Abstract
Myocardial infarction is a leading cause of mortality and morbidity worldwide. Although essential for successful recovery, myocardium reperfusion is associated with reperfusion injury. Icariin, a major flavonoid of Epimedium koreanum Nakai, has been proven to exert efficacy for improving cardiovascular function. We investigated the molecular effect and signal pathway of icariin on cardiac ischemia/reperfusion injury. In an in vivo model of infarct in rats, icariin (10 mg/kg) significantly attenuated myocardial infarct size induced by ischemia/reperfusion (I/R). From the TUNEL assay, icariin reduced the apoptotic cell induced by I/R and decreased blood indicators of creatine kinase, ischemia-modified albumin, and lactate dehydrogenase. All this effect was antagonized by the PI3K inhibitor LY294002. Meanwhile, icariin activated the PI3K/Akt/eNOS pathway. The PI3K inhibitor LY294002 suppressed icariin-mediated protective effect. These results suggest that icariin protects against myocardial ischemia reperfusion injury in rats by activating the PI3K/Akt/eNOS-dependent signal pathways and may be a useful drug for angiogenic therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease is a global health problem [1]. Ischemic heart disease with high morbidity and mortality rates has aroused medicine and public concern worldwide [2–4]. Myocardial infarction will be the leading cause of mortality by 2020 according to the World Health Organization [4]. Although prompt myocardial ischemia/reperfusion (I/R) might be effective against myocardial impairment, but it also will cause secondary injury via mitochondrial dysfunction, excessive release of glutamate, and overproduction of pro-inflammatory mediators and reactive oxygen species (ROS) that can provoke further myocardial damage [5]. Ischemic post-conditioning (IPC) is a procedure that has been studied in both animals and humans as a way of protecting the heart and brain cells from reperfusion injury after prolonged ischemia [6]. However, this treatment is not fit to patients with thrombolytic drugs. Therefore, novel treatment strategies are required to attenuate I/R injury and improve clinical outcome, and more feasible and effective drugs are needed in clinical IPC. At present, some drugs such as erythropoietin [7], adenosine, and hydrogen sulfide [8] have shown good protective effect at the beginning of reperfusion of the heart, but these drugs have not been widely used in clinical.
Icariin, a natural flavonoid glucoside isolated from a traditional Chinese medicine Epimedium, has been reported to have a variety of pharmacological activities [9]. Studies proved that icariin has male sexual function improvement, antioxidant [10, 11], antidepressant [12, 13], anti-inflammatory [14, 15], and neuroprotective effects [16] in vitro. Icariin also shows inhibitory activity on human tumors [17, 18]. Previous research has suggested that icariin markedly improved the cardiac function, which may be related to reducing mitochondrial oxidative stress injuries in streptozotocin (STZ)-induced diabetic rats [19]. In addition, study shows icariin ameliorates left ventricular dysfunction and cardiac remodeling through down-regulating matrix metalloproteinase-2 and -9 activity and myocardial apoptosis in rats with congestive heart failure [20]. A new study suggests icariin protects rat cardiac H9c2 cells from apoptosis by inhibiting endoplasmic reticulum stress [21]. These findings suggest that icariin may have powerful effects in positive inotropic action. However, the effect of icariin on myocardial injury after myocardial infarction has not been reported and the mechanism of heart protection is not clear.
Phosphoinositide 3-kinases (PI3Ks) are proteins coupled to a variety of cell surface receptors and play a key role in cell proliferation, survival, and apoptosis [22, 23]. Several studies have suggested that the PI3K/Akt signaling pathway plays a key role in cardiac protection against I/R injury [24]. It has been reported that icariin stimulated angiogenesis by activating the MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways [25]. The PI3K/Akt pathway was involved in the protective effects of icariin on LPS-induced acute inflammatory responses [26]. However, it is unclear whether the PI3K/AKT/eNOS pathway mediates the cardioprotection of icariin on cardiac ischemia/reperfusion injury.
In the present study, we have investigated the effect of icariin on cardiac ischemia/reperfusion injury in vitro and further explored the probable molecular mechanism of the process.
Materials and Methods
Animal
Adult male Spraque-Dawley rats (200–250 g) were used to prepare myocardial I/R model. Animals were kept at a constant temperature (23 ± 2 °C), humidity (60 ± 10 %), and a light/dark (12/12 h) cycle. Food and water were available ad libitum until the beginning of the experiment. All the experimental procedures were carried out following the guidelines of The People’s Hospital of Liaoning Province and have been approved by the Committee for animal experimentation of The People’s Hospital of Liaoning Province. Every effort was made to reduce the number of animals and their suffering.
Myocardial I/R operations were prepared according to a previous procedure [27]. Animals were randomly allocated into four groups (10 rats in each). Group A is sham operation group receiving normal saline without I/R, Group B is model group with I/R induced via ligation of LAD coronary artery for 30 min followed by 2 h reperfusion, while Group C received icariin (10 mg/kg, Melonepharma, Dalian, China) before reperfusion and Group D received icariin, 10 mg/kg with LY294002 (0.3 mg/kg, Beyotime, Shanghai, China) before reperfusion. Briefly, the rats were anesthetized with Chloral hydrate 3 mg/kg (i.m.), and artificial respiration was immediately started with room air (volume 2.0 ml/100 g, rate 80 strokes/min). The chest was opened by middle thoracotomy. After pericardiotomy, a 4-0 black silk ligature was placed under the left aortic descending coronary artery, and the ends of the tie was threaded through a small vinyl tube to form a snare for reversible left aortic descending coronary artery occlusion. Before the reperfusion procedures, rats received an i.p. injection of icariin or LY294002 or vehicle (normal sterile saline). The myocardium was reperfused by loosening the snare for 2 h. Following termination of the experiment, serum was collected for LDH measurement. Hearts of the other six rats of each group were collected, washed with ice cold saline, immersed immediately in liquid nitrogen, and kept at −80 °C.
CK, IMA and LDH Determination in Serum
Serum activity of CK and LDH were assayed enzymatically using commercially available kits provided by Jiancheng Bioengineering Institute, Nanjing, China. IMA was analyzed using the colorimetric method [28]. Briefly, 200 μl of serum were placed into a clean tube, 50 μl of 0.1 % CoCl2 was added and mixed gently. After leaving the mixture undisturbed for 10 min, 50 μl of 1.5 mg/ml dithiothreitol (DTT) was added and 50 μl of distilled water was used instead of DTT as a control. After 2 min, 1 ml of 0.9 % NaCl was added and the absorbance at 470 nm was determined using a spectrophotometer (UV752, YOKE, Shanghai, China).
Determination of Cardiac Infarct Size
At the end of 2-h reperfusion period, animals (n = 10) from each group were intracardiacally perfused with isotonic saline and sacrificed by spinal dislocation. Hearts were then sliced into 2 mm sections and incubated with 2 % tri-phenyl tetrazolium chloride (TTC) at 37 °C in PBS for 15 min. Normally, in viable cells, TTC is converted to red formazone pigment by NAD and lactate dehydrogenase (LDH), thus viable cells stain bright red. Since, infracted cells lose the enzyme as well as the cofactor, thus, they remain unstained or stain dull yellow. Heart sections were placed over glass plate and the infracted areas were traced by a 100 squares in 1 cm2 transparent plastic grid. In each slice, the average infarcted area as well as the non-infarcted area was determined. Infarcted area was expressed as a percentage of total heart area according to a previous procedure [29].
TUNEL-Based Assay
DNA fragmentation, a marker of apoptosis, was measured using TdT-mediated dUTP nick end labeling (TUNEL) staining with an In Situ Cell Death Detection KitH (Roche, Mannheim, Germany), as previously described. The heart was removed, postfixed in 4 % paraformaldehyde for 24 h, and then embedded in paraffin for slicing 5 μm sections. Briefly, the sections were postfixed in ethanol–acetic acid (2:1), rinsed, incubated with 1 % proteinase K (in 50 mM Tris/5 mM EDTA buffer) for 15 min, rinsed, incubated in 3 % H2O2, permeabilized with 0.1 % Triton X-100, and rinsed again. Subsequently, sections were incubated in the TUNEL mixture for 1 h at 37 °C, and rinsed. Five different random highpower fields (400× magnification) in peri-infarct region were counted for each condition using a light microscope, and TUNEL-positive apoptotic cells appeared as brown nuclear or cytoplasmic staining. The numbers of TUNEL-positive cells from three slices per heart were averaged. Cell counting was performed by a pathologist blinded to the experiment condition.
Immunoblotting
Heart homogenate proteins were collected and lysed in lysis buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1 mM dithiothreitol, 1 % Triton X-100, and 1 % protease inhibitor), boiling for 10 min, followed by brief sonication. Lysates were then cleared by centrifugation at 14,000×g for 10 min, and the supernatant was collected. Protein concentration was determined using the BCA kit (Pierce Chemical) as per the manufacturer’s instructions. 40 μg of protein was resolved by SDS-PAGE and transferred onto nitrocellulose membranes. Blots were incubated overnight at 4 °C with rabbit polyclonal antibodies against AKT (1:500 diluted, Santa Cruz, Dallas, TX, USA), p-AKT (1:200 diluted, Santa Cruz), Bax (1:500 diluted, Santa Cruz), Bcl-2 (1:500 diluted, Santa Cruz), PI3K p85 (1:500 diluted, Santa Cruz), p-eNOS (1:200 diluted, Bioss, Beijing, China) followed by horseradish peroxidase–conjugated secondary antibodies for 2 h. Detection was performed by chemiluminescence (ECL, 7SeaPharmTech, Shanghai, China). All blots were stripped and incubated with polyclonal anti-β-actin antibody (1:1,000 diluted, Santa Cruz) to ascertain equal loading of proteins.
Immunohistochemistry
In brief, the sections were de-paraffinized in xylene and rehydrated through graded alcohols, then boiled in 0.01 M citrate buffer (pH 6.0) for 10 min. Hydrogen peroxide, 0.3 %, was added to block any endogenous peroxidase activity. To block nonspecific binding, the sections were incubated with a goat serum blocking solution composed of 10 % normal goat serum in phosphate buffer saline, pH 7.4 and 0.05 % sodium azide. The sections were incubated with anti-caspase-3 (1:100 diluted, Santa Cruz) at 4 °C overnight. Polydetector secondary antibody (Beyotime) was used to avoid contaminating endogenous biotin or streptavidin. After washing, the antigen–antibody complex was applied and stained with diaminobenzidine. Counterstaining was performed lightly with hematoxylin. Pre-immune serum was used instead of the first antibody as a negative control. All the control slides yielded negative results. The evaluation of immunostaining was performed by a pathologist who was unaware of the fate of the tissue site.
Statistical Analysis
Histopathological scores between groups were compared using sum of ranks test. Quantitative data from experiments were expressed as mean ± SD, significance was determined by one-way analysis of AVONA followed by Dunnett’s test. P < 0.05 was considered statistically significant.
Result
Icariin Reduced Myocardial Infarct Volume in I/R Rats
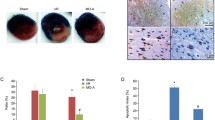
The area of infarction is a vital index of myocardial cardiac injury. I/R can induce heart infarction by inducing cell apoptosis. We examined the effect of icariin on infarct size by TTC staining (Fig. 1). In normal control animals subjected to I/R injury, the infarct size was found to be 23.88 ± 5.15 % and significantly different (P < 0.01) compared with sham control animal. Treatment with 10 mg/kg icariin significantly diminished the infarct size to 12.81 ± 3.39 %. It strongly suggested the beneficial effects of icariin in the rat model of I/R-induced myocardial injury. Treatment with 10 mg/kg icariin and LY294002 0.3 mg/kg only diminished the infarct size to 17.85 ± 3.94 %. Compared to Group C, the infarct size adds 5.04 % (P < 0.05).
Effects of icariin on infarct size in rat hearts subjected to ischemia, followed by 2 h of reperfusion. a White areas indicate infracted tissues using triphenyltetrazolium chloride staining. TTC-stained sections showing the ischemic region (white) and the infarct area (red). b Quantitative analysis of the infarct volume. Values are expressed as mean ± SEM of three independent experiments, each in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001 (Color figure online)
Icariin Reduces LDH, LDH, and CK Release
To evaluate the I/R condition influenced by icariin, some blood indicators of such as creatine kinase (CK), ischemia-modified albumin (IMA) and lactate dehydrogenase (LDH) are detected. Myocardial infarction, by rat cardiac ischemia followed by a 2-h reperfusion produced a significant (P < 0.001) increase in serum CK, IMA, and LDH, by 305.7, 258.5, and 174.5 % from sham group, respectively. Treatment with icariin reduced CK, IMA, and LDH to the level of sham group from I/R (Fig. 2), respectively. However, treatment of LY294002 and icariin contributes to the high level of LDH, IMA, and CK in I/R rat model.
Icariin reduces CK, IMA, and LDH release. Icariin reduced CK, IMA and LDH level after I/R injury in rat serum. PI3K inhibitor LY294002 cotreatment statistically increased the release of CK, IMA, and LDH compared with icariin treated alone. The columns and errors bars represent means and SEM. *P < 0.05, ***P < 0.001
Icariin Treatment Inhibited Apoptosis of Myocardial Cells After I/R
Cell apoptosis is one of the major outcomes after I/R. The observations that icariin protected against cell apoptosis in rats with I/R motivated us to further study the impacts of icariin on myocardial cell apoptosis. TUNEL is a method widely used for the detection of apoptosis, where apoptotic cells are distinguished by their color. With the TUNEL assay in vivo, we found that the number of TUNEL-positive cells was significantly increased in the hearts of rats in I/R (vehicle-treated) group compared with the sham-operated group (Fig. 3). No TUNEL-positive cells were observed in the sham-operated group. Treatment with icariin markedly and significantly reduced the number of TUNEL-positive cells. Increase in the number of apoptotic myocardial cells was found in rats with I/R, whereas the icariin treatment dramatically decreased cell apoptosis. We then decided to use a different approach based on the detection of activated caspase-3, which is considered a valuable and specific tool for identifying apoptotic cells in tissue sections. The expression of Caspase-3 indicated that there were more apoptotic cardiomyocytes 2 h after modeling in the ischemic cardiac tissues than in the control ones (Fig. 4a).
Icariin treatment inhibited apoptosis of myocardial cells after I/R. a Caspase-3 immunoreactivity in the ischemic myocardium was remarkably higher in Model group compared to the icariin group. b The expression of apoptotic markers Bcl-2 and Bax was affected by icariin. c Quantitative analysis of the level of Bcl-2 and Bax. d Effect of icariin on PI3K/AKT/eNOS pathway. e Relative protein levels were quantified by densitometry and shown in the histogram. Values are expressed as mean ± SEM of three independent experiments, each in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001
We also detected the markers of apoptosis such as the anti-apoptotic gene, Bcl-2, and the pro-apoptotic gene, Bax, in the cardiac tissues by Western blot analysis (Fig. 4b, c). Data showed that the expression of Bcl-2 was significantly decreased after modeling in I/R group as compared with control group. On the contrary, the expression of Bax was increased in the I/R group, and was reversed by icariin treatment. Altogether, these results indicated that icariin protects cardiomyocytes against apoptosis.
Effect of Icariin on PI3K/AKT/eNOS Pathway
The PI3K/Akt/eNOS signaling pathway has been recently shown to regulate the icariin-induced angiogenesis. With I/R model, we investigated whether icariin could activate the PI3K/Akt/eNOS pathway and affect the reperfusion injury using Western blot analyses. As shown in Fig. 4d, the ratio of P-Akt/Akt expression was significantly decreased in I/R model group compared to the sham group. However, the expression of PI3K was significantly induced by icariin. The PI3K inhibitor LY294002 administration inhibited the phosphorylation of Akt and abolished the icariin-induced phosphorylation of Akt. These findings strongly suggested that icariin could induce Akt phosphorylation by activating the PI3K pathway. It has been shown that Akt activation promotes the phosphorylation of eNOS, which are involved in angiogenesis. We next determined whether icariin regulates eNOS phosphorylation. As expected, icariin increased eNOS phosphorylation compared to model group. All the results suggested that icariin could affect the PI3K/Akt/eNOS pathway contributing to the protective effect of myocardial cell.
Discussion
A lot of evidence suggests that cardiomyocyte apoptosis contributes to the cell death in hearts undergoing I/R in vivo and in vitro [30]. For the reason that adult cardiac myocytes have lost the proliferation ability to produce more cardiac cells helping recover the cardiac function. Previously, despite unclear action pathway, others have demonstrated that icariin, extracted from Epimedium, have a protective effect and it can attenuate cell apoptosis in some in vitro model [16]. In this model, icariin attenuates cardiac remodeling through down-regulating myocardial apoptosis and matrix metalloproteinase activity in rats with congestive heart failure [20]. Icariin reduces mitochondrial oxidative stress injury in diabetic rat hearts [19]. These suggest that icariin is protective for the rat hearts. As a result, it is a promising candidate as a natural, alternative way of treating myocardial disease. Furthermore, it would be an attractive candidate due to its low toxicity/side effect profiles in comparison to the potential and in some cases serious adverse effects of existing drugs for treating myocardial I/R injury.
The aim of this study was to determine whether icariin could potentially protect against myocardial ischemia reperfusion injury in rats and its mechanism. Our results demonstrated that icariin exerts a protective effect on myocardial I/R injury in an in vivo rat heart model. We observed significant improvement of myocardial condition in the rat treatment with icariin during myocardial I/R challenge. This was reflected by a reduction in infarct size and serum indicator CK, IMA, and LDH. In vivo, icariin injection before reperfusion significantly decreased infarct size. Meanwhile, icariin was also shown to directly inhibit myocardial cell apoptosis by the TUNEL assay. The Bcl-2 family proteins are essential for the program of apoptosis and consist of pro-apoptotic and anti-apoptotic factors. Some of the proteins within this family, including Bcl-2 and Bcl-XL, inhibit apoptosis; others, such as Bax and Bak, promote apoptosis and in some instances are sufficient to cause apoptosis independent of additional signals [31]. The present study demonstrates that stimulation of cardiomyocytes with icariin increases the expression of Bcl-2 and decreases the expression of Bax. It provided that treatment with icariin immediately decreased myocardial I/R injury.
Protective signaling pathways involving activation of phosphatidylinositide-3 kinase (PI3K) and its target Akt/protein kinase B, mediate cytoprotective effects via phosphorylation of a number of proteins and induction of nitric oxide (NO) production, altogether resulting in limitation of apoptosis and improved myocyte survival. The PI3K/Akt signaling pathway plays an important role during cellular proliferation, function, and survival. Akt is identified as cytoprotective through intrinsic mechanisms during cell injury, and previous reports had shown that Akt up-regulates Bcl-2 expression through cAMP-response Element-binding Protein. In order to assess the anti-apoptotic mechanism of icariin in heart I/R models, we evaluated protein expression of Akt, p-Akt, and its downstream target protein eNOS after myocardial I/R injury.
From our study, we observed that the protein level of p-Akt and its upstream PI3K were significantly higher in the I/R + icariin group than the I/R model group and that the expression of eNOS was significantly increased in the I/R + icariin group, and PI3K inhibitor LY294002 reverses these effects. Thus, the protective effect provided by icariin in ischemic myocardial cell may be partly due to the activation of the PI3K/Akt/eNOS pathway. Therefore, the activation of PI3K/Akt/eNOS pathway, either with icariin or pharmacologically, could be a novel therapeutic method for preventing myocardial I/R injury.
However, our study is not very sufficient. There are several limitations in our study. We only used an acute cardiac I/R model and did not confirm our findings at later time points in a chronic cardiac I/R model.
Conclusion
In summary, this study has demonstrated that icariin protected myocardial function from myocardial I/R injury in rats. It decreased infarct volume, alleviated myocardial damage, and attenuated cardiac remodeling. These effects of icariin are correlated with decrease in serum levels of blood indicators CK, IMA, and LDH and activating of PI3K/Akt/eNOS pathway in ischemic myocardial tissue of rats, which is a potential mechanism for protecting the I/R injury and enhancing the resistance to injury in the early stage of I/R. Results from the present study provide insight into the effects and mechanisms of icariin which may be as an effective therapeutic drug to treat the myocardial I/R injury. Taken all together, we report a beneficial and novel role for icariin as a protective agent against consequences of myocardial I/R injury via PI3K/Akt/eNOS pathway.
References
Cannon, B. (2013). Cardiovascular disease: Biochemistry to behaviour. Nature, 2013(493), S2–S3.
Murray, C. J., & Lopez, A. D. (1997). Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet, 1997(349), 1498–1504.
Piper, H. M., Garcia-Dorado, D., & Ovize, M. (1998). A fresh look at reperfusion injury. Cardiovascular Research, 1998(38), 291–300.
Rohilla, A., Khan, M. U., & Khanam, R. (2012). Cardioprotective potential of simvastatin in the hyperhomocysteinemic rat heart. Journal of Advanced Pharmaceutical Technology & Research, 2012(3), 193–198.
Kawamoto, A., Gwon, H. C., Iwaguro, H., Yamaguchi, J. I., Uchida, S., Masuda, H., et al. (2001). Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation, 2001(103), 634–637.
Tsang, A., Hausenloy, D. J., Mocanu, M. M., & Yellon, D. M. (2004). Postconditioning: A form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circulation Research, 2004(95), 230–232.
Ehrenreich, H., Timner, W., & Siren, A. L. (2004). A novel role for an established player: Anemia drug erythropoietin for the treatment of cerebral hypoxia/ischemia. Transfusion and Apheresis Science, 2004(31), 39–44.
Kiss, L., Deitch, E. A., & Szabo, C. (2008). Hydrogen sulfide decreases adenosine triphosphate levels in aortic rings and leads to vasorelaxation via metabolic inhibition. Life Sciences, 2008(83), 589–594.
Li, S., Dong, P., Wang, J., Zhang, J., Gu, J., Wu, X., et al. (2010). Icariin, a natural flavonol glycoside, induces apoptosis in human hepatoma SMMC-7721 cells via a ROS/JNK-dependent mitochondrial pathway. Cancer Letters, 2010(298), 222–230.
Liu, Z. Q. (2006). Icariin: A special antioxidant to protect linoleic acid against free-radical-induced peroxidation in micelles. Journal of Physical Chemistry A, 2006(110), 6372–6378.
Liu, Z. Q., Luo, X. Y., Sun, Y. X., Wu, W., Liu, C. M., Liu, Z. Q., & Liu, S. Y. (2004). The antioxidative effect of icariin in human erythrocytes against free-radical-induced haemolysis. Journal of Pharmacy and Pharmacology, 2004(56), 1557–1562.
Pan, Y., Kong, L., Xia, X., Zhang, W., Xia, Z., & Jiang, F. (2005). Antidepressant-like effect of icariin and its possible mechanism in mice. Pharmacology, Biochemistry and Behavior, 2005(82), 686–694.
Pan, Y., Wang, F. M., Qiang, L. Q., Zhang, D. M., & Kong, L. D. (2010). Icariin attenuates chronic mild stress-induced dysregulation of the LHPA stress circuit in rats. Psychoneuroendocrinology, 2010(35), 272–283.
Zeng, K. W., Fu, H., Liu, G. X., & Wang, X. M. (2010). Icariin attenuates lipopolysaccharide-induced microglial activation and resultant death of neurons by inhibiting TAK1/IKK/NF-kappaB and JNK/p38 MAPK pathways. International Immunopharmacology, 2010(10), 668–678.
Wu, J., Zhou, J., Chen, X., Fortenbery, N., Eksioglu, E. A., Wei, S., & Dong, J. (2012). Attenuation of LPS-induced inflammation by ICT, a derivate of icariin, via inhibition of the CD14/TLR4 signaling pathway in human monocytes. International Immunopharmacology, 2012(12), 74–79.
Liu, B., Zhang, H., Xu, C., Yang, G., Tao, J., Huang, J., et al. (2011). Neuroprotective effects of icariin on corticosterone-induced apoptosis in primary cultured rat hippocampal neurons. Brain Research, 2011(1375), 59–67.
Wang, Q., Hao, J., Pu, J., Zhao, L., Lu, Z., Hu, J., et al. (2011). Icariin induces apoptosis in mouse MLTC-10 Leydig tumor cells through activation of the mitochondrial pathway and down-regulation of the expression of piwil4. International Journal of Oncology, 2011(39), 973–980.
Yang, J. X., Fichtner, I., Becker, M., Lemm, M., & Wang, X. M. (2009). Anti-proliferative efficacy of icariin on HepG2 hepatoma and its possible mechanism of action. American Journal of Chinese Medicine, 2009(37), 1153–1165.
Bao, H., & Chen, L. (2011). Icariin reduces mitochondrial oxidative stress injury in diabetic rat hearts. Zhongguo Zhong Yao Za Zhi., 2011(36), 1503–1507.
Song, Y. H., Cai, H., Gu, N., Qian, C. F., Cao, S. P., & Zhao, Z. M. (2011). Icariin attenuates cardiac remodelling through down-regulating myocardial apoptosis and matrix metalloproteinase activity in rats with congestive heart failure. Journal of Pharmacy and Pharmacology, 2011(63), 541–549.
Zhang, Q., Li, H., Wang, S., Liu, M., Feng, Y., & Wang, X. (2013). Icariin protects rat cardiac H9c2 cells from apoptosis by inhibiting endoplasmic reticulum stress. International Journal of Molecular Sciences, 2013(14), 17845–17860.
Wu, H., Yan, Y., & Backer, J. M. (2007). Regulation of class IA PI3Ks. Biochemical Society Transactions, 2007(35), 242–244.
Vanhaesebroeck, B., Leevers, S. J., Panayotou, G., & Waterfield, M. D. (1997). Phosphoinositide 3-kinases: A conserved family of signal transducers. Trends in Biochemical Sciences, 1997(22), 267–272.
Ha, T., Hua, F., Liu, X., Ma, J., McMullen, J. R., Shioi, T., et al. (2008). Lipopolysaccharide-induced myocardial protection against ischaemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovascular Research, 2008(78), 546–553.
Chung, B. H., Kim, J. D., Kim, C. K., Kim, J. H., Won, M. H., Lee, H. S., et al. (2008). Icariin stimulates angiogenesis by activating the MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways in human endothelial cells. Biochemical and Biophysical Research Communications, 2008(376), 404–408.
Xu, C. Q., Liu, B. J., Wu, J. F., Xu, Y. C., Duan, X. H., Cao, Y. X., & Dong, J. C. (2010). Icariin attenuates LPS-induced acute inflammatory responses: Involvement of PI3K/Akt and NF-kappaB signaling pathway. European Journal of Pharmacology, 2010(642), 146–153.
Ji, Y., Pang, Q. F., Xu, G., Wang, L., Wang, J. K., & Zeng, Y. M. (2008). Exogenous hydrogen sulfide postconditioning protects isolated rat hearts against ischemia-reperfusion injury. European Journal of Pharmacology, 2008(587), 1–7.
Bar-Or, D., Lau, E., & Winkler, J. V. (2000). A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia—A preliminary report. Journal of Emergency Medicine, 2000(19), 311–315.
Ertl, G., & Frantz, S. (2005). Wound model of myocardial infarction. American Journal of Physiology Heart and Circulatory Physiology, 2005(288), H981–H983.
Ma, X. L., Kumar, S., Gao, F., Louden, C. S., Lopez, B. L., Christopher, T. A., et al. (1999). Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation, 1999(99), 1685–1691.
Sedlak, T. W., Oltvai, Z. N., Yang, E., Wang, K., Boise, L. H., Thompson, C. B., & Korsmeyer, S. J. (1995). Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proceedings of National Academy of Sciences of the United States of America, 1995(92), 7834–7838.
Acknowledgments
This study was supported by a grant from the National High Technology Research and Development Program of China (863 Program, No. 2011AA02A111).
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhai, M., He, L., Ju, X. et al. Icariin Acts as a Potential Agent for Preventing Cardiac Ischemia/Reperfusion Injury. Cell Biochem Biophys 72, 589–597 (2015). https://doi.org/10.1007/s12013-014-0506-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0506-3