Abstract
Chronic atrophic gastritis (CAG) is a critical initial step in gastric cancer tumorigenesis accompanied by high malignancy. Erianin has been proposed as a promising agent in treating precancerous lesions of gastric cancer. Considering that little work has been implemented concerning the specific role and possible regulatory mechanism of Erianin in CAG, the goal of the study is to disclose the effects and mechanism of erianin on the malignant transformation in the process of CAG. CAG cell model was generated in human gastric epithelium GES-1 cells induced by Nmethyl-N’-nitro-N-nitrosoguanidine (MNNG). CCK-8 method determined cell viability. ELISA and corresponding assay kits severally appraised the contents of inflammatory cytokines and oxidative stress markers. Cellular reactive oxygen species (ROS) formation was measured by flow cytometry analysis using DCFH-DA probe. GFP-LC3 immunofluorescence staining and Western blotting evaluated autophagy. Also, Western blotting analyzed the expression of components in mitogen activated protein kinase (MAPK)/mechanistic target of rapamycin (mTOR) signaling. The results manifested that MNNG treatment diminished the viability and autophagy whereas intensified the inflammation and oxidative stress in GES-1 cells, which were all reversed by Erianin. Besides, Erianin blocked mTOR/MAPK signaling in MNNG-exposed GES-1 cells. Autophagy inhibitor 3-methyladenine (3-MA) or p38 MAPK agonist asiatic acid partially counteracted the protection elicited by Erianin against viability loss, inflammatory reaction as well as oxidative stress in MNNG-induced GES-1 cells. Combined with the findings, Erianin might mediate autophagy to improve MNNG-elicited CAG via MAPK/mTOR signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic atrophic gastritis (CAG) is a prevalent serious and insidious illnesses of digestive system, notoriously featured by atrophy of the epithelium or thinning of the gastric mucosa, loss or atrophy of gastric mucosal glands, and thickening of the mucosal base, or coupled with intestinal metaplasia and dysplasia [1]. Patients with CAG may suffer from indigestion, belching, abdominal discomfort, loss of appetite and weight [2]. CAG is well established as a predisposing factor for gastric carcinogenesis which is recognized as one of the major drivers of cancer incidence and fatality, representing a huge health and psychological burden for patients globally [3, 4]. Current treatment modalities mainly include eradication of Helicobacter pylori, antacid and mucosal protective agents [5]. Considering the tight association between CAG and gastric cancer (GC), the active exploration of effective drugs is conducive to obstruct the malignant transformation from CAG into GC.
Recent researches have concentrated on the active role of Traditional Chinese medicine (TCM) in treating CAG, in view of favorable clinical effects and minimal side effects [6, 7]. Dendrobium chrysotoxum Lindl is a species of Dendrobium which is a medicinal and edible plant indexed in the Chinese Pharmacopoeia (2010 version) [8]. As a bibenzyl compound present in Dendrobium chrysotoxum Lindl, Erianin has also been supported to harbor a broad spectrum of biological properties such as anti-angiogenic, anti-oxidative, anti-tumor, anti-inflammatory antibacterial effects [9, 10]. Moreover, Wang et al. have proposed Erianin as a promising candidate in the prevention and therapy for precancerous lesions of gastric cancer [11]. Nonetheless, the functional role of Erianin in CAG is indistinct.
Mitogen activated protein kinase (MAPK) and mechanistic target of rapamycin (mTOR) are both crucial intracellular signal transduction pathways implicated in cell survival, proliferation, metabolism, and other cellular functions [12, 13]. Mounting evidence has exposed the involvement of MAPK and mTOR signaling cascades in the progression of GC [14,15,16]. Coincidentally, Erianin has been reported to inactivate MAPK signaling in hepatocellular carcinoma [17] and mTOR signaling in lung cancer [18].
Collectively, the goal of the current work was to specify the impacts and response mechanism of Erianin in CAG.
Materials and Methods
Culture and Treatment of Cells
Human gastric epithelium GES-1 cells (BeNa Culture Collection, BNCC; Beijing, China) were subjected to high glucose Dulbecco’s modified Eagle medium (DMEM; Macgene, Beijing, China) comprising 10% fetal bovine serum (FBS; Clark Bio, Claymont, DE, USA) in a moist incubator equilibrated with 5% CO2. GES-1 cells were cultivated with 40 μM Nmethyl-N’-nitro-N-nitrosoguanidine (MNNG; Aladdin, Shanghai, China) [19] and treated by 20, 40 and 80 nM Erianin (Aladdin, Shanghai, China) [11]. Besides, GES-1 cells were pretreated by 2.5 μM autophagy inhibitor 3-methyladenine (3-MA; Tocris Bioscience, Bristol, UK) for 2 h or 30 μM p38 MAPK agonist asiatic acid (Aladdin, Shanghai, China) for 1 h.
Cell Counting Kit-8 (CCK-8)
GES-1 cells inoculated into 96-well plates (5000 cells/well) were treated by 10, 20, 30, 40 and 50 μM MNNG or 20, 40 and 80 nM Erianin in the presence or absence with 40 μM MNNG, or pretreated by 2.5 μM 3-MA or 30 μM asiatic acid prior to incubation with MNNG at the concentration of 40 μM and Erianin at the concentration of 80 nM. 10 μL CCK-8 solution (MCE, Shanghai, China) was pipetted to cells for 2 h incubation, followed by the absorbance detection under the microplate reader (Fluorocount, Packard, Germany) at 450 nm.
Enzyme-linked Immunosorbent Assay (ELISA)
In compliance with the standard protocol of ELISA kits (Multi Sciences, Hangzhou, China), tumor necrosis factor-alpha (TNF-α; cat. no. EK182HS), interleukin-1beta (IL-1β; cat. no. EK101BHS), interleukin-6 (IL-6; cat. no. EK106HS), interleukin-8 (IL-8; cat. no. EK108HS) and interleukin-10 (IL-10; cat. no. EK110HS) contents were determined after GES-1 cells were centrifuged at 300 x g for 10 min. Under a microplate reader, the absorbance detection was carried out at 450 nm.
Measurement of Oxidative Stress Levels
In compliance with the standard protocol of relevant assay kits (Abbkine, Wuhan, China), superoxide dismutase (SOD; cat. no. KTB1030), glutathione (GSH; cat. no. KTB1600) and malondialdehyde (MDA; cat. no. KTB1050) concentrations were detected after GES-1 cells were centrifuged at 12000 x g for 5 min. Under a microplate reader, the absorbance detection was carried out at 450 nm.
Dichloro-dihydro-fluorescein Diacetate (DCFH-DA) Assay
Reactive oxygen species (ROS) accumulation was assayed by CheKine™ Reactive Oxygen Species (ROS) Detection Fluorometric Assay Kit (Abbkine, Wuhan, China) referring to the guidance provided by manufacturer. GES-1 cells were probed with DCFH-DA probe (10 μmol/l) at 37 ˚C for half an hour without light exposure. Following PBS washing, the fluorescence intensity was subjected to flow cytometry analysis (Ex, 488 nm; Em, 525 nm) (Aceabio, San Diego, CA, USA) or was observed under a fluorescence microscope (Keyence Corporation).
Immunofluorescence (IF) Staining
Following respective cell immobilization and permeabilization with 4% paraformaldehyde for half an hour and 0.1% Triton X-100 for 10 min, GES-1 cells were saturated with PBS harboring 1% BSA for 1 h, followed by being processed for immunofluorescence with LC3B antibody (cat. no. ab232940; Abcam) overnight at 4 °C prior to the addition of secondary antibody conjugated with Alexa Fluor 488 (cat. no. ab150077; Abcam) for 1 h at room temperature. 1 mg/ml DAPI was applied for the nuclear staining. Fluorescence intensity was recorded under a fluorescence microscope.
Western Blot
Following the employment of RIPA buffer (BioRad, Hercules, CA, USA), the extracted proteins were cultivated with 12% SDS-PAGE and passed to the PVDF membranes. Afterwards, the non-specific binding sites of the membranes were impeded by 5% BSA and the membranes were labeled by primary antibodies resisting p62 (cat. no. ab109012; 1/10000; Abcam), Beclin-1 (cat. no. ab210498; 1/1000; Abcam), LC3B (cat. no. ab192890; 1/2000; Abcam), p-p38 MAPK (cat. no. #AF4001; 1/1000; Affinity Biosciences), p38 MAPK (cat. no. ab308333; 1/1000; Abcam), p-mTOR (cat. no. ab109268; 1/1000; Abcam), mTOR (cat. no. ab32028; 1/1000; Abcam) and β-actin (cat. no. ab8227; 1/1000; Abcam) as well as HRP-linked secondary antibody (ab6721; 1/2000; Abcam). By means of ECL substrate (Advansta, USA), color development was performed.
Statistical Analyses
All experimental values analyzed by GraphPad Prism 8.0 software (San Diego, CA, USA) were reported as the mean ± SD. Multiple data sets were compared by One-way ANOVA as well as Tukey’s post hoc test. P-values less than 0.05 were denoted significant in statistics.
Results
Erianin Improves MNNG-stimulated Decline on GES-1 Cell Viability
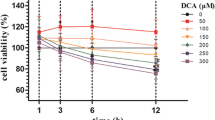
Prior to the generation of CAG cell model induced by MNNG, GES-1 cell viability was measured upon exposure to MNNG at the concentrations of 10, 20, 30, 40 and 50 μM. As described in Fig. 1A, a prominent downward trend was observed in GES-1 cell viability in a concentration-dependent manner when treated by varying doses of MNNG and 40 μM MNNG in which the best survival rate of GES-1 cells was demonstrated was employed to induce cellular models of CAG. Similarly, the impacts of Erianin on GES-1 cell viability were also estimated and Erianin with increasing concentrations (20, 40 and 80 nM) was discovered to exert no significant effects on GES-1 cell viability (Fig. 1B). Further, the experimental data from CCK-8 assay illuminated that the diminished viability of GES-1 cells challenged with MNNG was heightened by Erianin in a concentration-dependent manner (Fig. 1C).
Erianin improves MNNG-stimulated decline on GES-1 cell viability. A CCK-8 method appraised the viability of GES-1 cells challenged with increasing concentrations of MNNG. **P < 0.01, ***P < 0.001 vs. 0 μm MNNG. B CCK-8 method appraised the viability of GES-1 cells treated by ascending doses of Erianin. GES-1 cells were administrated with increasing concentrations of Erianin upon exposure to 40 μM MNNG. C CCK-8 method assayed cell viability. ***P < 0.001 vs. Control; ## P < 0.01 vs. MNNG
Erianin Relieves MNNG-elicited Inflammatory Reaction and Oxidative stress in GES-1 Cells
In addition, the results from ELISA presented that MNNG exposure resulted in the elevation on TNF-α, IL-6, IL-1β, IL-8 activities and the decline on IL-10 activity. In GES-1 cells treated by MNNG, Erianin concentration-dependently reduced TNF-α, IL-6, IL-1β, IL-8 activities whereas elevated IL-10 activity (Fig. 2A). It was also noticed that MDA and ROS contents were both raised whilst SOD and GSH contents were both lowered by MNNG in GES-1 cells, which were all reversed by increasing doses of Erianin (Fig. 2B-D).
Erianin relieves MNNG-elicited inflammatory reaction and oxidative stress in GES-1 cells. GES-1 cells were administrated with increasing concentrations of Erianin upon exposure to 40 μM MNNG. A Inflammatory levels by ELISA. B Relevant assay kits determined oxidative stress levels. C, D DCFH-DA fluorescence probe evaluated ROS generation. ***P < 0.001 vs. Control; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. MNNG
Erianin Aggravates the Autophagy of MNNG-treated GES-1 Cells
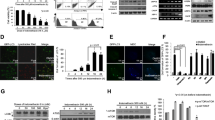
As depicted by immunofluorescence staining, Further Western blot analysis hinted that LC3 fluorescence intensity was attenuated in MNNG-exposed GES-1 cells, which was then intensified by Erianin in a dose-dependent manner (Fig. 3A). Besides, p62 expression was ascending and Beclin1, LC3II/I expression were descending in GES-1 cells challenged with MNNG. However, Erianin markedly decreased p62 expression while promoted Beclin1, LC3B expression in MNNG-induced GES-1 cells in a concentration-dependent manner (Fig. 3B).
Erianin aggravates the autophagy of MNNG-treated GES-1 cells. GES-1 cells were administrated with increasing concentrations of Erianin upon exposure to 40 μM MNNG. A Immunofluorescence staining detected LC3 expression. B Western blot examined the expression of proteins associated with autophagy. ***P < 0.001 vs. Control; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. MNNG
Erianin Blocks AMPK/mTOR Signaling in MNNG-challenged GES-1 Cells
Interestingly, through Western blot, MNNG exposure was found to raise p-p38 MAPK and p-mTOR expression in GES-1 cells. In MNNG-treated GES-1 cells, the addition of Erianin evidently down-regulated p-p38 MAPK/p38 MAPK and p-mTOR/mTOR expression again (Fig. 4). Based on these findings, Erianin at the concentration of 80 nM showed the most prominent effect, therefore being adopted for the follow-up experiments.
Erianin blocks AMPK/mTOR signaling in MNNG-challenged GES-1 cells. GES-1 cells were administrated with increasing concentrations of Erianin upon exposure to 40 μM MNNG. Western blot examined the expression of proteins associated with AMPK/mTOR signaling. ***P < 0.001 vs. Control; ##P < 0.01, ###P < 0.001 vs. MNNG
Erianin Exerts Anti-inflammatory and Anti-oxidant properties in MNNG-induced CAG Cell Model Dependent on MAPK/mTOR Signaling-mediated Autophagy
To identify the potential downstream mechanism of Erianin in MNNG-stimulated cellular model of CAG associated with autophagy mediated by MAPK/mTOR signaling, autophagy inhibitor 3-MA or p38 MAPK agonist asiatic acid was adopted. The data from CCK-8 assay expounded that the reinforced viability of MNNG-treated GES-1 cells imposed by Erianin was partially eliminated by inhibition of autophagy or inactivation of p38 MAPK (Fig. 5A). As expected, 3-MA or asiatic acid enhanced p-p38 MAPK/p38 MAPK and p-mTOR/mTOR expression again which were both repressed in Erianin-treated GES-1 cells challenged with MNNG (Fig. 5B). Additionally, LC3 fluorescence intensity was strengthened, p62 expression was depleted and Beclin1, LC3II/I expression in MNNG-treated GES-1 cells were raised by Erianin, which were all restored by pretreatment with 3-MA or asiatic acid (Fig. 5C, D). Meanwhile, relative to the MNNG group, TNF-α, IL-6, IL-1β, IL-8 concentrations were eliminated and IL-10 concentration was enhanced in MNNG + 80 nM Erianin group. However, 3-MA or asiatic acid improved TNF-α, IL-6, IL-1β and IL-8 concentrations while lowered IL-10 concentration in comparison with the MNNG + 80 nM Erianin group (Fig. 6A). Furthermore, Erianin prominently suppressed MDA, ROS levels and boosted SOD, GSH levels in MNNG-exposed GES-1 cells, which were all abrogated by 3-MA or asiatic acid (Fig. 6B–D).
Erianin modulates autophagy in MNNG-induced CAG cell model via MAPK/mTOR signaling. Asiatic acid or 3-MA was to pretreat Erianin-administrated GES-1 cells exposed to 40 μM MNNG. A CCK-8 method assayed cell viability. B Western blot examined the expression of proteins associated with AMPK/mTOR signaling. C Immunofluorescence staining detected LC3 expression. D Western blot examined the expression of proteins associated with autophagy. ***P < 0.001 vs. Control; ##P < 0.01, ###P < 0.001 vs. MNNG; &P < 0.05, &&P < 0.01, &&&P < 0.001 vs. MNNG+80nM Erianin
Erianin exerts anti-inflammatory and anti-oxidant properties in MNNG-induced CAG cell model dependent on MAPK/mTOR signaling-mediated autophagy. Asiatic acid or 3-MA was to pretreat Erianin-administrated GES-1 cells exposed to 40 μM MNNG. A Inflammatory levels by ELISA. B Relevant assay kits determined oxidative stress levels. C, D DCFH-DA fluorescence probe evaluated ROS generation. ***P < 0.001 vs. Control; ###P < 0.001 vs. MNNG; &&P < 0.01, &&&P < 0.001 vs. MNNG+80Nm Erianin
Discussion
Chronic atrophic gastritis (CAG) is a frequently-occurring intractable disease in the digestive system with diverse etiologies [1]. N-nitrosamines are highly toxic substances present in environmental sources and food, excess intake of which is viewed as an initiating factor of CAG [20]. N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) is a sort of N-nitrosamine ingredient, which can be applied in laboratory as chemical mutagen and carcinogen [21]. In particular, MNNG chiefly expedites the conversion of nitrosamine and other carcinogens from nitrate in the stomach, which leads to CAG, even GC [21]. The malignant transformation of GES-1 cells stimulated by MNNG has been proved to motivate the developmental process of precancerous lesions in the gastric mucosa [22]. Hence, MNNG may be utilized to imitate CAG-induced cell injury in gastric epithelium cells. Here, the viability of GES-1 cells was assessed exposed to varying concentrations of MNNG and it was observed that GES-1 cell viability was concentration-dependently diminished by MNNG treatment. Moreover, 40 μM MNNG was employed to induce cellular models of CAG to maintain the cell survival.
Dendrobium chrysotoxum Lindl has been commonly applied to clinical treatment of chronic gastritis, a major bisbenzyl compound of which, Erianin, has also been reported to have great therapeutic potential in precancerous lesions of gastric cancer [11]. A great body of evidence has demonstrated that Erianin may play the tumor-suppressing role in oral squamous cell carcinoma [23, 24], osteosarcoma [25], colorectal cancer [26] through inhibiting cell proliferation. In our study, the possibly protective role of Erianin was suggested in MNNG-induced CAG cell model in gastric epithelial cells, as manifested by the boosted viability of MNNG-challenged GES-1 cells by increasing doses of Erianin. Inflammatory response has been commonly determined as a crucial factor driving the occurrence and progression of CAG and that the sustained inflammatory response reflected by the secretion of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) in the gastric mucosa is the main inducement of CAG [27]. Mounting evidence has documented the potent anti-inflammatory activity of Erianin in ulcerative colitis [28] and NLRP3-related diseases [29]. The present experimental results expounded that the ascending concentrations of pro-inflammatory enzymes including TNF-α, IL-6, IL-1β, IL-8 and the descending concentration of anti-inflammatory IL-10 in GES-1 cells exposed to MNNG were all dose-dependently reversed by administration with Erianin. Oxidative stress is closely involved in the pathological process of CAG [30]. As reported, the level of oxidants like MDA is increased in the blood of CAG patients in comparison with healthy individuals [31]. Importantly, Erianin has been mentioned to protect against diabetic nephropathy [32] and ulcerative colitis [28] through reducing oxidative damage. In the current work, we discovered that increasing doses of Erianin repressed MDA and ROS levels whereas raised the levels of antioxidants like SOD and GSH. All these findings underlined the anti-inflammatory and ant-oxidative stress properties of Erianin in MNNG-stimulated in vitro model of CAG for the first time.
Autophagy is the process of cellular self-digestion in response to extrinsic stimuli, by which damaged macromolecules and organelles are targeted to lysosomes through autophagic vesicles to maintain cell differentiation, homeostasis, and survival [33]. Beclin1 is a core factor in triggering the initiation of autophagy [34]. Lipidated LC3 is a marker of autophagosomes, while p62 is a substrate of autophagy [35]. Previous study has introduced that autophagy plays the regulatory role during the process of CAG [36]. Notably, Erianin has been reported to serve as an autophagy inducer in oral squamous cell carcinoma [24] and osteosarcoma [25]. Through investigation, Erianin was also noted to activate autophagy in MNNG-challenged GES-1 cells, evidenced by the intensified LC3 fluorescence intensity, the down-regulated p62 expression and the up-regulated Beclin1, LC3B expression, implying that the activation of autophagy mediated by Erianin might play the protective role in CAG. This finding was completely contrary to the study conducted by Zhu et al. which has reported the high autophagy level in the precancerous lesions of gastric cancer rat model [37].
Recent literatures have clarified that Erianin can function in hepatocellular carcinoma and lung cancer through blocking MAPK signaling and mTOR signaling [17, 18]. As expected, in MNNG-treated GES-1 cells, Erianin also robustly lowered p-p38 MAPK/p38 MAPK and p-mTOR/mTOR expression. Furthermore, Liu et al. have proposed that Rhein inactivates MAPK signaling in the gastric mucosa of mice with CAG [38]. Intriguingly, it is generally believed that the MAPK pathway is an upstream inhibitor of autophagy [39] and mTOR signaling is also identified as a master regulator of autophagy [40, 41]. As a result, our experimental results illuminated that the pretreatment with autophagy inhibitor 3-MA or p38 MAPK agonist asiatic acid partially reversed the impacts of Erianin on the viability, MAPK/mTOR signaling, autophagy, inflammatory reaction as well as oxidative stress in GES-1 cells upon exposure to MNNG.
However, there are some limitations to this study. More signaling pathways that might be mediated by Erianin in CAG need to be investigated in future studies. Also, considering the discrepancies in the autophagy level in CAG and precancerous lesions of gastric cancer, the autophagy level needs to be detected again in animal experiments in our future studies.
Taken together, Erianin relieves inflammatory reaction and oxidative damage initiated by MNNG during the progression of CAG, the main mechanism of which is associated with activation of autophagy mediated by blockade of MAPK/mTOR signaling. This finding may provide a pharmacological basis for treating CAG by Erianin, and would possibly open a new venue for Erianin as a therapeutic strategy in CAG, and may highlight the importance of Erianin derivatives or newly identified Erianin targets in treating CAG .
References
Li, Y., Xia, R., Zhang, B. & Li, C. (2018). Chronic Atrophic Gastritis: A Review. Journal of Environmental Pathology Toxicology and Oncology, 37(3), 241–259.
Luo, C., Sun, Z., Li, Z., Zheng, L. & Zhu, X. (2019). Notoginsenoside R1 (NGR1) Attenuates Chronic Atrophic Gastritis in Rats. Medical Science Monitor, 25, 1177–1186.
Lahner, E., Conti, L., Annibale, B. & Corleto, V. D. (2020). Current Perspectives in Atrophic Gastritis. Current Gastroenterology Reports, 22(8), 38
Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C., & Lordick, F. (2020). Gastric cancer. Lancet, 396(10251), 635–648.
den Hollander, W. J. & Kuipers, E. J. (2012). Current pharmacotherapy options for gastritis. Expert Opinion on Pharmacotherapy, 13(18), 2625–2636.
Yang, L., Liu, X., Zhu, J., Zhang, X., Li, Y., & Chen, J., et al. (2023). Progress in traditional Chinese medicine against chronic gastritis: From chronic non-atrophic gastritis to gastric precancerous lesions. Heliyon, 9(6), e16764.
Liu, X., Wang, S., Li, J., Zhang, J. & Liu, D. (2022). Regulatory effect of traditional Chinese medicines on signaling pathways of process from chronic atrophic gastritis to gastric cancer. Chinese Herbal Medicines, 14(1), 5–19.
Wang, Y. H.(2021). Traditional uses, chemical constituents, pharmacological activities, and toxicological effects of Dendrobium leaves: A review. Journal of Ethnopharmacology, 270, 113851
Li, G., Zhang, H., Lai, H., Liang, G., Huang, J. & Zhao, F. et al. (2023). Erianin: A phytoestrogen with therapeutic potential. Frontiers in Pharmacology, 14, 1197056
Zhang, Y., Zhang, Q., Wei, F. & Liu, N. (2019). Progressive study of effects of erianin on anticancer activity. OncoTargets and Therapy, 12, 5457–5465.
Wang, Y., Chu, F., Lin, J., Li, Y., Johnson, N. & Zhang, J. et al. (2021). Erianin, the main active ingredient of Dendrobium chrysotoxum Lindl, inhibits precancerous lesions of gastric cancer (PLGC) through suppression of the HRAS-PI3K-AKT signaling pathway as revealed by network pharmacology and in vitro experimental verification. Journal of Ethnopharmacology, 279, 114399.
Gaestel, M. (2015). MAPK-Activated Protein Kinases (MKs): Novel Insights and Challenges. Frontiers in Cell and Developmental Biology, 3, 88.
Saxton, R. A., & Sabatini, D. M. (2017). mTOR Signaling in Growth, Metabolism, and Disease. Cell, 168(6), 960–976.
Zhang, Q., Wang, X., Cao, S., Sun, Y., He, X. & Jiang, B. et al. (2020). Berberine represses human gastric cancer cell growth in vitro and in vivo by inducing cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and Akt signaling pathways. Biomedicine and Pharmacotherapy, 128, 110245
Wang, K., Tang, J., Fan, S., Su, H., Yu, R., & Zhang, Y., et al. (2023). ABBV-744 induces autophagy in gastric cancer cells by regulating PI3K/AKT/mTOR/p70S6k and MAPK signaling pathways. Neoplasia, 45, 100936.
Yang, L., Liu, Y. N., Gu, Y. & Guo, Q. (2023). Deltonin enhances gastric carcinoma cell apoptosis and chemosensitivity to cisplatin via inhibiting PI3K/AKT/mTOR and MAPK signaling. World Journal of Gastrointestinal Oncology, 15(10), 1739–1755.
Yang, L., Hu, Y., Zhou, G., Chen, Q. & Song, Z. (2020). Erianin suppresses hepatocellular carcinoma cells through down-regulation of PI3K/AKT, p38 and ERK MAPK signaling pathways. Bioscience Reports, 40(7), BSR20193137
Zhang, H. Q., Xie, X. F., Li, G. M., Chen, J. R., Li, M. T. & Xu, X. et al. (2021). Erianin inhibits human lung cancer cell growth via PI3K/Akt/mTOR pathway in vitro and in vivo. Phytotherapy Research, 35(8), 4511–4525.
Tong, Y., Liu, L., Wang, R., Yang, T., Wen, J. & Wei, S. et al. (2021). Berberine Attenuates Chronic Atrophic Gastritis Induced by MNNG and Its Potential Mechanism. Frontiers in Pharmacology, 12, 644638
Gushgari, A. J., & Halden, R. U. (2018). Critical review of major sources of human exposure to N-nitrosamines. Chemosphere, 210, 1124–1136.
Lu, L., Chen, B., Zhang, X., Xu, Y., Jin, L. & Qian, H. et al. (2023). The effect of phytochemicals in N-methyl-N-nitro-N-nitroguanidine promoting the occurrence and development of gastric cancer. Frontiers in Pharmacology, 14, 1203265.
Wen, J. X., Tong, Y. L., Ma, X., Wang, R. L., Li, R. S., & Song, H. T., et al. (2021). Therapeutic effects and potential mechanism of dehydroevodiamine on N-methyl-N’-nitro-N-nitrosoguanidine-induced chronic atrophic gastritis. Phytomedicine, 91, 153619.
Luo, Q., Li, X., Gan, G., Yang, M., Chen, X. & Chen, F. (2021). PPT1 Reduction Contributes to Erianin-Induced Growth Inhibition in Oral Squamous Carcinoma Cells. Frontiers in Cell and Developmental Biology, 9, 764263
Chen, Y. T., Hsieh, M. J., Chen, P. N., Weng, C. J., Yang, S. F. & Lin, C. W. (2020). Erianin Induces Apoptosis and Autophagy in Oral Squamous Cell Carcinoma Cells. The American Journal of Chinese Medicine, 48(1), 183–200.
Wang, H., Zhang, T., Sun, W., Wang, Z., Zuo, D. & Zhou, Z. et al. (2016). Erianin induces G2/M-phase arrest, apoptosis, and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death and Disease, 7(6), e2247
Miao, Q., Deng, W. Q., Lyu, W. Y., Sun, Z. T., Fan, S. R. & Qi, M. et al. (2023). Erianin inhibits the growth and metastasis through autophagy-dependent ferroptosis in KRAS(G13D) colorectal cancer. Free Radical Biology and Medicine, 204, 301–312.
Waldum, H. & Fossmark, R. (2023). Inflammation and Digestive Cancer. International Journal of Molecular Sciences, 24(17), 854–863.
Dou, B., Hu, W., Song, M., Lee, R. J., Zhang, X. & Wang, D. (2020). Anti-inflammation of Erianin in dextran sulphate sodium-induced ulcerative colitis mice model via collaborative regulation of TLR4 and STAT3. Chemico-Biological Interactions, 324, 109089
Zhang, X., Hu, L., Xu, S., Ye, C., & Chen, A. (2021). Erianin: A Direct NLRP3 Inhibitor With Remarkable Anti-Inflammatory Activity. Frontiers in Immunologyl, 12, 739953.
Park, J. M., Han, Y. M., Oh, J. Y., Lee, D. Y., Choi, S. H. & Kim, S. J. et al. (2021). Fermented kimchi rejuvenated precancerous atrophic gastritis via mitigating Helicobacter pylori-associated endoplasmic reticulum and oxidative stress. Journal of Clinical Biochemistry and Nutrition, 69(2), 158–170.
Tsukanov, V. V., Smirnova, O. V., Kasparov, E. V., Sinyakov, A. A., Vasyutin, A. V. & Tonkikh, Y. L. (2018). Changes in the indices of prooxidant and antioxidant systems in blood plasma in men with atrophic gastritis and gastric cancer. Terapevticheskii arkhiv, 90(2), 24–27.
Chen, M. F., Liou, S. S., Kao, S. T. & Liu, I. M. (2019). Erianin protects against high glucose-induced oxidative injury in renal tubular epithelial cells. Food and Chemical Toxicology, 126, 97–105.
Parzych, K. R. & Klionsky, D. J. (2014). An overview of autophagy: morphology, mechanism, and regulation. Antioxidants and Redox Signaling, 20(3), 460–473.
Tran, S., Fairlie, W. D., & Lee, E. F. (2021). BECLIN1: Protein Structure, Function and Regulation. Cells, 10(6), 1522.
Bresciani, A., Spiezia, M. C., Boggio, R., Cariulo, C., Nordheim, A., & Altobelli, R., et al. (2018). Quantifying autophagy using novel LC3B and p62 TR-FRET assays. PLoS One, 13(3), e0194423.
Wang, T., Liu, K., Wen, L., Yang, Y., Yin, X. & Liu, K. et al. (2020). Autophagy and Gastrointestinal Diseases. Advances in Experimental Medicine and Biology, 1207, 529–556.
Zhu, F., Zhang, X., Wen, J., Liu, Y. & Zhu, Y. (2024). Celastrus orbiculatus extract reverses precancerous lesions of gastric cancer by inhibiting autophagy via regulating the PDCD4-ATG5 signaling pathway. Journal of Pharmacy and Pharmacology, 76(3), 257–268.
Liu, S., Shu, L., Yang, J., Liu, Y., Zhang, S. & Wang, N. et al. (2023). Rhein Exhibits Anti-Inflammatory Effects in Chronic Atrophic Gastritis via Nrf2 and MAPK Signaling. Turkish Journal of Gastroenterology, 34(5), 525–532.
Zhou, Y. Y., Li, Y., Jiang, W. Q. & Zhou, L. F. (2015). MAPK/JNK signalling: a potential autophagy regulation pathway. Bioscience Reports, 35(3), e00199
Kim, Y. C. & Guan, K. L. (2015). mTOR: a pharmacologic target for autophagy regulation. Journal of Clinical Investigation, 125(1), 25–32.
Wang, Y. & Zhang, H. (2019). Regulation of Autophagy by mTOR Signaling Pathway. Advances in Experimental Medicine and Biology, 1206, 67–83.
Author information
Authors and Affiliations
Contributions
K.W. conceived the study. Q.J. and G.F. wrote the main manuscript text, performed the experiments, analyzed the data and prepared the figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, Q., Fan, G. & Wu, K. Potential Action Mechanism of Erianin in Relieving MNNG-triggered Chronic Atrophic Gastritis. Cell Biochem Biophys (2024). https://doi.org/10.1007/s12013-024-01536-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s12013-024-01536-x