Abstract
Background
Cardiomyocyte apoptosis plays an important role in alcoholic cardiac injury. However, the association between calcium-sensing receptor (CaSR) and alcohol-induced cardiomyocyte apoptosis remain unclear. Therefore, we investigated the role and its moleculer mechanism of CaSR in rat cardiomyocyte apoptosis induced by alcohol.
Methods
Alcohol-induced cardiomyocyte apoptosis in vivo and in vitro model of rats were applied in this study. The expression of CaSR, endoplasmic reticulum stress markers and apoptosis were tested by immunohistological staining, western blot, TUNEL and flow cytometry, respectively. [Ca2+]i were detected by confocal laser scanning microscopy.
Results
Compared with the control group, alcohol intake (AI) led to abnormal arrangements of cardiomyocytes and obvious increase of myocardial apoptosis. Moreover, AI also significantly upregulated protein expression of CaSR, GRP94, caspase-12 and CHOP. Alcohol induced apoptosis of cultured cardiomyocytes of rats in a dose-dependent way. Activation of CaSR markedly enhanced cardiomyocyte apoptosis and ERS induced by alcohol, ERS inducer also significantly increased cardiomyocyte apoptosis without activating CaSR. Furthermore, GdCl3 augmented alcohol-induced increase of [Ca2+]i in cardiomyocytes, which was attenuated by NPS2390 but not 4-PBA pre-treatment.
Conclusions
Alcohol could induce cardiomyocyte apoptosis in rats in vivo and in vitro, which was mediated probably via activating CaSR, and then ERS and the increase of the cytosolic [Ca2+]i. This provides a potential target for preventing cardiomyocyte apoptosis and cardiomyopathy induced by alochol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alcoholic cardiomyopathy is caused by long-term heavy drinking with an important cause of dilated cardiomyopathy [1]. Alcohol intake (AI) may lead to progressive cardiac injury, and then impaired contractile dysfunction and enlarged cardiac cavity, at last resulting in heart failure [2]. Therefore, exploring molecular and cellular mechanism in the development of alcoholic cardiac injury contributes to provide new treatment targets for alcoholic cardiomyopathy.
Many studies have indicated the pathogenesis mechanism of alcoholic myocardial injury involves myocardial apoptosis [3], changes in intracellular calcium ([Ca2+]i) [4] and endoplasmic reticulum stress (ERS) [5]. Increasing evidence from aminals and patients has demonstrated that alcohol can result in a higher percentage of myocardial apoptosis [6, 7]. Moreover, apoptosis plays an important role in the development of heart failure (HF) and cardiac remodeling [8, 9]. Endoplasmic reticulum (ER) is responsible for protein synthesis, folding, maturation and transport. ERS, which is induced by the perturbations of ER function and structure, could cause cell death [10] by activating distinct signals, such as the activation of transcription factors [11], ER-resident caspases [12] and the Bax/Bcl-2 family proteins [13, 14]. Recently, ERS was demonstrated to be pathologically involved in cardiac diseases and damage under numerous conditions, including MI [9] and AI [14]. However, the relation of ERS with alcohol-induced myocardial apoptosis is unclear, and then the detailed mechanisms need further research.
Calcium-sensing receptor (CaSR) is a member of G protein-coupled receptors family [15]. It is expressed in all kind of tissues and cells and regulates cell functions, such as proliferation, differentiation and apoptosis [16, 17]. In 2003, Wang R et al. firstly reported CaSR is functionally expressed in rat cardiac tissue [18]. Since then, increasing studies have demonstrated that CaSR plays an important role in the development of cardiovascular diseases, for example: CaSR is involved in ischemia-reperfusion injury [19], hypertension [20], diabetic cardiomyopathy [21] and lipopolysaccharide-induced myocardial cell injury [22]. Moreover, our previous studies indicated that CaSR took part in ventricular remodeling including myocardial fibrosis after myocardial infarction [23]. However, the association between CaSR and alcoholic cardiomyocye apoptosis remains unknown. In this study, we used a rat alcohol intake (AI) in vivo model and alcohol-induced cardiomyocyte apoptosis in vitro model of rats to investigate the role and its mechanism of CaSR in alcohol induced cardiomyocyte apoptosis, which will provide a new target for the treatment of alcohol-related heart disease.
Methods
Experimental Animals and Groups
Wistar Rats (male, 180 ± 20 g) were provided by the Animal Research Institute of Harbin Medical University, and the experimental procedure was approved by the Institution Animal Care and Use Committee of Harbin Medical University, Animal Management Rule of the People’s Republic of China (Ministry of Health, People’s Republic of China, document No. 55, 2001) and Ethics Committee of the First Affiliated Hospital of Harbin Medical University (permit ethical number: 2015-3-6) [24]. All rats were housed in a temperature-controlled room under a 12 h/12 h-light/dark with access to a standard chow diet and tap water ad libitum. Upon completion of the acclimation for one week, rats were randomly assigned into the following two groups: (i) The AI group (n = 20): rats were administrated with 5 ml/kg 5%, 10% and 20% alcohol (vol/vol) twice per day by gavage and ad libitum during the first, second and third weeks, respectively; then, 40% alcohol (vol/vol) was given (5 ml/kg twice per day) by gavage and ad libitum from the fourth week until the 12th week. (ii) The control group (n = 20): rats received the same volume of an isocaloric isonitrogenous solution administered intragastrically instead of alcohol as a control for 12 weeks. Rats were housed individually in standard plastic cages and maintained under identical conditions.
HE Staining
After 12 weeks of administration, rats were anaesthetized. Immediately after sacrificing, hearts were harvested, and fixed in 4% buffered paraformaldehyde for 24 h. The tissues were then embedded and sectioned (4 μm). Heart sections were stained with HE and viewed by light microscopy (Olympus BX60, Beijing, China).
Immunohistological Staining
Expression of CaSR in rat myocardium was determined by immunohistochemistry in both groups. Briefly, heart sections were stained with an antibody against CaSR (1:100, Alomone Labs Ltd., Hadassah Ein Kerem, Jerusalem). After washing, the sections were incubated with biotin-conjugated goat anti-rabbit IgG (1:200, ZSGB-Bio, Beijing, China) and then with avidin-biotin-peroxidase complex from a Vector ABC Elite Kit (Sigma, USA). After incubating with DAB solution, nuclei were counterstained with haematoxylin. Rabbit IgG (1 mg/ml) was instead of the primary antibody in a negative control. Five areas of LV tissues were chosen randomly to count the CaSR positive cells under × 400 magnification as previously described [25].
TUNEL Assay
TUNEL assay (Roche, USA, 11684817910) was performed to determine the cardiomyocyte apoptosis in heart as described previously [26]. Homogeneously stained areas without signs of necrosis were counted as positive for apoptosis. Five areas of LV tissues were chosen randomly to count the TUNEL positive cells under × 400 magnification. Calculation was performed by an observer who blinded to the treatment groups.
Isolation and Culture of Cardiomyocytes
1- to 3-day-old neonatal rat hearts were isolated to achieve cardiomyocytes as previously described by Ieda 26. Briefly, after hearts were digested, cardiomyocytes were collected by selective adhesion after 90 min pre-culture in a humidified incubator (95% air-5% CO2). BrdU (5-Bromo-2’-deoxyuridine) was supplemented to increase the purity. The culture medium was replaced after 48 h, and the cells were further cultured for 24 h. Then, the cardiomyocytes were cultured with low-serum DMEM (1.5% FBS) for 12 h before experiments.
Detection of Cultured Cardiomyocyte Apoptosis of Rats Induced by Different Concentrations of Alcohol
Apoptosis was measured after cultured rat cardiomyocytes exposure to different concentrations of alcohol (0 mM, 50 mM, 100 mM and 200 mM), using the Annexin V/PI kit (BioLegend, California, USA, 640914) under guidance of the manufacturer’s protocol. Briefly, after collecting and resuspending in 1 × binding buffer at a concentration of 1 × 106 cells/ml, the cells were incubated with 5 μl of both Annexin V-FITC and PI respectively for 15 min at room temperature in the dark, followed by the addition of another 400 μl of 1 × binding buffer. Cells were analysed by flow cytometry (BD Biosciences) within 1 h. Apoptotic cells were stained positively for Annexin V and negatively for PI.
Cardiomyocyte Treatment
After being cultured with low-serum DMEM for 12 h, to explore the relationship between ERS and apoptosis, cardiomyocytes were grouped as follows: (i) The control group: a control vehicle was added to the cardiomyocytes at the same osmolality; (ii) The alcohol group: the cardiomyocytes were treated with 100 mM alcohol for 24 h; (iii) The alcohol + dithiothreitol (DTT) group: the cardiomyocytes were pre-incubated with DTT (an ERS inducer, Sigma-Aldrich, St. Louis, MO, USA) 2 mM for 3 h before with 100 mM alcohol treatment for 24 h.
To clarify the relationship between CaSR and ERS, the cardiomyocytes were divided into four groups: (i) The control group: a control vehicle was added to the cardiomyocytes at the same osmolality; (ii) The alcohol group: the cardiomyocytes were treated with 100 mM alcohol for 24 h; (iii) The alcohol + GdCl3 group: the cardiomyocytes were pre-incubated with 100 μM GdCl3 (an activator of CaSR, Sigma-Aldrich, St. Louis, MO, USA) for 4 h before with 100 mM alcohol treatment for 24 h; (iv) The alcohol + GdCl3 + NPS2930 group: after pre-incubating the cardiomyocytes with NPS2390 (an inhibitor of CaSR, 10 mM, Sigma-Aldrich, St. Louis, MO, USA) for 1 h, 200 μM GdCl3 was added for 4 h, followed by 100 mM alcohol for 24 h.
All samples were collected for further detection at the end of in vitro experiments.
Western Blotting
After treatment, four LVs of each group and cell lysates of treated cardiomyocytes were collected to obtain protein with lysis buffer. Then, the lysates were centrifuged at 12,000 rpm for 20 min at 4 °C to remove the nuclei. The protein concentration was determined by BCA protein assay kit (Beyotime, P0012). The proteins were electrophoresed and transferred to PVDF membranes. After blocking for 2 h, the membranes were incubated with primary antibodies, which were as following: CaSR (1:1000), anti-GRP94 (1:1000, Abcam, ab13509), anti-caspase12 (1:1000, Abcam, ab18766), anti-CHOP (1:1000, Abcam, ab10444), anti-Bax (1:500, Santa Cruz, sc-7480), anti-caspase-3 (1:500, Santa Cruz, sc-7148), anti-Bcl-2 (1:500, Santa Cruz, sc-492), and anti-β-actin (1:1000, Santa Cruz, sc-1616) at 4 °C overnight separately. The membranes were washed and incubated with fluorescence-conjugated goat anti-mouse or anti-rabbit IgGs (1:10,000, Rockland, 611144122) for 1 h. Odyssey Infrared Imaging System and Odyssey v3.0 software were used to quantify the bands. β-actin was used as loading control.
Measurement of the Intracellular Ca2+ Concentration ([Ca2+]i)
The different groups of cardiomyocyte samples were incubated with 10 μM Flou-3/AM (Sigma Chemical, St. Louis, MO, USA, 73881) at 37 °C for 30 min. Then, to remove the residual dye the Ca2+-free PBS were used to rinse the cardiomyocytes and further incubated in DMEM. Spectrofluorometric measurements were recorded for 5 min and collected using the Delta Scan System spectrofluorometer (Photon Technology International, London, Ontario), with exciting at 488 nm and emission at 530 nm.
Data Analysis
The data are presented as the mean ± SEM. If the data were normally distributed, the differences between two groups were evaluated using t-tests; significance between multiple groups was evaluated by one-way ANOVA followed by Tukey’s test. If the data were not normally distributed, the Mann-Whitney test was used for 2 groups, and the Kruskal-Wallis test was used for more than 3 groups. A value of P < 0.05 was considered statistically significant.
Results
AI Provoked Myocardial Apoptosis in Rats
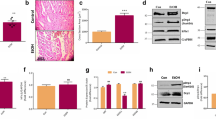
No animals died during the experiment. Compared with those in the control group, rat myocardium in the AI group developed an irregular, disorganized cardiomyocyte pattern (Fig. 1A). As shown in Fig. 1B, TUNEL staining results showed that there were more immunopositive cells in the AI group than the control group. Further semi-quantitative result analysis revealed that there was significant difference in TUNEL positive cell percentage between the two groups (Fig. 1C). As shown in Fig. 1D–F, the expression levels of pro-apoptosis protein caspase-3 and Bax were higher in the AI group than those in the control group. However, the anti-apoptosis protein Bcl-2 markedly decreased in rat myocardial tissues of the AI group compared to the control group.
Effect of AI on myocardial morphology and myocardial apoptosis. A HE staining of the control and AI rat myocardium (a, c: ×100; b, d: ×400). a, b: Control group; c, d: AI group. Scale bar = 200 μm. B TUNEL staining of rat myocardium (×400). a: Control group; b: AI group. Scale bar = 200 μm. C Statistical analysis of the TUNEL positive cells. At least 20 fields of vision were counted. *P < 0.05 versus the control group. D Expression of apoptosis-associated proteins in the control and AI groups. Four samples of each group were collected randomly to detect Bax, Bcl-2 and caspae-3 expression. E, F Statistical analysis of the expression of Bax/Bcl-2 and caspase-3. The graphs show the mean ± SEM of the four samples of each group. *P < 0.001 versus the control group
AI Upregulated CaSR Expression and ERS in Rats
Immunohistological staining revealed that compared with the control group, the immunopositive areas of CaSR were significantly larger in the myocardial section of rats in the AI group (Fig. 2A). Western blot results indicated that protein expression of CaSR was significantly upregulated in the AI group compared with the control group (Fig. 2B, C). In addition, the expressions of ERS pathway markers GRP94, caspase-12 and CHOP were significantly upregulated in the AI group compared with those in the control group (Fig. 2D–G).
AI provoked CaSR expression and ERS in rats. A CaSR immunohistochemistry of control and AI myocardium (×400). a: Control group; b: AI group. Scale bar = 200μm. B Protein expression of CaSR in control and AI myocardium. C Statistical analysis of the expression of CaSR. The graphs show the mean ± SEM of the four samples of each group. *P < 0.05 versus the control group. D Expression of ERS pathway markers in control and AI myocardium. Four samples of each group were collected randomly to detect the markers. E–G Statistical analysis of the expression of GRP-94, caspase-12 and CHOP. The graphs show the mean ± SEM of the four samples of each group. *P < 0.05 versus the control group
Alcohol Dose-dependently Induced Cardiomyocyte Apoptosis In Vitro
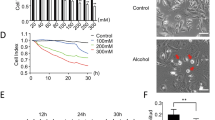
Cardiomyocytes were exposed to different alcohol doses (0 mM, 50 mM, 100 mM, 200 mM) for 24 h. Then, cardiomyocyte apoptosis was detected by flow cytometry. The results demonstrated that the level of myocardial apoptosis induced by 50 mM alcohol was obviously higher compared to that induced by 0 mM, moreover, myocardial apoptosis was further aggravated by 100 mM alcohol than 50 mM alcohol, which increased by 3 times. The 200 mM alcohol induced the cardiomyocyte apoptosis pecentage was 20.3%, which increased by 3.3% compared with the 100 mM alcohol (Fig. 3A, B). Therefore, cardiomycyte apoptosis model induced by 100 mM alcohol was applied in the following study.
Alcohol induced cardiomyocyte apoptosis in a dose-dependent way. A Cardiomyocyte apoptosis accessed by flow cytometry using an Annexin V/PI kit. Cells were treated as indicated, n = 3. B Statistical analysis of the apoptotic cells. The graphs show the mean ± SEM of the three experiments. *P < 0.05, **P < 0.005, and ***P < 0.001 versus the 0 mM alcohol group
Activation of ERS Amplified Alcohol-induced Apoptosis of Cultured Cardiomyocytes in Rats
To explore the relationship between ERS and apoptosis, ERS inducer DTT was used in in vitro experiment, and the expression of CaSR, ESR markers and apoptosis related proteins were detected by western blot. The results showed that the expressions of GRP94, caspase-12, CHOP, Bax/Bcl-2 and caspase-3 were significantly increased in alcohol-treated cardiomyocytes compared to control cardiomyocytes, moreover, their expression levels were upregulated by DTT compared to the alcohol treatment alone (Fig. 4). Interestingly, the protein expression of CaSR was significantly upregulated in alcohol-treated cardiomyocytes compared to control cardiomyocytes, while DTT did not strengthen CaSR expression of cardiomyocytes compared with alcohol treatment alone (Fig. 4A, B). These results implied that alcohol could provoke cardiomyocyte apoptosis probably via activation of ERS.
CaSR activation enhanced alcohol-induced ERS and apoptosis of cultured cardiomyocytes in rats. A Expression of CaSR and ERS markers in the different groups. B–E Statistical analysis of the expression of CaSR, GRP-94, caspase-12 and CHOP. The graphs show the mean ± SEM from three independent experiments. *P < 0.05, **P < 0.005, and ***P < 0.001 versus the control group; #P < 0.05, ##P < 0.005, and ###P < 0.001 versus the alcohol group; and @P < 0.05 and @@P < 0.005 versus the GdCl3 group. F Expression of apoptotic markers in different groups. G, H Statistical analysis of the expression of Bax/Bcl-2 and caspase-3. The graphs show the mean ± SEM from three independent experiments. ***P < 0.001 versus the control group; #P < 0.05 and ##P < 0.005 versus the alcohol group; and @P < 0.05 and @@P < 0.005 versus the alcohol + GdCl3 group
ERS was Mediated by CaSR During the Alcohol-induced Cardiomyocyte Apoptosis
To clarify the relationship between CaSR and ERS, we tested the expression of CaSR, ERS proteins and apoptosis related markers in the presence of alcohol with CaSR activator (GdCl3) and inhibitor (NPS2390). The results revealed that compared to the control group, CaSR expression was markedly upregulated by alcohol. Moreover, GdCl3 significantly enhanced CaSR protein expression of cardiomyocytes induced by alcohol treatment while NPS2390 markedly attenuated CaSR protein expression (Fig. 5). Similarly, ERS proteins (GRP94, caspase-12 and CHOP) and apoptosis related markers (Bax/Bcl-2 and caspase-3) increased in the alcohol group than those in the control group. Protein expressions of GRP94, caspase-12, CHOP, Bax/Bcl-2 and caspase-3 were augmented by GdCl3, but reduced by NPS2390 (Fig. 5A, C–H). These data suggested that alcohol could induce rat cardiomyocyte apoptosis through activation of CaSR-mediated ERS.
ERS activation amplified alcohol-induced apoptosis of cultured cardiomyocytes in rats. A Expression of CaSR and ERS markers in the different groups. B–E Statistical analysis of the expression of CaSR, GRP-94, caspase-12 and CHOP. The graphs show the mean ± SEM from three independent experiments. **P < 0.005, and ***P < 0.001 versus the control group; ##P < 0.005, and ###P < 0.001 versus the alcohol group. F Expression of apoptotic markers in different groups. G, H Statistical analysis of the expression of Bax/Bcl-2 and caspase-3. The graphs show the mean ± SEM from three independent experiments. **P < 0.005 and ***P < 0.001 versus the control group; #P < 0.05 versus the alcohol group
Alcohol-induced Increase in Cytosolic [Ca2+]i was Mediated by CaSR Rather than ERS in Cultured Cardiomyocytes of Rats
To investigate whether ERS activation depends on [Ca2+]i or not, confocal laser scanning microscopy was used to detect cytosolic [Ca2+]i in rat cardiomyocytes which were exposed to alcohol, CaSR activator (GdCl3) and inhibitor (NPS2390), and ERS inhibitor (4-PBA). The results demonstrated that alcohol led to an significant increase of [Ca2+]i in cultured cardiomyocytes of rats compared with the control, which was further enhanced by GdCl3. However, the increase of [Ca2+]i in cultured cardiomyocytes induced by alcohol was attenuated by pre-treatment with NPS2390 but not 4-PBA (Fig. 6).
CaSR activation augmented the [Ca2+]i in alcohol-treated cardiomyocytes in vitro. A CaSR activation augmented the [Ca2+]i in alcohol-treated cardiomyocytes. Statistical analysis of the fluorescence intensities of [Ca2+]i in different treated cardiomyocytes. n = 3, ***P < 0.001 versus the control group of the same time point; #P < 0.05 and ##P < 0.001 versus the alcohol group of the same time point; and @P < 0.05 versus the alcohol + GdCl3 group of the same time point. B The schematic diagram of the mechanism of alcohol induced cardiomyocyte apoptosis by CaSR-ERS pathway. In cardiomyocytes, CaSR induced by alcohol triggers [Ca2+]i increase; [Ca2+]i promotes ERS activation, which leads to cardiomyoctye apoptosis
Discussion
Our present data showed that AI provoked myocardial apoptosis, also increased protein expression of CaSR and ERS in rats. Moreover, the in vitro experimental data indicated alcohol can induce the cardiomyocyte apoptosis of rats in a dose-dependent way. Further study showed that alcohol-induced apoptosis was mediated probably by activation of CaSR, the increase of the cytosolic [Ca2+]i and ERS.
Cardiomyocyte apoptosis participates in the pathogenesis of many diseases including atherosclerosis, myocardial ischemia and alcoholic cardiomyopathy [27]. In this study, we found that AI induced myocardial apoptosis as shown in TUNEL-stained positive cells, increased expression of caspase-3 and Bax, and decreased expression of Bcl-2 in rats. Moreover, we also found alcohol dose-dependently induced cardiomyocyte apoptosis in vitro. It was reported that AI also increased apoptosis in hearts from humans [28], male and female mice [29, 30] and male rats [31]. In addition, alcohol increased cardiomyocyte loss and the susceptibility of cells to undergo apoptosis [21, 32]. In this study, we found cardiomyocytes apoptosis in AI rats at 12 weeks. However, Zhang RH et al. reported that the apoptosis increased within 6 weeks (4% vol/vol liquid diet) in mice [33]. Raymond AR et al. reported apoptosis increased within 16 weeks (5% vol/vol alcohol in drinking water) in rats [31]. These can be a consequence of methodological variations and different animals.
In our study, we showed that the expression of CaSR was upregulated in the process of myocardial apoptosis induced by AI. Moreover, in vitro study showed that alcohol induced activation of CaSR, suggesting that CaSR was involved in alcohol-induced cardiomyocyte apoptosis. It has been reported that CaSR activates cardiomyocyte apoptosis in diabetic rats [34], hypoxia/reperfusion [35] and HF [36]. Fernandez-Sola et al. reported that CaSR R990G gene expression is increased in chronic pancreatitis patients with moderate-to-heavy alcohol consumption [28]. To our knowlegde, we firstly reported that CaSR played a role in alcohol induced myocardial apoptosis.
Our in vivo results suggested that AI could provoke protein expressions of GRP94, caspase-12 and CHOP in myocardial tissue of rats, indicating activation of ERS takes part in alcoholic myocardiopathy. At the same time, alcohol-induced ERS activation was also observed during alcohol-induced cardiomyocyte apoptosis in vitro. In previous studies, alcohol exposure significantly increases the expression of ERS markers and activates signaling pathways associated with ERS [37]. Moreover, CaSR can promote hypoxia/reoxygenation-induced apoptosis of cultured cardiomyocytes in rats [38]. CaSR can also promote lipopolysaccharide-induced cardiomyocyte injury through ERS [22]. To elucidate the relationship between CaSR and ERS in alcohol-induced cardiomyocyte apoptosis, activator and inhibitor of CaSR and agonist of ERS were used to treat cardiomyocytes in vitro. The results demonstrated that GdCl3, an agonist of CaSR, further enhanced the activation of CaSR, ERS markers and cardiomyocyte apoptosis induced by alcohol. While ERS activator DTT increased the alcohol induced expressions of GRP94, caspase-12, CHOP and apoptosis markers (Bax/Bcl-2 and caspase-3) but not protein expression of CaSR. Caspase-12, which is localized in the ER membrane, is cleaved and activated in response to the [Ca2+]i mobilization [39]. Caspase-12 knockout mice resist to ERS, suggesting that caspase-12 is involved in cell death caused by ERS [40]. CHOP also plays a role in cell apoptosis caused by ERS [41], and overexpression of CHOP makes cells sensitive to ERS through down-regulation of Bcl-2 expression. Therefore, we assumed that CaSR could induce activation of the ERS pathway and aggravate cardiomyocyte apoptosis in AI.
Through activation of the phospholipase C (PLC) signaling, CaSR accumulates inositol phosphate (IP) and mobilizes Ca2+. Moreover, CaSR-activated Ca2+ leakage [42] and massive Ca2+ release from the ER are potent inducers of ERS [43]. In order to elucidate the mechanism of CaSR mediated ERS, [Ca2+]i was detected in alcohol treated cardiomyocytes with the activator and inhibitor of CaSR and inhibitor of ERS. We demonstrated that alcohol stimulated the increase of [Ca2+]i which was consistent with previous studies [41]. GdCl3, a CaSR agonist, further promoted the alcohol-induced increase of [Ca2+]i in cultured cardiomyocytes while NPS2390, an antagonist of CaSR, reversed its effect. Interestingly, 4-PBA, a inhibitor of ERS, pre-treatment did not inhibit the alcohol-induced [Ca2+]i increase in the presence of the CaSR activator GdCl3, suggesting alcohol induced [Ca2+]i increase depends on activation of CaSR more than ERS. Therefore, activation of ERS during alcohol-induced rat cardiomyocyte apoptosis can be caused by CaSR-mediated increase of [Ca2+]i.
Conclusions
In summary, we demonstrated that AI provoked myocardial apoptosis, upregulation of CaSR and activation of ERS in rats. Moreover, alcohol could induce rat cardiomyocyte apoptosis in a dose dependent way. Alcohol induced apoptosis was mediated by activation of CaSR, and then increase of the cytosolic [Ca2+]i and ERS. These will provide a potential target for preventing cardiomyocyte apoptosis and myocardiopathy induced by alochol.
References
Steiner, J. L. & Lang, C. H. (2017). Etiology of alcoholic cardiomyopathy: mitochondria, oxidative stress and apoptosis. The International Journal of Biochemistry & Cell Biology, 89, 125–135.
Movva, R., & Figueredo, V. M. (2013). Alcohol and the heart: to abstain or not to abstain? International Journal of Cardiology, 164(3), 267–276.
Regula, K. M., & Kirshembaum, L. A. (2005). Apoptosis of ventricular myocytes; a means to an end. Journal of Molecular and Cellular Cardiology, 38(1), 3–13.
Thomas, A. P., Sass, E. J., & Tun-Kirchmann, T. T., et al. (1989). Ethanolinhibits electrically-induced calcium transients in isolated rat cardiac myocytes. Journal of Molecular Cellular Cardiology, 21(6), 555–565.
Li, S. Y., & Ren, J. (2008). Cardiac overexpression of alcohol dehydrogenase exacerbates chronic ethanol ingestion-induced myocardial dysfunction and hypertrophy: role of insulin signaling and ER stress. Journal of Molecular and Cellular Cardiology, 44(6), 992–1001.
Fernández-Solà, J., Fatjó, F., & Sacanella, E., et al. (2006). Evidence of apoptosis in alcoholic cardiomyopathy. Human Pathology, 37(8), 1100–1110.
Jankala, H., Eriksson, C. J. P., & Eklund, K. K., et al. (2002). Combined calcium carbimide and ethanol treatment induces high blood acetaldehyde levels, myocardial apoptosis and altered expression of apoptosis-regulating genes in rat. Alcohol and Alcoholism, 37(3), 222–228.
Xin, W., Li, X. Y., & Lu, X. C., et al. (2011). Involvement of endoplasmic reticulum sress-associated apoptosis in a heart failure model induced by chronic myocaridal ischemia. International Journal of Molecular Medicine, 27(4), 503–509.
Liu, M., Wang, X. R., & Wang, C., et al. (2013). Panax quinquefolium saponin attenuates ventricular remodelling after acute myocardial infarction by inhibiting chop-mediated apoptosis, Panax quinquefolium saponin. Shock, 40(4), 339–344.
Schroder, M., & Kaufman, R. J. (2005). The mammalian unfolded protein response. Annual Review of Biochemistry, 74, 739–789.
Reddy, R., Mao, C., & Baumeister, P., et al. (2003). Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. Journal of Biologcal Chemistry, 278(23), 20915–20924.
Breckenridge, D., Germain, M., & Mathai, J., et al. (2003). Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene, 22(53), 8608–8618.
Scorrano, L., Oakes, S. A., & Opferman, J. T., et al. (2003). BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science, 300(5616), 135–139.
White, C., Li, C., & Yang, J., et al. (2005). The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP(3)R. Nature Cell Biology, 7(10), 1021–1028.
Wang, M., Yao, Y., & Kuang, D., et al. (2006). Activation of family C G-protein coupled receptors by the tripeptide glutathione. Journal of Biological Chemistry, 281(13), 8864–8870.
Brown, E. M., Vassilev, P. M., & Quinn, S., et al. (1999). G-protein-coupled, extracellular Ca2+ -sensing receptor: a versatile regulator of diverse cellular functions. Vitamins and Hormones, 55, 1–71.
Lin, K. I., Chattopadhyay, N., & Bai, M., et al. (1998). Elevated extracellular calcium can prevent apoptosis via the calcium-sensing receptor. Biochemical and Biophysical Research Communication, 249(2), 325–331.
Wang, R., Xu, C. Q., & Zhao, W. M., et al. (2003). Calcium and polyamine regulated calcium-sensing receptors in cardiac tissues. European Journal of Biochemistry, 270(12), 2680–2688.
Zhang, W. H., Fu, S. B., & Lu, F. H., et al. (2006). Involvement of calcium-sensing receptor in ischemia/reperfusion-induced apoptosis in rat cardiomyocytes. Biochemical and Biophysical Research Communication. 347, 872–881.
Wang, L. N., Wang, C., & Lin, Y., et al. (2008). Involvement of calcium-sensing receptor in cardiac hypertrophy- induced by angiotensinII through calcineurin pathway in cultured neonatal rat cardiomyocytes. Biochemical and Biophysical Research Communication, 369(2), 584–589.
Qi, H., Cao, Y., & Huang, W., et al. (2013). Crucial role of calcium-sensing receptor activation in cardiac injury of diabetic rats. PLoS One., 8(5), e65147.
Han, G., Wang, H. Y., & Han, Z. W., et al. (2018). Relationship between CaSRs and LPS-injured cardiomyocytes. International Journal of Clinical and Experimental Pathology, 11(4), 1965–1971.
Liu, W. X., Zhang, X., & Zhao, M., et al. (2015). Activation in M1 but not M2 macrophages contributes to cardiac remodeling after myocardial infarction in rats: a critical role of the calcium sensing receptor/NRLP3 inflammasome. Cell Physiology and Biochemistry, 35, 2483–2500.
Fogle, R. L., Lynch, C. J., & Palopoli, M., et al. (2010). Impact of chronic alcohol ingestion on cardiac muscle protein expression. Alcoholism: Clinical and Experimental Research, 34(7), 1226–1234.
Ieda, M., Tsuchihashi, T., & Ivey, K. N., et al. (2009). Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Developmental Cell, 16(2), 233–244.
Olson, E. R., Shamhart, P. E., & Naugle, J. E., et al. (2018). Angiotensin II-induced extracellular signal-regulated kinase 1/2 activation is mediated by protein kinase c delta and intracellular calcium in adult rat cardiac fibroblasts. Hypertension, 51(3), 704–711.
Kartkaya, K., Kanbak, G., & OGlakci, A., et al. (2014). Protective effect of calpain inhibitor N-acetyl-L-leucyl-L-leucyl-L-norleucinal on acute alcohol consumption related cardiomyopathy. Molecular Biology Reports, 41(10), 6743–6753.
Fernández-Solà, J., Lluis, M., & Sacanella, E., et al. (2011). Increased myostatin activity and decreased myocyte proliferation in chronic alcoholic cardiomyopathy. Alcoholism Clinical and Experimental Research, 35(7), 1220–1229.
Zhang, B., Turdi, S., & Li, Q., et al. (2010). Cardiac overexpression of insulin-like growth factor 1 attenuates chronic alcohol intake-induced myocardial contractile dysfunction but not hypertrophy: roles of Akt, mTOR, GSK3beta, and PTEN. Free Radical Biology and Medicine, 49(7), 1238–1253.
Stewart, A., Maity, B., & Anderegg, S. P., et al. (2015). Regulator of G protein signaling 6 is a critical mediator of both reward-related behavioral and pathological responses to alcohol. Proceedings of the National Academy of Sciences USA, 112(7), E786–E795.
Raymond, A. R., Becker, J., & Woodiwiss, A. J., et al. (2016). Ethanol-associated cardiomyocyte apoptosis and left ventricular dilation are unrelated to changes in myocardial telomere length in rats. Journal of Cardiac Failure, 22(4), 294–302.
Guo, J., Li, H. Z., & Zhang, W. H., et al. (2010). Increased expression of calcium-sensing receptor induced by ox-LDL amplified apoptosis of cardiomyocyts during stimulated ischaemia-reperfusion. Clinical and Experimental Pharmacology and Physiology, 37(3), e128–e135.
Zhang, R. H., Gao, J. Y., & Guo, H. T., et al. (2013). Inhibition of CYP2E1 attenuates chronic alcohol intake-induced myocardial contractile dysfunction and apoptosis. Biochimica et Biophysica Acta, 1832(1), 128–141.
Lu, F. H., Fu, S. B., & Leng, X. N., et al. (2013). Role of calcium-sensing receptor in cardiomyocyte apoptosis via the sarcoplasmic reticulum and mitochondrial death pathway in cardiac hypertrophy and heart failure. Cellular Physiology and Biochemistry, 31(4-5), 728–743.
Oyadomari, S., Koizumi, A., & Takeda, K., et al. (2002). Targeted disruption of the chop gene delays endoplasmic reticulum stress-mediated diabetes. The Journal of Clinical Investigation, 109(4), 525–532.
Gan Rt, Hu. G. X., & Zhao, Y. J., et al. (2012). Post-conditioning protecting rat cardiomyoctes from apoptosis via attenuating calcium sensing receptor-induced edo(sarco)plasmic reticulum stress. Molecular and Cellular Biochemistry, 361(1-2), 123–134.
Kaufman, R. J. (2002). Orchestrating the unfolded protein response in health and disease. Journal of Clinical Investigation, 110(10), 1389–1398.
Lu, F., Tian, Z., & Zhang, W., et al. (2010). Calcium-sensing receptors induce apoptosis in rat cardiomyocytes via the endo(sarco) plasmic reticulum pathway during hypoxia/reoxygenation. Basic and Clinical Pharmacology and Toxicology., 106(5), 396–405.
Nakagawa, T., Zhu, H., & Morishima, N., et al. (2000). Caspase-12 mediates endoplasmic reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature, 403(6765), 98–103.
Jo, D. G., Jun, J. I., & Chang, J. W., et al. (2004). Calcium binding of ARC mediates regulation of caspase 8 and cell death. Molecular and Cellular Biology, 24(22), 9763–9770.
Hajnóczky, G., Buzas, C. J., & Pacher, P., et al. (2005). Alcohol and mitochondria in cardiac apoptosis: mechanisms and visualization. Alcoholism: Clinical and Experimental Research, 29(5), 693–701.
Xu, J., Zhou, Q., & Xu, W., et al. (2012). Endoplasmic reticulum stress and diabetic cardiomyopathy. Experimental Diabetes Research, 2012, 827971.
Ferri, K. F., & Kroemer, G. (2001). Organelle-specific initiation of cell death pathways. Nature Cell Biology, 3(11), E255–E263.
Acknowledgements
The authors thank Feng XIN, Lijuan YAN and Liping LIU for the technical work in this research. This work was supported by the National Natural Science Foundation of China (81370319, 81700318).
Author Contributions
W.L. designed research and wrote the paper; W.L., M.Z. and X.Z. performed research; W.L., J.C. and X.Z. analysed data and revised the manuscript; Y.L. and X.Y. supervised the experiments, revised and approved the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Ethical Approval
The authors are accountable for all aspects of the work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, W., Zhao, M., Zhang, X. et al. Alcohol Intake Provoked Cardiomyocyte Apoptosis Via Activating Calcium-Sensing Receptor and Increasing Endoplasmic Reticulum Stress and Cytosolic [Ca2+]i. Cell Biochem Biophys 81, 707–716 (2023). https://doi.org/10.1007/s12013-023-01167-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-023-01167-8