Abstract
Severe acute pancreatitis (SAP) is a common acute abdominal disease. This study was designed to investigate the preventive effects of curcumin on SAP and its possible mechanism of action. We observed increased volume of ascites, serum AMY, IL-6, and TNF-α levels, and expression of TLR-4 and NF-κB mRNA and protein in a rat model of SAP. Application of curcumin resulted in lower ascites volume and serum AMY. The levels of serum cytokines IL-10 and TNF-α were also significantly reduced after curcumin treatment, as evident from ELISA analysis. RT-PCR analysis showed down-regulation of TLR4 and NF-κB expressions as a function of curcumin treatment. Our results demonstrate the protective effect of curcumin in a rat model of SAP via the involvement of TLR-4/NF-κB signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe acute pancreatitis (SAP), which has acute onset, rapid progression, and bad outcomes, is a common clinical acute abdominal disease [1, 2]. The traditional view of the mechanism for SAP includes self-digestion induced by trypsin, and obstacles in pancreatic microcirculation and infection [3–5]. However, the detailed molecular mechanism of SAP pathogenesis is still not clear. Recent reports have highlighted the role of the activation of inflammatory factors and the signal pathway in pancreatic tissues in the process of SAP [6, 7]. Activation of IL-6, TNF-α, and inflammatory infiltration cells in early SAP phase can lead to pathological injury not only to the pancreatic tissue, but also in extra-pancreatic organs, through activating downstream inflammation mediators by amplifying a series of cascade reaction [8]. The injuries would further induce systemic inflammatory response syndrome (SIRS) or even multiple organ dysfunction syndrome (MODS) [9, 10].
Toll-like receptors (TLRs) are key modulators of the intrinsic immune response [11]. Members of the TLR family recognize and bind to their corresponding ligand to activate signal transduction pathways and thus produce different biological functions in response to the various stimuli [12]. TLR4 is the first reported TLR by which the mediated signal pathway can non-specifically bind in pathogen-associated molecular patterns (PAMPs) [13]. NF-kB, a nuclear transcription factor present in a wide variety of cells, shares an upstream/downstream relationship with TLR4 [14]. NF-kB mainly functions at the level of regulating inflammation, cell survival, and apoptosis [15]. Under normal circumstances, members of the NF-kB forms homomeric or heteromeric dimers in cytoplasm, which exert the function of activation/inhibition of transcriptions [16]. With the activation of the TLR4-mediated signal transduction pathway, the NF-kB activation and transcription of related proinflammatory cytokines is then stimulated [17]. Several studies have confirmed that the expression and activation of TLR4 and NF-kB were upregulated, and a large amount of neutrophils and inflammatory mediators were detected in the SAP rat model induced through various ways [18]. Thus, it is highly likely that the TLR-4/NF-kB signaling pathway is closely related to the occurrence and development of SAP.
Curcumin is the main active ingredient extracted from the rhizome of herbs [19]. A number of experimental studies have demonstrated the protective properties of curcumin for anti-inflammatory, antiviral, antioxidant, anti-cancer, anti-fibrosis, and liver- and kidney-protection [19]. Curcumin exerts its inhibitory effects on both acute and chronic inflammation, by reducing the infiltration of neutrophils, inhibiting lipid peroxidation, and downregulating NF-κB [20, 21].
In the present study, we investigate the expression of TLR-4 and NF-κB signaling pathway in SAP rat model and evaluate the effect of curcumin on the prevention and treatment of SAP (Research projects in education department of sichuan province, NO.15ZB0254).
Materials and Methods
Animal Preparation, Grouping, and Model Building
54 healthy clean male SD rats (aged 3–4 months, weight 180–250 g) were purchased from experimental animal center of our facility. All procedures were approved by local ethics committee and were performed in accordance with the Guideline for the Care and Use of Laboratory Animals of the first affiliated hospital of Chengdu medical college. Rats were fed for adaption in the laboratory 1 week before experiment, and were allowed to drink water but restricted to food intake 12 h prior to the experiment.
Rats were randomly divided into 3 groups: normal group, severe acute pancreatitis model group (SAP Group), and curcumin pretreating group. Each group was further subdivided into 3 groups as 3, 6, 12 h according to the time course in the experiment (each sub-group had 6 rats).
SAP Model Procedure
SD rats were anesthetized with intraperitoneal injection of 4 % chloral hydrate (0.6 ml/kg). After routine shaving and draping, surgical incision was made into the abdomen from the lower abdominal midline under sterile conditions. A 1-ml-small syringe needle tip was then inserted into the pancreatic duct through duodenal papilla opening. Occlusions in pancreatic puncture and biliary bile duct respectively were made with two pieces of small artery clipping. 5 % sodium taurocholate was then pressurized at 0.1 ml/min in 4 min. The injection was suspended for 5 min to confirm pancreatic tissue edema in these animals after which the vascular clamp was removed and the abdomen was closed layer by layer.
Animals in the normal group underwent the same operational procedure but without injection of sodium taurocholate in pancreatic duct. Rats in curcumin group were intraperitoneally injected with curcumin solution (100 mg/kg) 30 min before the establishment of model. All animals after surgery were subcutaneously injected with saline (0.2 ml/kg) in order to make up for the missing liquid. Animals were allowed to drink water but restricted to food.
Ascitic Volume, Serum AMY Determination, and IL-6, TNF-α Measurement
Dry cotton balls were prepared for measuring the ascite weight. After the rats were sacrificed at each certain time point, ascites were suctioned by dry cotton balls. Weights of dry and wet cotton balls were recorded before and after the suction. The volume of ascites was calculated based on the difference of the wet and dry cotton weight (density of the ascite was assumed to be 1 mg/ml).
Blood samples were obtained from each group 3, 6, 12 h after the model was built and was stored at 4 °C overnight. The sera were separated and stored at −20 °C. Serum samples were analyzed with automatic biochemical analyzer (Automatic Analyzer 7600) through G7-PNP method to detect the level of serum AMY.
Enzyme-Linked Immunosorbent Assay (ELISA) kits were used to detect the level of IL-6 and TNF-α in rat serum by strictly following the manufacturer instructions [22].
Detection of Expression of TLR-4/NF-kB Through Semi-Quantitative RT-PCR
The cDNA sequences of NF-κB and TLR-4 were retrieved from the Genbank to design primers aided by Oligo software. Specificity of all primers was confirmed by NCBI BLAST software, and the primers were synthesized by Shenggong Biological Engineering Technology Co., Ltd. (Shanghai, China). Sequences of RT-PCR primers are shown in Table 1.
Semi-quantitative PCR was performed with the use of SYBR green chemistry on an ABI PRISM 7000 sequence detection system (Applied Biosystems) as previously reported [22]. Regression analysis of the mean values of four multiplex RT-PCRs for the log10 diluted cDNA was used to generate the standard curves. Mean values generated at individual time points were compared with Student’s t test.
Western Blotting
Total proteins were extracted from the rat SAP Model cells using RIPA buffer (Thermo). Proteins were separated by SDS-PAGE on a 10 % gel. Primary antibodies are rabbit NF-κB (Abcam), TLR-4 (Santa Cruz), and GAPDH (Santa Cruz). Secondary antibody is HRP-conjugated anti-rabbit antibody (Jackson Labs). The bound antibody was assayed with a chemofluorescence detection kit (Amersham, Piscataway, NJ). The representative images are shown.
Statistical Analysis
The ascetic volume and Schimidt scores are presented as the Mean ± standard deviation (SD). STATA 12.0 Software (STATA, Texas, Data Analysis and Statistical Software, USA) was employed to determine the statistical significance. All inspection levels were set to 0.05. Two-by-two comparison was proceeded with SNK (Student- Newman-Kewls) method. P < 0.05 was taken as statistically significant.
Results
Effect of Curcumin on Ascitic Volume and Serum AMY Level
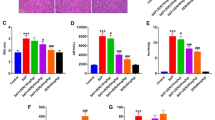
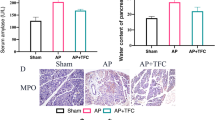
The volume of ascites in SAP group is significantly higher than that in the normal group and curcumin group, respectively (P < 0.01, P < 0.01), especially at 12 h. Moreover, volume of ascites in SAP group increases during the time course of model building, while volume of ascites in C group shows a decrease from 3 to 12 h (P < 0.01, Fig. 1a).
The data were transferred into log for convenient analysis. A statistical difference among the three groups (each P < 0.01) could be seen. The serum AMY levels in both the SAP group and the curcumin group were higher than that in the normal group. Generally speaking, serum AMY level in the normal and SAP groups both increased. In SAP group, AMY level increases at 3 h, reaching the peak at 6 h, and decreases at 12 h. But the level at 12 h still remained higher than that at 3 h. It is interesting to note that the serum AMY level of curcumin group significantly decreased from 3 to 6 h (P < 0.05), but slightly from 6 to 12 h (P > 0.05 Fig. 1b).
Effect of Curcumin on Serum IL-6 and TNF-α Levels
Compared with the normal group, the serum IL-6 level in the SAP group and curcumin group is significantly higher (P < 0.01). Moreover, serum IL-6 level at each time point in curcumin group could be seen as significantly (P < 0.01) lower than that in the SAP group. In the SAP group, the increase is most significant at 6 h, while in the curcumin group, the whole overall trend of serum IL-6 level appears to be declining (Fig. 2a).
Trend of serum TNF-α in the three groups was similar to the IL-6 level in the three groups. Levels in SAP group and curcumin group were all significantly higher than that in the normal group (P < 0.01). Compared to the SAP group, there was a significant decrease of serum TNF-α at each time point in the curcumin group (P < 0.01, Fig. 2b). 6 h is a very significant time point for peaking of the levels of both IL-6 and TNF-α in both SAP and curcumin groups (P < 0.01).
The Expression of TLR-4 and NF-κB mRNA and Protein in Pancreatic Tissue
Compared with the normal control group, the mRNA expression of NF-κB and TLR-4 in the pancreatic tissue of SAP model shows a significant increase (P < 0.01). However, significant reduction (P < 0.01) in the gene expression of both TLR-4 and NF-κB in the curcumin group compared to the SAP group could be seen (Figs. 3, 4).
To further determine the expression of TLR and NF-κB at the proteins levels, Western Blot assays were applied. The protein expression of NF-κB and TLR-4 in the pancreatic tissue of SAP model shows a significant increase when compared with the normal control group (P < 0.01). However, significant reduction (P < 0.01) in the protein of both TLR-4 and NF-κB in curcumin group compared to SAP group could be seen (Fig. 5).
Discussion
We describe the effects of curcumin on the prevention and treatment of SAP in rat animal model. The expression of TLR-4 and NF-κB signaling pathway in SAP rat model was also studied with or without the presence of curcumin. We observed that volume of ascites and serum level of AMY increased after the SAP model was built and decreased when curcumin was injected. Moreover, both IL-6 and TNF-α levels also showed the similar trend. It was interesting to note 6 h as the possible peak time for the development of SAP. In addition, the expression of TLR-4 and NF-κB mRNA in pancreatic tissue was also found to be downregulated in the presence of curcumin, while both of these were activated and upregulated in untreated SAP model.
The molecular mechanism of SAP pathogenesis is not clearly understood [5]. However, it is generally believed that SAP is a SIRS [9] and MODS [10] as a result of trypsin activation and release-induced pancreatic self-digestion, hemorrhage, and necrosis [6]. In the pathology of SAP, synthesis and release of a large number of cytokines and inflammatory mediators play a very important role in the pancreatic gland damage and subsequent SIRS and MODs [5, 6, 10]. Thus, blocking the related signal pathway and inhibiting the transcription and translation of mediators of inflammation-related genes at the molecular level would in turn decrease the synthesis and release of cytokines and inflammatory mediators which can effectively alleviate the damage to pancreatic tissue and ameliorate inflammatory process [7].
Toll-like receptor 4 (TLR-4), reported for the first time in 1997, is a kind of inflammatory trans-membrane receptor [23]. When the corresponding ligands interact with TLR-4, downstream signal transduction pathway is activated immediately, resulting in specific biological functions [24]. TLR4 mainly recognizes LPS and some gram-negative bacteria PAMPs by forming homologous dimers [25, 26]. TLR4 expressed by epithelia in pancreatic ducts and acinus activates a series of signal molecules through a combination of corresponding ligands via the MyD88 signal pathway [27]. Signal molecules, including TNF receptor-associated factor 6 (TRAF-6), IL-10 receptor-associated kinase (IRAK) and TGF-β-activated kinase (TAK1), would eventually lead to the activation of transcription factors such as nuclear factor κB (NF-κB) [28] and initiate target gene transcription and release of inflammatory mediators such as IL-10 and TNF-α thereby regulating the inflammation process [29]. Studies from Gukovsky et al. [30] confirmed that in normal rat pancreatic cells, NF-κB activation is not obvious. However, in the model of acute pancreatitis, NF-κB is activated, especially in patients with SAP, suggesting that NF-κB plays an important role in the development and also prognosis of SAP [31]. Our results also showed that after the SAP model was built, NF-κB and TLR-4 were upregulated along with IL-10 and TNF-α, further confirming the previous theories.
Curcumin is one of the main active ingredients made from rhizome of herbs [19]. Current studies have confirmed that curcumin exerts various effects including anti-inflammatory, antiviral, antioxidant, anti-cancer, and anti-fibrosis [21]. By reducing the infiltration of neutrophils, inhibiting lipid peroxidation and downregulation of NF-κB [21], inhibitory effect of curcumin on acute and chronic inflammation can be achieved. Earlier results have demonstrated that curcumin has obvious inhibitory effects on the activation of TLR-4/NF-κB signaling pathway [30]. In the present study, intraperitoneal injection of curcumin displayed a protective effect on SAP in rats. PCR results showed that TLR-4/NF-κB protein expression in curcumin group was significantly lower than that in the SAP group, suggesting that curcumin may block TLR-4/NF-κB expression upstream of the signaling pathways at the molecular level. Moreover, volume of ascites and serum AMY level in rats of curcumin group were significantly reduced when compared with that in the SAP control group. In addition, IL-10 and TNF-α levels also declined in the curcumin group, further suggesting that the therapeutic effect of curcumin was probably achieved through the TLR-4/NF-κB signaling pathway.
To conclude, the effect of curcumin was studied in the established rat model of SAP and was found to have a positive effect on the treatment for SAP. Experimental evidences to understand the mechanism of curcumin action revealed the importance of TLR-4/NF-κB signaling pathway in the activation and development of SAP.
References
Phillip, V., Steiner, J. M., & Algul, H. (2014). Early phase of acute pancreatitis: Assessment and management. World Journal of Gastrointestinal Pathophysiology, 5(3), 158–168.
da Costa, D. W., et al. (2014). Staged multidisciplinary step-up management for necrotizing pancreatitis. British Journal of Surgery, 101(1), e65–e79.
Kota, S. K., et al. (2013). Metabolic pancreatitis: Etiopathogenesis and management. Indian Journal of Endocrinology Metabolism, 17(5), 799–805.
Closa, D. (2013). Free radicals and acute pancreatitis: Much ado about… something. Free Radical Research, 47(11), 934–940.
Waldthaler, A., Schutte, K., & Malfertheiner, P. (2010). Causes and mechanisms in acute pancreatitis. Digestive Diseases, 28(2), 364–372.
Armstrong, J. A., et al. (2013). Oxidative stress in acute pancreatitis: lost in translation? Free Radical Research, 47(11), 917–933.
Escobar, J., et al. (2009). Cross-talk between oxidative stress and pro-inflammatory cytokines in acute pancreatitis: A key role for protein phosphatases. Current Pharmaceutical Design, 15(26), 3027–3042.
Fisic, E., et al. (2013). The role of IL-6, 8, and 10, sTNFr, CRP, and pancreatic elastase in the prediction of systemic complications in patients with acute pancreatitis. Gastroenterology Research Practice, 2013, 282645.
Khanna, A. K., et al. (2013). Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE-II, CTSI Scores, IL-6, CRP, and Procalcitonin in predicting severity, organ failure, pancreatic necrosis, and mortality in acute pancreatitis. HPB Surgery, 2013, 367581.
Gasparovic, V., et al. (2014). Severe acute pancreatitis as a part of multiple dysfunction syndrome. Collegium Antropologicum, 38(1), 125–128.
Barak, B., Feldman, N., & Okun, E. (2014). Toll-like receptors as developmental tools that regulate neurogenesis during development: An update. Frontiers in Neuroscience, 8, 272.
Liu, X., et al. (2014). The relationship between SNPs in the genes of TLR signal transduction pathway downstream elements and rheumatoid arthritis susceptibility. Tsitologiia i Genetika, 48(3), 24–29.
Murad, S. (2014). Toll-like receptor 4 in inflammation and angiogenesis: a double-edged sword. Frontiers in Immunology, 5, 313.
Xu, Y., et al. (2014). oxLDL/beta2GPI/anti-beta2GPI complex induced macrophage differentiation to foam cell involving TLR4/NF-kappa B signal transduction pathway. Thrombosis Research, 134(2), 384–392.
Wullaert, A., Bonnet, M. C., & Pasparakis, M. (2011). NF-kappaB in the regulation of epithelial homeostasis and inflammation. Cell Research, 21(1), 146–158.
Ghosh, G., et al. (2012). NF-kappaB regulation: Lessons from structures. Immunological Reviews, 246(1), 36–58.
Zhang, Y., et al. (2014). Activation of vascular endothelial growth factor receptor-3 in macrophages restrains TLR4-NF-kappaB signaling and protects against endotoxin shock. Immunity, 40(4), 501–514.
Xiping, Z., et al. (2009). Effects of Salvia miltiorrhizae on ICAM-1, TLR4, NF-kappaB and Bax proteins expression in multiple organs of rats with severe acute pancreatitis or obstructive jaundice. Inflammation, 32(4), 218–232.
Lestari, M. L., & Indrayanto, G. (2014). Curcumin. Profiles of Drug Substances, Excipients and Related Methodology, 39, 113–204.
Lu, X., et al. (2014). The effect of Chinese herbal medicine on non-biliogenic severe acute pancreatitis: A systematic review and meta-analysis. Journal of Ethnopharmacology, 155(1), 21–29.
Jurenka, J. S. (2009). Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Alternative Medicine Review, 14(2), 141–153.
Bienvenu, J., et al. (1993). Analytical performances of commercial ELISA-kits for IL-2, IL-6 and TNF-alpha. A WHO study. European Cytokine Network, 4(6), 447–451.
Vaure, C., & Liu, Y. (2014). A comparative review of toll-like receptor 4 expression and functionality in different animal species. Frontiers in Immunology, 5, 316.
Zhao, H., et al. (2014). Role of Toll-like receptor-4 in renal graft ischemia-reperfusion injury. Am Journal of Physiology Renal Physiology, 306(8), F801–F811.
Lien, E., & Ingalls, R. R. (2002). Toll-like receptors. Critical Care Medicine, 30(1 Suppl), S1–S11.
Cohen-Sfady, M., et al. (2005). Heat shock protein 60 activates B cells via the TLR4-MyD88 pathway. Journal of Immunology, 175(6), 3594–3602.
Zhai, Y., et al. (2004). Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. Journal of Immunology, 173(12), 7115–7119.
Beutler, B., et al. (2006). Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annual Review of Immunology, 24, 353–389.
Doyle, S. L., Jefferies, C. A., & O’Neill, L. A. (2005). Bruton’s tyrosine kinase is involved in p65-mediated transactivation and phosphorylation of p65 on serine 536 during NFkappaB activation by lipopolysaccharide. Journal of Biological Chemistry, 280(25), 23496–23501.
Gukovsky, I., et al. (2003). Curcumin ameliorates ethanol and nonethanol experimental pancreatitis. American Journal of Physiology. Gastrointestinal and Liver Physiology, 284(1), G85–G95.
Wang, X., et al. (2003). Gastrointestinal dysmotility in patients with acute pancreatitis. Journal of Gastroenterology and Hepatology, 18(1), 57–62.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhong, K. Curcumin Mediates a Protective Effect Via TLR-4/NF-κB Signaling Pathway in Rat Model of Severe Acute Pancreatitis. Cell Biochem Biophys 73, 175–180 (2015). https://doi.org/10.1007/s12013-015-0664-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-015-0664-y