interaction between chromatin and other nuclear components [9]. The nuclei of stem cells also showed a fluid-like phenotype with remarkable deformability, which was attributed to low lamin A/C expression and relatively slack chromatin [9, 10]. As a result, stem cells can readily migrate through solid tissues by remodeling their cellular and nuclear morphology, while accommodating a large number of active genes in their nuclear interior. During differentiation, cell structures and functions change and heterochromatin begins to appear at the periphery of the nucleus due to the binding of lamins and nuclear binding proteins [31]. Increased expression of histone proteins and lamin A/C restricts chromatin dynamics, leading to the poor deformability and plasticity of nuclei in ESCs [8].
Nucleoskeletal Proteins
In addition to lamins, a number of other nuclear structural proteins also play a role in connecting the cytoskeleton to the nucleus. These include LINC complexes, the lamin B receptor (LBR), emerin, and others [13]. LBR and emerin directly bind lamins, which allows these complexes to bind to the INM [13, 32]. MAN-1, LAP2α and retinoblastoma (Rb) protein not only act as linkers between lamins and INM but also regulate gene expression by interacting with chromatin [14]. LINC complexes are mainly composed of SUN proteins and nesprin proteins [19, 33]. Consisting of SUN1 and SUN2, SUN proteins are located at the INM and interact with lamins, chromatin, and nuclear pore complexes [34]. SUN proteins bind nesprins on the ONM via their conserved KASH domain. An N-terminal actin-binding domain in the giant isoforms of nesprin-1 and nesprin-2 enables a physical link between the nuclear interior and cytoskeletal components, including actin microfilaments, microtubules and intermediate filaments [34]. Additionally, nuclear actin facilitates the association between chromatin and lamins; however, this effect is not readily detected by phalloidin, possibly because of the resulting changed configuration [15].
Nucleoskeleton-related proteins interact with chromatin, lamins, nuclear pore complexes and cytoskeleton, joining them into a complex conformation. For example, during ESC differentiation, increased expression of syn1/nesprin-1 results in a restructuring of the nucleus and chromatin [8]. Although LINC complexes have a minimal impact on 2D migration, they play an important role in regulating cell migration in a 3D collagen I matrix by influencing the organization of actin cap fibers [34]. Abnormal LINC complexes lead to reduced mechanotransduction, nuclear migration and chromosome positioning [33]. Similar effects (reduced mechanical sensation, cell motility and mechanotransduction) have also been found in cells with deficient perinuclear actin caps [35]. These phenomena demonstrate the pivotal role of a normal distribution and configuration of the nucleoskeleton in cell functions.
Nuclear Mechanical Hallmarks in Stem Cells
ESCs have a Young’s modulus as low as 0.35 kPa and are recognized as one of the softest stem cells. While HSCs and MSCs have a higher Young’s modulus, 2.4 ± 1.6 kPa and 1.1 ± 0.42 kPa, respectively, these cell types are still more pliable than mature cells. For instance, the stiffness of human umbilical vein endothelial cells (HUVECs) is approximately 10 kPa, while shear stress makes HUVECs exhibit an even higher Young’s modulus (12.0–18 kPa). Similar results have been obtained in endothelial cells (ECs) from bovine thoracic aortas. Data show that shear stress makes the nuclei in ECs significantly stiffer than in unstressed cells (0.62 ± 0.15 kPa and 0.42 ± 0.12 kPa, respectively). Furthermore, with a stiffness reaching approximately 3.5 kPa and 12.8 ± 0.3 kPa, respectively, osteoblasts and vessel smooth muscle cells also show stiff mechanical characteristics. Interestingly, tumor cells show a similar cell stiffness to stem cells. The Young’s modulus of lung carcinoma and breast ductal adenocarcinoma is 0.56 ± 0.09 and 0.50 ± 0.08 kPa, respectively. Of note, malignant tumors are more pliable than less invasive cells. For example, leukemia lymphoid (Jurkat) cells are significantly softer than leukemia myeloid cells (HL60), with a Young’s modulus of 0.02–0.08 kPa and 0.2–1.4 kPa, respectively. Similar results have been found comparing a less invasive ovarian cancer cell line (HEY) and more highly invasive ovarian cancer cells (HEYA8) (0.884 ± 0.529 kPa and 0.494 ± 0.222, respectively). To summarize, stem cells and tumor cells have a similar cell stiffness, which is lower than that of differentiated cells (Table 2).
As discussed above, the low stiffness of stem cells may be related to nuclear mechanics because the nucleus is a dominant regulator of cell stiffness. Despite their high ratio of nucleus to cytoplasm, stem cells typically have more pliable and plastic nuclei than mature cells. For example, the nuclei in human ESCs are highly deformable and stiffen 6-fold after terminal differentiation, whereas the nuclei in adult stem cells possess an intermediate stiffness [9]. Micropipette aspiration assays have been conducted to compare the mechanical properties of HSCs, bone marrow-derived MSCs and perivascular-derived MSCs. The results suggest that HSCs exhibit fluid-like mechanical properties, whereas MSCs from bone marrow and perivascular niches are mechanically stable [42]. However, another study found that the stiffness of HSCs, at 2.4 ± 1.6 kPa, was higher than that of MSCs, at approximately 1.1 ± 0.42 kPa [10]. Further research found that although HSCs are stiffer than MSCs at the incipient loading phase, they display higher viscoelasticity for a longer time [9, 10, 31]. Because the nucleus is stiffer than the cytoplasm, we speculate that HSCs, which have a higher ratio of nucleus to cytoplasm, are stiffer during the incipient period, whereas they are more pliable and fluid-like at later phases. Furthermore, the nucleoskeletal component lamin A/C, which is expressed abundantly in almost all differentiated cell types, is not expressed in either ESCs or HSCs and is only expressed in MSCs at intermediate levels [18, 42]. This finding may at least partially explain the lower Young’s modulus of stem cells compared with differentiated cells. To further test this hypothesis, Dahl et al. knocked down lamin A/C expression in human epithelial cells and found that the deformability of these cells was similar to HSCs [24]. Therefore, a lower expression of lamin A/C can explain the observation that the nuclei of stem cells are softer and more plastic. Moreover, Shin et al. found that lamins contribute to both the trafficking and the differentiation of HSCs [43]. Harada et al. also found that lamins impede 3D migration but promote survival against migration-induced stresses [44]. These findings further demonstrate the role of nuclear lamin in cell trafficking and uncover the link between lamins and nuclear rheology.
Rheological features are also essential parameters for describing nuclear mechanics. Chromatin plays a more significant role than lamin A/C in controlling the rheological properties of the nucleus. Compared with differentiated cells, the chromatin in stem cells was relatively loose and showed fluid-like characteristics [45]. Additionally, chromatin is sensitive to divalent cations, such as Ca2+ and Mg2+, which cause it to condense [24]. Interestingly, chromatin condensation by divalent cations resulted in stiffer nuclei during the incipient phase. Nevertheless, it exhibited remarkable resistance at later stages, as demonstrated in micropipette aspiration experiments [24].
Intriguingly, cancer cells have high proliferation and migration potentials, similar to stem cells. Abundant evidence suggests that the similarities between the biological properties of tumor cells and stem cells are associated with their analogous nuclear mechanical structures [40, 41, 46]. As an essential contributor to nuclear mechanics, lamin A/C is expressed equally in stem cells and tumor cells in some tissues, but this expression is dissimilar to mature cells [12]. For example, lamin A/C is expressed in colon stem cells although it is not expressed in normal colon cells [19]. Therefore, we postulate that lamin A/C is a novel biomarker of colon epithelial stem cells. Patients with tumor cells that express lamin A/C have a significantly worse prognosis than patients with lamin A/C negative tumors. Furthermore, a low expression level of lamin A significantly increases cell motility and invasiveness without influencing cell proliferation [19]. Tumors that express lamin A/C display stem-cell-like phenotypes. In addition, up-regulation of lamin A/C stimulates expression of the actin binding protein T-plastin, which subsequently inhibits the expression and activity of the cell adhesion molecule E-cadherin [12, 19]. These results may explain the strong motility and migration potentials of both tumor cells and stem cells. Furthermore, the expression and activity levels of other nucleoskeleton-related proteins, such as LAP2α, LBR, and emerin, are also altered in tumor cells [19]. The process of cancerization is considered the reverse of the process of stem cell differentiation. Alterations in nuclear mechanics and abnormal expression levels of lamins and nucleoskeleton-related proteins in tumor cells may change their chromatin configuration. Such changes may re-activate silent genes in heterochromatin, inducing dysfunctional gene expression [19]. For example, an abnormal distribution and expression of lamin A, emerin and LAP2α resulted in the dysregulation of proliferation in tumor cells [19, 23, 47].
Nuclear mechanics is also important for stem cell function and development. The integrity of the nuclear lamina provides a solid foundation for normal cell motility, nuclear dynamics and cell polarization, which was found to be mediated by lamin A/C during cell migration [13]. During early differentiation periods, histone proteins and lamin A help regulate chromatin protein dynamics [8]. Heterochromatin separates gradually from euchromatin by binding with lamins and histone proteins. In addition, structural changes on the nuclear envelope also occur during the differentiation of ESCs, a process that is regulated by the increased expression of syne-1 and nesprin-1 [48]. Swift et al. showed that lamin A responds to matrix elasticity and influences downstream differentiation [49]. Buxboim et al. further explained the mechanism of lamin A/C mechanosensing [50]. In the absence of lamin A/C binding to chromatin, chromatin is capable of mobilizing in the central parts of the nuclei where most gene expression and RNA transcription take place. The high compliance of the nuclei of stem cells reflects a high transcriptional accessibility of chromatin [18]. Additionally, stem cells take advantage of their plastic nuclei to readily squeeze through solid tissues, which may explain the persistent migration of stem cells [48]. During differentiation, increasing levels of lamin A/C not only compress the nucleus but also constrain chromatin into relatively small zones by directly binding to it [6, 19]. Thus, heterochromatin is progressively formed at the nuclear periphery and only part of the chromatin dwells in the nuclear interior [24]. When condensed chromatin and lamin A/C accumulate at the periphery of the nucleus, a stiffer and more solid-like nucleus arises [19]. As a result, the potential of differentiated cells is limited and specialized because only a small portion of the chromatin remains active in the central nucleus.
Nuclear Mechanics and Stem Cell Differentiation
Nuclear Mechanics and Stem Cell Differentiation toward Osteoblasts and Adipocytes
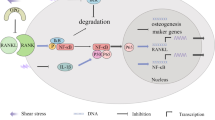
The ability to give rise to osteoblasts and adipocytes is a fundamental trait of MSCs. Although a variety of mechanical stimulations have been reported to mediate stem cell differentiation, the impact of intrinsic nuclear mechanics on stem cell differentiation has not been studied extensively. When MSCs were treated with lamin A/C siRNA and cultured in osteogenic-inducing culture media, a significant reduction in the expression of alkaline phosphatase (ALP), osteocalcin (OCN), bone sialo-protein (BSP), osterix (OSX) and alizarin red staining was observed [22]. By contrast, the potential of these cells to give rise to an adipogenic lineage was increased. Interestingly, a decreased osteogenic potential of stem cells was always accompanied by an increased adipogenic potential. This shift in the balance between osteogenic and adipogenic lineages may be caused by an indirect suppression of the expression and physical binding of lamin A/C to Runt-related transcription factor 2 (Runx2); adipocyte-specific transcript factor γ2 (PPARγ2) interacts with Runx2 to control lineage specification [51].
Runx2 is recognized as an essential nuclear effector that actively participates in the regulation of osteogenesis via the activation of LEF1/TCFs and β-catenin complexes [52]. Although the absence of lamin A/C did not affect Runx2 expression, it significantly promoted the expression of MAN-1, a nuclear envelope protein [22, 53]. Elevated levels of free MAN-1 in the nucleus impair the DNA-binding activity of Runx2 [53]. Further investigation is needed to examine whether the mechanism for the increase in free MAN-1 is an increase in the transcription level or the protein stability of MAN-1. Regardless, the accumulated free MAN-1 induced by lamin A/C deficiency may inhibit osteogenesis by diminishing Runx2 activity.
The tumor growth factor-β (TGF-β)/Smad pathway is a central regulator of mesenchymal tissue homeostasis and has been implicated in the positive regulation of osteogenic differentiation [24, 34]. For instance, in senile osteoporosis, the decreased expression of TGF-β in MSCs in aged mice led to a shortage of osteoblasts in the bone matrix [24]. It has been reported that MAN-1 is closely associated with the maintenance of Smad proteins and antagonizes TGF-β in the nucleus [54]. Notably, lamin A/C binds directly to both TGF-β-induced Smad 2/3 and Rb proteins in embryonic fibroblasts [23, 53]. Therefore, the regulatory mechanism of the TGF-β signaling pathway could be mediated by both lamin A/C and MAN-1. In addition, the canonical Wnt/β-catenin pathway is another essential signaling pathway for the regulation of stem cell homeostasis, particularly during osteogenesis and adipogenesis. Excessive emerin protein binds β-catenin and subsequently diminishes the accumulation of β-catenin in the nucleus. Hence, the transcriptional activation of TCF/LEF that is induced by β-catenin is compromised, resulting in a decrease in the secretion of osteopontin (OPN) and OCN [22, 23, 53].
Nuclear Mechanics of Stem Cells during Myoblast Differentiation
In response to injured muscles, a strong mobilization of somatic stem cells to the injured sites is needed for their participation in tissue repair and regeneration. To assess the role of lamin A/C on this process, MSCs expressing lamin A/C mutations (HGPS-MSCs) were obtained from iPSCs derived from Hutchinson-Gilford progeria syndrome (HGPS) patients who expressed a truncated and farnesylated form of lamin A/C called progerin [2]. Notably, a remarkable increase in nuclear lobulations and malformations and misdistribution of lamin-associated polypeptide 2α (LAP2α) was observed in HGPS-MSCs [2]. Additionally, HGPS-MSCs died much faster than normal MSCs in ischemic limbs, likely as a result of their unusual nuclei and accumulated DNA damages [2, 55, 56]. Moreover, the capability of HGPS-MSCs to promote tissue regeneration in ischemic hind limbs was also compromised [56]. This was possibly due to the decreased viability of the HGPS-MSCs and a decreased myogenetic potential. Another report showed that although the expression of lamin A/C and lamin B1 in C2C12 myoblasts remained unchanged during myogenesis, the solubility and distribution of lamin A/C was different. Moreover, the expression of lamin B2 was enhanced, with a twofold decrease in the expression of LAP2α [19]. This change in the solubility and redistribution of lamin A/C could be another mechanism for the regulation of nuclear mechanics and myogenesis. Furthermore, C2C12 myoblasts transfected with wild-type lamins had decreased myogenetic potential, whereas C2C12 myoblasts transfected with mutant lamins lost their potential for myogenetic commitment [19]. Given that the dramatically enhanced expression of lamin A/C was accompanied by changes in the expression of myocardial markers in 5-azacytidine-induced human adipose-derived stem cells, lamin A/C may be considered a biomarker for cardiomyocyte differentiation of stem cells [57]. Regardless of the discrepancy between lamin A/C expression in stem cells and C2C12 myoblasts during myogenesis, which could be attributed to differences in cell type, normal nuclear mechanics and its related components appear indispensable for the myogenetic commitment of stem cells.
Myogenetic transcription factor, MyoD, is a prerequisite for the activation of MyoD-dependent target genes and subsequent initiation of muscle differentiation. The interaction of dephosphorylated Rb with MyoD in the nucleus is required to execute MyoD functions. In fibroblasts, lamin A/C and LAP2α form a complex with Rb in the nucleus; when Rb is not in a complex it is depleted by proteasomal degradation [19]. The ectopic expression of MyoD in mutant myoblasts partially ameliorates their depressed myogenetic differentiation potential [23]. However, mice deficient in lamin A/C have delayed myogenetic differentiation kinetics because of the deregulation of the Rb/MyoD pathway that results from the loss of lamin A/C or emerin [12].
Effects of the Nuclear Mechanics of Stem Cells on the Differentiation of Epidermal Cells
Intriguingly, studies have suggested that nuclear mechanics is vital for the homeostasis of MSCs. However, there are few papers that study the relationship between nuclear mechanics and epithelial stem cells. Epithelial stem cells in Zmpste24-null mice displayed aberrant nuclear composition because of a deficiency in lamins and reduced proliferation associated with the Wnt signaling pathway and microphthalmia transcription factor [28]. The ability of HGPS-MSCs to mediate vascular circulation in ischemic hind limbs is reduced compared with normal MSCs, possibly because of the reduced expression of paracrine factors, including VEGF, bFGF and IL-6, from the HGPS-MSCs. These factors are all associated with epidermal differentiation; therefore, whether an abnormal expression of lamins in epithelial stem cells could impact the differentiation potential of these cells in ischemic hind limbs is an intriguing topic [2]. Moreover, exciting research on the adult stem cells found in colonic crypts unexpectedly showed that the putative epithelial stem cells in the basal crypts were positive for lamin A/C but negative for the proliferation biomarker PCNA [58]. Conversely, very weak or absent lamin A/C expression was detected in the rapidly proliferating cells in the colonic crypts [58]. Therefore, lamin A/C could be regarded as a biomarker of epithelial stem cells. Of note, colorectal cancer, one of the most prevalent cancers in the Western world, is widely considered to be caused by stem cell dysfunction [58].
Conclusions and Perspectives
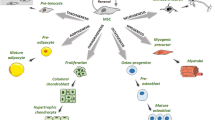
Nuclear mechanics are regulated by chromatin, lamin proteins and nucleoskeleton-related proteins and are implicated in multiple of biological behaviors [13, 14, 59]. Stem cell differentiation is affected by nuclear mechanics through two mechanisms. First, the ability of cells to sense and conduct mechanical signals changes throughout the differentiation process due to differences in the expression of mechanical components [23]. Stem cells with sufficient mechanotransduction give rise to mechanically sensitive cells, such as osteoblasts and myoblasts, whereas stem cells on a soft environment preferably differentiate into mechanically insensitive cells, such as adipocytes and neurocytes. Second, the appropriate expression and configuration of lamins, chromatin and nucleoskeleton-binding proteins at different stages of differentiation changes the nuclear mechanics of stem cells and allows stem cells to differentiate into mature cells (Fig. 2) [23, 31, 48].
Although there is increasing evidence on the intimate relationship between nuclear mechanics and stem cell differentiation, research focusing on nuclear mechanics and stem cell differentiation is at an incipient phase and the following standing issues need to be addressed: (1) What is the exact molecular mechanism for the ability of stem cells to sense and transmit mechanical signals from the extracellular environment to the nucleus? (2) How do nuclear mechanical components regulate gene expression and stem cell differentiation by physically modulating the configuration of chromatin? (3) Based on the similar biological characteristics and nuclear mechanics between stem cells and tumor cells, tumorigenesis is postulated to be a reversal of the process of stem cell differentiation. However, until the molecular mechanisms are better understood, the real relationship between these two processes cannot be validated.
References
Zhang, F., & Pasumarthi, K. B. (2008). Embryonic stem cell transplantation: promise and progress in the treatment of heart disease. BioDrugs, 22(6), 61–74.
Zhang, J. (2011). A human iPSC model of Hutchinson Gilford progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell, 8(1), 31–45.
Paolo, B., Mara, R., et al. (2001). Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells, 19, 180–192.
Engler, A. J., et al. (2006). Matrix elasticity directs stem cell lineage specification. Cell, 126(4), 77–89.
Guilak, F., et al. (2009). Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell, 5(1), 17–26.
Dahl, K. N., Booth-Gauthier, E. A., & Ladoux, B. (2010). In the middle of it all: mutual mechanical regulation between the nucleus and the cytoskeleton. Journal of Biomechanics, 43(1), 2–8.
Shivashankar, G. V. (2011). Mechanosignaling to the cell nucleus and gene regulation. Annual Review of Biophysics, 40, 361–378.
Melcer, S., et al. (2012). Histone modifications and lamin A regulate chromatin protein dynamics in early embryonic stem cell differentiation. Nature Communications, 3, 910.
Pajerowski, J. D., et al. (2007). Physical plasticity of the nucleus in stem cell differentiation. PNAS, 104(40), 15619–15624.
Ribeiro, A. J., & Dahl, K. N. (2012). The nuclear as a central structure in defining the mechanical properties of stem cells. In 32ed Annual International Conference of the IEEE EMBS (pp. 831–834).
Pekovic, V., & Hutchison, C. J. (2008). Adult stem cell maintenance and tissue regeneration in the ageing context: the role for A-type lamins as intrinsic modulators of ageing in adult stem cells and their niches. Journal of Anatomy, 213(1), 5–25.
Zwerger, M., Ho, C. Y., & Lammerding, J. (2011). Nuclear mechanics in disease. Annual Review of Biomedical Engineering, 13, 397–428.
Dahl, K. N., Ribeiro, A. J., & Lammerding, J. (2008). Nuclear shape, mechanics, and mechanotransduction. Circulation Research, 102(11), 1307–1318.
Martins, R. P., et al. (2012). Mechanical regulation of nuclear structure and function. Annual Review of Biomedical Engineering, 14, 431–455.
Zhong, Z., Wilson, K. L., & Dahl, K. N. (2010). Beyond Lamins: other structural components of the nucleoskeleton. Methods in Cell Biology, 98, 97–119.
Philip, J. T., & Dahl, K. N. (2008). Nuclear mechanotransduction: response of the lamina to extracellular stress with implications in aging. Journal of Biomechanics, 41(15), 3164–3170.
Vaziri, A., & Mofrad, M. R. (2007). Mechanics and deformation of the nucleus in micropipette aspiration experiment. Journal of Biomechanics, 40(9), 2053–2062.
Constantinescu, D., et al. (2006). Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells, 24(1), 177–185.
Coffinier, C., Fong, L. G., & Young, S. G. (2010). LINCing lamin B2 to neuronal migration growing evidence for cell-specific roles of B-type lamins. Nucleus, 1(5), 407–411.
Lee, J. S., et al. (2007). Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophysics Journal, 93(7), 2542–2552.
Deguchi, S., et al. (2005). Flow-induced hardening of endothelial nucleus as an intracellular stress-bearing organelle. Journal of Biomechanics, 38(9), 1751–1759.
Akter, R., et al. (2009). Effect of lamin A/C knockdown on osteoblast differentiation and function. Journal of Bone and Mineral Research, 24(2), 283–293.
Prokocimer, M., et al. (2009). Nuclear lamins: key regulators of nuclear structure and activities. Journal of Cell and Molecular Medicine, 13(6), 1059–1085.
Verstraeten, V. L., & Lammerding, J. (2008). Experimental techniques for study of chromatin mechanics in intact nuclei and living cells. Chromosome Research, 16(3), 499–510.
Horn, H. F., et al. (2013). The LINC complex is essential for hearing. Journal of Clinical Investigation, 123(2), 740–750.
Ho, C. Y., & Lammerding, J. (2012). Lamins at a glance. Journal of Cell Science, 125(9), 2087–2093.
Friedl, P., Wolf, K., & Lammerding, J. (2011). Nuclear mechanics during cell migration. Current Opinion in Cell Biology, 23, 55–64.
Espada, J., et al. (2008). Nuclear envelope defects cause stem cell dysfunction in premature-aging mice. Journal of Cell Biology, 181(1), 27–35.
Houben, F., et al. (2009). Disturbed nuclear orientation and cellular migration in A-type lamin deficient cells. Biochemical Biophysics Acta, 1793(2), 312–324.
Dahl, K. N., et al. (2006). Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. PNAS, 103(27), 10271–10276.
Hampoelz, B., & Lecuit, T. (2011). Nuclear mechanics in differentiation and development. Current Opinion in Cell Biology, 23(6), 668–675.
Wheeler, M. A., et al. (2007). Distinct functional domains in nesprin-1α and nesprin-2β bind directly to emerin and both interactions are disrupted in X-linked Emery–Dreifuss muscular dystrophy. Experimental Cell Research, 313(13), 2845–2857.
Alexandre, M., & Tom, M. (2010). LINC complexes in health and disease. Nucleus, 1(1), 40–52.
Khatau, S. B., et al. (2012). The distinct roles of the nucleus and nucleus-cytoskeleton connections in three-dimensional cell migration. Scientific Reports, 2, 488.
Shyam, B. K., Dong-Hwee, K., et al. (2010). The perinuclear avtin cap in health and disease. Nucleus, 1(4), 337–342.
Poh, Y. C., et al. (2010). Embryonic stem cells do not stiffen on rigid substrates. Biophysics Journal, 99(2), L19–L21.
Kuznetsova, T. G., et al. (2007). Atomic force microscopy probing of cell elasticity. Micron, 38(8), 824–833.
Takai, E., et al. (2005). Osteoblast elastic modulus measured by atomic force microscopy is substrate dependent. Annuals of Biomedical Engineering, 33(7), 963–971.
Qiu, H., et al. (2005). Short communication: vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circulation Research, 107(5), 615–619.
Cross, S. E., et al. (2008). AFM-based analysis of human metastatic cancer cells. Nanotechnology, 19, 384003.
Xu, W., et al. (2012). Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PloS One, 7(10), e46609.
Ribeiro, A. J., et al. (2012). Mechanical characterization of adult stem cells from bone marrow and perivascular niches. Journal of Biomechanics, 45(7), 1280–1287.
Shin, J. W., Spinler, K. R., Swift, J., et al. (2013). Lamins regulate cell trafficking and lineage maturation of adult human hematopoietic cells. Proceedings of the National Academy of Sciences of the United States of America, 110(47), 18892–18897.
Harada, T., Swift, J., Irianto, J., et al. (2014). Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. Journal of Cell Biology, 204(5), 669–682.
Christiaan, H. R., Merel, L. R., et al. (2011). Robust nuclear lamina-besed cell classification of aging and senescent cells. Aging, 3(12), 1192–1201.
Swaminathan, V., et al. (2011). Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Research, 71(15), 5075–5080.
Meshorer, E., & Gruenbaum, Y. (2008). Gone with the Wnt/Notch: stem cells in laminopathies, progeria, and aging. Journal of Cell Biology, 181(1), 9–13.
Smith, E. R., et al. (2011). Increased expression of Syne1/nesprin-1 facilitates nuclear envelope structure changes in embryonic stem cell differentiation. Development Dynamics, 240(10), 2245–2255.
Swift, J., Ivanovska, I. L., Buxboim, A., et al. (2013). Nuclear lamin-A scales with tissue stiffess and enhances matrix-directed differentiation. Science, 341(6149), 1240104.
Buxboim, A., Swift, J., Irianto, J., et al. (2014). Matrix elasticity regulates lamin-A, C phosphorylation and turnover with feedback to actomyosin. Current Biology, 24(16), 1909–1917.
Platt, I. D., & El-Sohemy, A. (2009). Regulation of osteoblast and adipocyte differentiation from human mesenchymal stem cells by conjugated linoleic acid. Journal of Nutrition Biochemistry, 20(12), 956–964.
Gaur, T., et al. (2005). Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. Journal of Biology Chemistry, 280(39), 33132–33140.
Li, W., et al. (2011). Decreased bone formation and osteopenia in lamin a/c-deficient mice. PloS One, 6(4), e19313.
Pan, D., et al. (2005). The integral inner nuclear membrane protein MAN1 physically interacts with the R-Smad proteins to repress signaling by the transforming growth factor-beta superfamily of cytokines. Journal of Biology Chemistry, 280(16), 15992–16001.
Ylva, R., Tomas, M., et al. (2011). stem cell depletion in Hutchinson-Gilford progeria syndrome. Aging Cell, 10, 1011–1020.
Katherine, L. W., Michael, S. Z., & Kenneth, K. L. (2001). Lamins and disease: insights into nuclear infrastructure. Cell, 104, 647–650.
Rodriguez-Serrano, F., et al. (2010). Promotion of human adipose-derived stem cell proliferation mediated by exogenous nucleosides. Cell Biology International, 34(9), 917–924.
Willis, N. D., Wilson, R. G., & Hutchison, C. J. (2008). Lamin A: a putative colonic epithelial stem cell biomarker which identifies colorectal tumors with a more aggressive phenotype. Biochemical Society Transactions, 36(6), 1350–1353.
Ivanovska, I., et al. (2010). Physical plasticity of the nucleus and its manipulation. Methods in Cell Biology, 98, 207–220.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 11272365), the Research Fund for the Doctoral Program of Higher Education of China (No. 20130191110029) and the Fundamental Research Funds for the Central Universities (106112015CDJZR238807).
Conflicts of interest
The authors indicate no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mao, X., Gavara, N. & Song, G. Nuclear Mechanics and Stem Cell Differentiation. Stem Cell Rev and Rep 11, 804–812 (2015). https://doi.org/10.1007/s12015-015-9610-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-015-9610-z